Accumulating evidence suggests that sports-related concussion has long-term sequelae. Tremblay et al. show that ageing retired athletes with a history of concussions exhibit diffuse abnormalities in white matter tracts. The abnormalities are associated with cognitive decline relative to matched controls, suggesting that concussions exacerbate the ageing process.
Keywords: sports-related concussions, ageing, white matter, neuropsychology
Abstract
Sports-related concussions have been shown to lead to persistent subclinical anomalies of the motor and cognitive systems in young asymptomatic athletes. In advancing age, these latent alterations correlate with detectable motor and cognitive function decline. Until now, the interacting effects of concussions and the normal ageing process on white matter tract integrity remain unknown. Here we used a tract-based spatial statistical method to uncover potential white matter tissue damage in 15 retired athletes with a history of concussions, free of comorbid medical conditions. We also investigated potential associations between white matter integrity and declines in cognitive and motor functions. Compared to an age- and education-matched control group of 15 retired athletes without concussions, former athletes with concussions exhibited widespread white matter anomalies along many major association, interhemispheric, and projection tracts. Group contrasts revealed decreases in fractional anisotropy, as well as increases in mean and radial diffusivity measures in the concussed group. These differences were primarily apparent in fronto-parietal networks as well as in the frontal aspect of the corpus callosum. The white matter anomalies uncovered in concussed athletes were significantly associated with a decline in episodic memory and lateral ventricle expansion. Finally, the expected association between frontal white matter integrity and motor learning found in former non-concussed athletes was absent in concussed participants. Together, these results show that advancing age in retired athletes presenting with a history of sports-related concussions is linked to diffuse white matter abnormalities that are consistent with the effects of traumatic axonal injury and exacerbated demyelination. These changes in white matter integrity might explain the cognitive and motor function declines documented in this population.
Introduction
Surveys including head injury for which no medical assistance is sought estimate that the incidence of sports concussions is somewhere between 1.6 to 3.8 million annually in the USA alone (Langlois et al., 2006), thus making any potential long-term consequence of the injury sizeable in both human and economic terms. The consequences of sport-related injuries have received even more intensified scrutiny in the popular media as notorious professional athletes that had suffered multiple injuries, regardless of severity, showed early signs of neurodegeneration (McKee et al., 2009, 2013). Unfortunately, research addressing the potential association between concussion and neurodegeneration is sparse (Klein et al., 1996; Monti et al., 2013), partly because the characterization of the long-term effects of sport-related concussion has only been acknowledged by the scientific community within the last decade. Indeed, while the acute effects of concussions have been extensively studied to make better informed return-to-play decisions (review in Giza et al., 2013), the chronic effects of these mild head traumas have only recently been addressed systematically (review in De Beaumont et al., 2012a). Moreover, the potential interaction between the long-term effects of sports concussions and the ageing process has only been touched on by a few investigators thus far (Guskiewicz et al., 2005; De Beaumont et al., 2009, 2013; Broglio et al., 2012; Didehbani et al., 2013; Hart et al., 2013; Strain et al., 2013; Tremblay et al., 2013).
Little is known about the synergistic effects between ageing and a history of sports concussions and how this interaction might exacerbate cognitive decline later in life. We know, however, that the normal ageing process is associated with significant changes in both grey and white matter in the brain (Raz and Rodrigue, 2006; Fjell et al., 2013). Grey matter volume loss is particularly salient within the parenchyma of both the frontal and temporal lobes (Raz et al., 1997; Tisserand et al., 2002; Masliah et al., 2006; Fjell et al., 2009). White matter is also highly vulnerable to the ageing process, with an estimated volume loss of 45% between the ages of 20 and 80 years (Salat et al., 1999; Marner et al., 2003). Age-related white matter anomalies are thought to partly underlie the decline in cognitive function typical of the normal ageing process, which encompasses domains of information processing speed, psychomotor speed, postural stability, memory, attention and executive functions (Gunning-Dixon et al., 2009; Madden et al., 2009).
The long-term pathophysiology of sports concussion shows some overlap with normal age-related neurocognitive downturn. Namely, accelerated age-related cognitive decline is observed in functions such as episodic memory and attention in retired athletes with a remote history of sports concussions relative to same-aged peers without a history of concussion (De Beaumont et al., 2009). Moreover, retired athletes with a history of concussions exhibit significant decline in motor execution speed as well as sequential motor learning compared to age- and education-matched controls (De Beaumont et al., 2013). Importantly, these functional manifestations were found to be correlated with electrophysiological and metabolic anomalies in brain regions implicated in the generation of these behaviours. More recently, structural investigations have revealed that the ventricular system, a usual target of age-related effects, was significantly enlarged in aged, but otherwise healthy retired athletes with a history of concussions (Tremblay et al., 2013). However, apart from an interaction between age and the cortical thickness of areas most susceptible to the effects of ageing, not a single grey matter anomaly, including atrophy and morphometry measures of all cortical and subcortical structures of the brain, was uncovered when comparing former concussed athletes with non-concussed controls. Thus we are presented with a paradox: how can the ventricular system be enlarged without any change in grey matter? This leaves white matter as the final substrate to account for the balance, which is already known to change as a part of the ageing process.
The development of powerful neuroimaging tools has enabled us to investigate the integrity of whole-brain white matter volume. Diffusion tensor imaging (DTI) allows the quantification of water molecules’ diffusion across brain tissues (Basser and Jones, 2002) where the direction and amplitude of this diffusion can be modelled (Assaf and Pasternak, 2008; Basser and Pierpaoli, 2011). Fractional anisotropy is a measure of diffusion along the longitudinal axis of the axon. A decline in fractional anisotropy is thought to indicate damage to white matter (Mac Donald et al., 2007a). Another measure, mean diffusivity, reflects overall diffusion amplitude regardless of directionality, which is expected to be elevated in membrane-free spaces such as the lateral ventricles, but restricted in cell-dense regions such as grey matter and white matter. Finally, metrics of axial and radial diffusivity, respectively reflect diffusion amplitude along the main direction of the tensor, and diffusion amplitude along the directions orthogonal to the main directions of the tensor. Axial and radial diffusivity are thought to reflect axonal and myelin loss, respectively (Song et al., 2002; Sun et al., 2006).
DTI technique has recently been improved by a novel computational method that allows a robust between-subject analysis of all major white matter tracts in the human brain. Tract-based spatial statistics (TBSS) involves co-registering fibre tracts of all subjects to a common ‘skeleton’ and performing statistics on every voxel of this 3D network, including correlational analyses between white matter integrity metrics and neurocognitive measurements (Smith et al., 2006). Therefore, whole-brain white matter anomalies patterns affecting specific tracts can be related to particular cognitive/motor impairments in clinical populations. Multiple groups trying to uncover the nature of acute and chronic symptoms in concussed athletes have applied this technique (Zhang et al., 2010; Cubon et al., 2011; Koerte et al., 2012; Chamard et al., 2013; Hart et al., 2013; Strain et al., 2013) or older techniques (Zhang et al., 2003, 2006; Chappell et al., 2006; Henry et al., 2011; Bazarian et al., 2012; Marchi et al., 2013; Virji-Babul et al., 2013). Diffuse anomalies during the acute and sub-acute periods are described in most cases along various major interhemispheric, associative, and projection fibre tracts, although no consistent spatial pattern of injury seems to emerge from those studies (Gardner et al., 2012).
Recently, Hart and colleagues (2013) investigated white matter integrity in retired athletes with a history of sports concussions. Participants from the latter study were ex-NFL players, of whom half were affected by neuropsychiatric (i.e. depression) or neurological (i.e. dementia or mild cognitive impairment, MCI) conditions. The authors separated subjects into cognitively normal and cognitively impaired groups of concussed players for statistical comparison. When looking at diffusion-based measures of white matter integrity, the authors found significant differences between cognitively impaired athletes and a matched control group of non-athletes, but found no difference between clinically normal retired players with concussion history and the control group. Therefore, this investigation recapitulated what is already known of the effects of MCI, dementia, and depression on white matter integrity, failing to dissociate the effects of such clinical conditions and those of sports concussions. Studies showing a high level of control on confounding factors such as clinical comorbidity are still required to unravel the effects of ageing with a history of concussion on white matter tissue integrity.
The current study presents the first investigation of white matter integrity on aged but clinically normal retired athletes with a history of sports-related concussions. In this study, we have focused on eliminating confounding factors such as drug abuse, clinical comorbidity and genetic predisposition that are currently obfuscating the long-term effects of concussions (Mccrory et al., 2013). We used TBSS to assess whole-brain white matter integrity in conjunction with an exhaustive neuropsychological test battery to investigate plausible neurocognitive correlations. In addition, previously acquired structural imaging data of ventricular and grey matter integrity are re-analysed in the context of the current investigation to examine their relationship to white matter findings.
Materials and methods
Participants
The sample of participants is the same as the one described in a previous study by our group (Tremblay et al., 2013). All participants included in this study were former male university-level athletes between the ages of 51 and 75 years, recruited with the help of university athletics organizations. Participants played either for their respective university’s ice hockey (70%) or American football (30%) teams. Participants were excluded if they presented any of the following characteristics: a history of alcohol and/or substance abuse, a current or a history of neurological or psychiatric condition (e.g. MCI, Alzheimer's disease, depression), a medical condition requiring daily medication or radiotherapy (malignant cancer, diabetes, hypertension, and/or other cardiovascular diseases), a learning disability (e.g. dyslexia), or traumatic brain injury (TBI) unrelated to contact sports (e.g. fall, motor vehicle accident, assault). All participants had to score ≤9 on the Beck Depression Inventory II to rule out the presence of depressive symptoms. Moreover, participants were not to have sustained sports-related concussions since the end of their university athletic career. To better control for data contamination due to the protective properties of regular physical activity on age-related cognitive function (Lindsay et al., 2002), participants had to report engaging regularly in physical activity at least three times a week at the time of testing and to have maintained this level of activity since the end of their athletic career. In addition, all participants had to have a body mass index (BMI) <30 kg/m2, according to the criteria for obesity of the World Health Organization.
Participants were divided into two groups as a function of their sports-related concussion history. The experimental group consisted of 15 former university-level athletes with a mean age of 60.87 years [standard deviation (SD) 7.51] and a mean level of education of 16.67 years (SD 4.07) who sustained their last sports concussion in early adulthood (mean 24.00 and SD 4.55). A standardized concussion history questionnaire (Collins et al., 2002; De Beaumont et al., 2009) was administered in an interview setting by an experienced sports physician to obtain detailed information about the number of previous concussions, their approximate date, the description of the accident, and the nature and duration of on-field post-concussion severity markers (confusion and/or disorientation, retrograde and/or anterograde amnesia, and loss of consciousness). Concussion was defined according to the definition provided by the 2009 Consensus Statement on Concussion in Sports (Mccrory et al., 2009) as ‘a complex pathophysiological process affecting the brain, induced by traumatic biomechanical forces, that results in the rapid onset of short-lived impairment of neurologic function that may or may not include loss of consciousness (LOC).’ The number of reported concussions sustained by the experimental group ranged from 1 to 5 (mean 2.08 and SD 1.31) and the time elapsed since the last concussion spanned from 29 to 53 years (mean 37.08 and SD 7.10). All brain injuries classified as ‘mild’ on the Glasgow Coma Scale (scores ranging from 13 to 15) and did not lead to chronic complications (e.g. post-concussion syndrome or other persistent clinical symptoms).
The control group consisted of 15 former university-level athletes with a mean age of 58.13 (SD 5.28) and a mean level of education of 17.27 (SD 3.45) who had no prior history of concussion. The two groups did not differ according to age [t(28) = 1.15, P = 0.259], level of education [t(28) = 0.44, P = 0.666], or frequency of the APOE ε 4 allele (Fisher’s exact test P = 1.00). The study was approved by the local ethics committees and all participants provided written informed consent before testing in accordance with the Declaration of Helsinki (World Medical Association, 2013).
Neuropsychological testing
Experimental procedures are as described in a previous article (Tremblay et al., 2013). Participants underwent two testing sessions. The first session included the interview with the sports physician to assess inclusion criteria and group classification using a general health questionnaire and a concussion history questionnaire. Following examination by the physician, a neuropsychologist blinded to group membership administered an exhaustive neuropsychological test battery aiming to assess age-related neurocognitive decline. The battery included tests of general cognitive ability (Mini-Mental State Examination), verbal fluency (semantic and phonemic), verbal and visual episodic memory (Rey Auditory Verbal Learning Test and Taylor Complex Figure Test), attention (Eriksen Flanker Task) and processing speed (Trail Making Test A and B and Symbol Digit Modalities Test). A computerized Serial Reaction Time Task was also administered to assess sequence-specific and non-specific motor learning (De Beaumont et al., 2013) for method). The Beck Depression Inventory-II was administered after the completion of the neuropsychological test battery.
Genotyping
At the end of the first session, saliva samples were collected to establish the APOE genotype of participants. DNA extraction from saliva samples was performed using Oragene OG-250 s kits (DNA Genotek) and participants were genotyped for APOE 112 (rs429358)-158 (rs7412) polymorphisms. PCR amplification was carried out as previously described (Petersen et al., 2005). APOE polymorphisms were subsequently determined via an established pyrosequencing protocol (Petersen et al., 2005).
Neuroimaging
The second session consisted of the neuroimaging evaluation. All magnetic resonance examinations were performed on a Siemens 3 T Magnetom TIM TRIO scanner with a 12-channel head coil (Siemens). T1 and T2-weighted images of the whole brain were acquired using an MP-RAGE and a turbo-spin echo sequence, respectively. Specific parameters of the acquisition sequences are described elsewhere (Tremblay et al., 2013). From the T1 images, we investigated peripheral grey matter tissue morphology using an optimized voxel-based morphometry analysis (Good et al., 2001) using the tools from the FSL toolbox (Smith et al., 2004). From the same images, we quantified gross brain tissue volumes of grey matter, white matter, and CSF, normalized for subjects’ intracranial volume, using the SIENAX tool (Smith et al., 2002). We also assessed sub-cortical grey matter volume and morphology using the FIRST tool from the same toolbox (Patenaude et al., 2011). Lastly, we assessed cortical grey matter integrity using a cortical thickness analysis implemented in the CIVET pipeline from the McConnell Brain Imaging Centre at McGill University (Lyttelton et al., 2007).
For the purpose of DTI analysis, diffusion-weighted volumes were acquired using gradients applied in 32 non-colinear directions. The following parameters were used: 75 contiguous slices; slice thickness = 2 mm; field of view = 256 × 256 mm2; matrix size of 128 × 128; voxel size = 2 mm isotropic; repetition time = 9200 ms; echo time = 84 ms; and b values of b = 0 and b = 1000 s/mm2.
DTI analysis
The diffusion images were preprocessed using the Imeka pipeline (www.imeka.ca). The pipeline involved: (i) denoising the diffusion images using NLMeans tools (Wiest-Daesslé et al., 2008); (ii) upsampling the diffusion images to 1 mm isotropic to fit the T1 image resolution; (iii) masking of the white matter using segmented anatomical MRI of the subject with FAST from the FSL package (www.fmrib.ox.ac.uk/fsl/); and (iv) computing the fractional anisotropy, mean, axial and radial diffusivity maps using the diffusion tensor model with the software MRtrix (www.brain.org.au/software/mrtrix/).
Voxel-wise analysis of the fractional anisotropy, mean, axial and radial diffusivity data was carried out using TBSS in the FMRIB Software Library. Image analysis using TBSS involved a number of steps: (i) non-linear alignment of all subjects’ fractional anisotropy images to the most representative subject of the current aged cohort; (ii) affine-transformation of the aligned images into standard MNI152 1 mm space; (iii) averaging of the aligned fractional anisotropy images to create a 4D mean fractional anisotropy image; (iv) thinning of the mean fractional anisotropy image to create a mean fractional anisotropy ‘skeleton’ representing the centre of all white matter tracts, and in this way removing partial volume confounds; and (v) thresholding of the fractional anisotropy skeleton at fractional anisotropy >0.3 to suppress areas of extremely low mean fractional anisotropy and excluding those with considerable interindividual variability. Similar steps for processing non-fractional anisotropy images were then carried out to obtain the mean, axial and radial diffusivity images. Non-parametric permutation-based statistics were employed using ‘Randomize’ with threshold-free cluster enhancement and 5000 permutations. A threshold of P < 0.05 was then applied on the results, corrected for multiple comparisons. Age and intracranial volume were included as covariates of no interest in all TBSS analyses.
Results
Demographics and cognitive function
The demographic information and the neuropsychological assessment results for each group are summarized in Table 1. These results were presented in previous studies (De Beaumont et al., 2013; Tremblay et al., 2013) and are reproduced here to justify the correlational analyses carried out between the cognitive variables and the new DTI findings. None of the participants presented any signs of depression (BDI-II scores ≤ 9), dementia (MMSE ≥ 27), or any other symptoms of neurological or psychiatric conditions sufficient to fulfil the criteria for a clinical diagnosis. However, neuropsychological assessment revealed subclinical cognitive alterations in the concussed group in memory and executive function domains. Relative to controls, former athletes with concussions showed reduced semantic verbal fluency and episodic memory on both delayed recall and recognition conditions of the Taylor Complex Figure Test, while performance on the copy trial was not different across groups. They also exhibited higher retroactive interference on the Rey Auditory Verbal Learning Test. Finally, concussed participants benefited significantly less from motor training on the Serial Reaction Time Task, as demonstrated by a significant group difference on sequence-specific learning.
Table 1.
Demographic, neuropsychological and imaging data
| Measures | Controls mean (SD) | Concussed mean (SD) | P-value |
|---|---|---|---|
| n | 15 | 15 | |
| Age | 58.13 (5.28) | 60.87 (7.51) | 0.26 |
| Hockey players (%) | 70 | 70 | |
| Education (years) | 17.27 (3.45) | 16.67 (4.06) | 0.67 |
| APOE ε-4 (% positive) | 2 (13,33) | 2 (13,33) | 1.0a |
| MMSE | 29.40 (1.12) | 29.20 (0.86) | 0.31b |
| BDI-II | 2.93 (3.08) | 3.43 (3.29) | 0.67b |
| TCFT (# items drawn) | |||
| Copy | 35.86 (0.36) | 35.33 (1.29) | 0.16 |
| Immediate recall | 30.14 (2.58) | 27.80 (3.95) | 0.07 |
| Delayed recall | 30.18 (2.47) | 27.33 (3.83) | 0.03 |
| Recognition | 20.36 (1.28) | 18.60 (2.19) | 0.02 |
| RAVLT (# of words) | |||
| Trials 1 to 5 total | 54.87 (7.83) | 52.33 (5.79) | 0.32 |
| Delayed recall | 12.20 (2.01) | 11.00 (2.67) | 0.18 |
| Recognition | 13.07 (1.91) | 12.67 (1.99) | 0.58 |
| Proactive interference | 1.00 (1.36) | 0.93 (1.44) | 0.90 |
| Retroactive interference | 0.93 (1.03) | 2.13 (1.85) | 0.04 |
| SDMT (# of correct digits) | 54.00 (8.60) | 49.53 (7.43) | 0.14 |
| Colour Trails Test (s) | |||
| Form A | 35.13 (10.61) | 32.43 (6.43) | 0.42 |
| Form B | 74.13 (26.86) | 73.00 (16.58) | 0.89 |
| Verbal fluency (words) | |||
| Phonemic | 46.00 (7.80) | 49.67 (11.31) | 0.31 |
| Semantic | 52.93 (8.67) | 46.40 (7.69) | 0.04 |
| SRTT (gain in ms) | |||
| Sequence specific | 94.19 (39.39) | 48.75 (63.43) | 0.04 |
APOE ε 4 = proportion of participants with a ε 4 allele; MMSE = Mini-Mental State Examination; BDI-II = Beck Depression Inventory II; RAVLT = Rey Auditory Verbal Learning Test; SDMT = Symbol Digit Modalities Test; SRTT = Serial Reaction Time Task; TCFT = Taylor Complex Figure Test.
aFisher’s exact test.
bMann-Whitney’s U.
Conventional imaging
Neuroradiological examination of T1 and T2-weighted images revealed benign alterations of brain parenchyma consistent with the effects of normal ageing. Slightly dilated Virchow Robin spaces were found in 33% of controls and in 20% of patients. Benign leukoencephalopathy of probable arteriosclerosis origin were detected in 47% of controls and 40% of patients. These observations were considered to be within the normal range for age and of no clinical significance. No other anomalies affecting white matter, cortical or subcortical grey matter, or the ventricular system were found in either group. No microbleeds or overt signs of diffuse axonal injury were detectable in the current sample using conventional imaging techniques.
Structural imaging
These results were presented in previous studies (De Beaumont et al., 2013; Tremblay et al., 2013) and are reproduced here to justify the correlational analyses carried out between the structural imaging variables and the new DTI findings. The structural analysis of peripheral grey matter morphometry using FSL-VBM revealed no group differences across the whole brain. Moreover, peripheral grey matter volume as well as cortical thickness measurements revealed no main effect of group. Subcortical grey matter morphometry and volumetry did not lead to significant group differences either. Overall, at the achieved statistical power, no grey matter abnormalities were uncovered when comparing group means. However, when looking at the effect of age on cortical thickness, a significant group by age interaction was uncovered on the cortical thickness of various clusters situated over frontal and temporal regions (see Fig. 3 in Tremblay et al., 2013).
Figure 3.
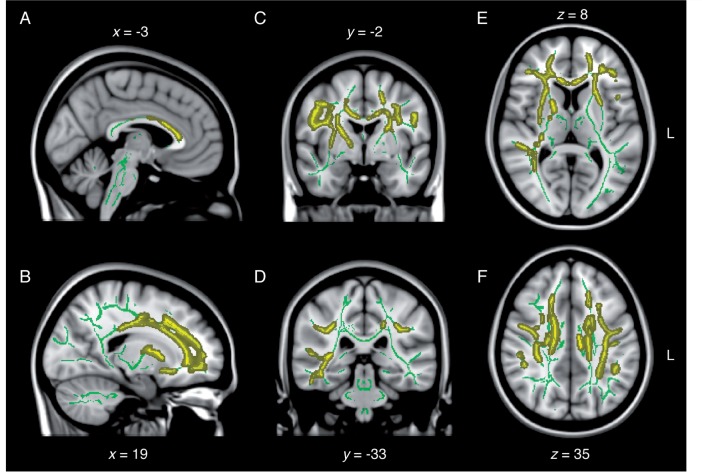
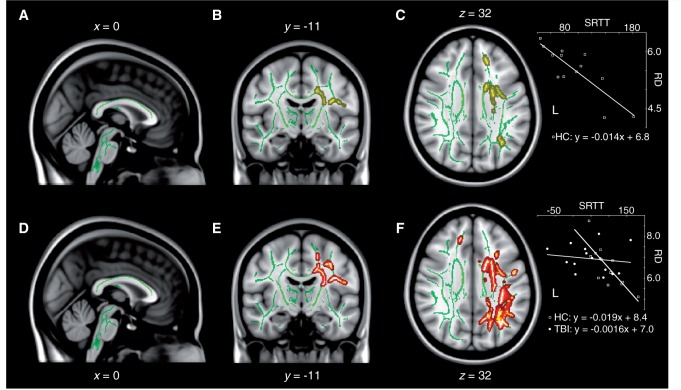
Diffuse increase in radial diffusivity following remote concussions. Sagittal (A and B), coronal (C and D), and axial (E and F) slices of the TBSS group contrast on radial diffusivity maps (controls > concussed; yellow). The contrasts are overlaid on the mean fractional anisotropy skeleton (in green) and the standard MNI152 T1 1 mm brain template. The results are thresholded at P < 0.05, corrected for multiple comparisons. See Supplementary Fig. 3 for effect size maps.
When looking at the ventricular system, a significant expansion of the lateral ventricles was observed in the concussed group, which correlated significantly with abnormal delayed recall and recognition performance of the Taylor Complex Figure Test.
Diffuse white matter anomalies revealed by DTI
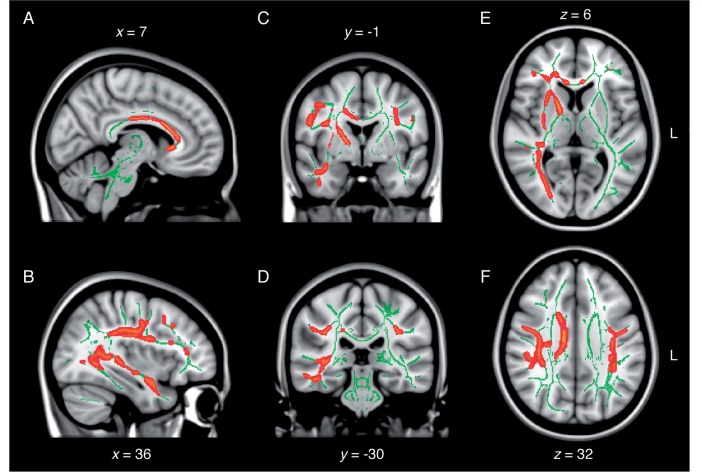
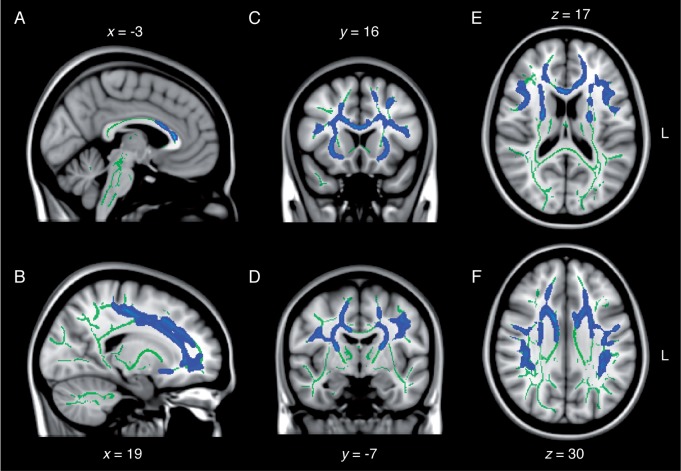
TBSS revealed a diffuse pattern of white matter anomalies throughout the concussed ageing brain in comparison to the non-concussed ageing brain. In general, group differences implicated decreased fractional anisotropy, increased mean diffusivity as well as increased radial diffusivity in the concussed group. Specifically, lower fractional anisotropy was observed in inter-hemispheric fibres of the corpus callosum (anterior body and genu) and forceps minor, and in intra-hemispheric association fibres of the right inferior longitudinal fasciculus, the right inferior fronto-occipital fasciculus and bilateral superior longitudinal fasciculi (Fig. 1). Projection fibres showing signs of lower fractional anisotropy included the anterior limb of the right internal capsule, the right external capsule, as well as the right corona radiata probably involving the corticospinal tract in its dorsal aspect (Fig. 1). The same contrast revealed a similar pattern of anomalies pertaining to increased mean diffusivity in major fibre tracts in the concussed brain (Fig. 2). Namely, the anterior body and the genu of the corpus callosum, as well as the forceps minor, exhibited higher mean diffusivity compared to controls. Moreover, association fibres such as bilateral uncinate fasciculi, the anterior aspects of the inferior fronto-occipital fasciculi, and superior longitudinal fasciculi in their frontal and parietal aspects showed similar anomalies (Fig. 2). Affected projection fibres included the external capsule, the anterior and posterior limb of the internal capsule, and the frontal aspects of corona radiata involving bilateral corticospinal tracts.
Figure 1.
Diffuse decrease in fractional anisotropy following remote concussions. Sagittal (A and B), coronal (C and D), and axial (E and F) slices of the TBSS group contrast on fractional anisotropy maps (controls < concussed; red). The contrasts are overlaid on the mean fractional anisotropy skeleton (in green) and the standard MNI152 T1 1 mm brain template. The results are thresholded at P < 0.05, corrected for multiple comparisons. See Supplementary Fig. 1 for effect size maps. L = left.
Figure 2.
Diffuse increase in mean diffusivity following remote concussions. Sagittal (A and B), coronal (C and D), and axial (E and F) slices of the TBSS group contrast on mean diffusivity maps (controls < concussed; blue). The contrasts are overlaid on the mean fractional anisotropy skeleton (in green) and the standard MNI152 T1 1 mm brain template. The results are thresholded at P < 0.05, corrected for multiple comparisons. See Supplementary Fig. 2 for effect size maps. L = left.
As opposed to fractional anisotropy maps, mean diffusivity maps did not involve inferior temporal or occipital aspects of major fibre tracts (e.g. inferior longitudinal fasciculus), but did exhibit a higher hemispheric symmetry of frontal and parietal anomalies. Radial diffusivity maps revealed extensive anomalies encompassing nearly all fibre tracts affected by abnormal mean diffusivity, with the exception of the dorsal most portion of the right corona radiata underneath the right medial primary motor cortex and the right superior frontal gyrus (Fig. 3). Furthermore, radial diffusivity was abnormally elevated in the temporal and occipital aspects of the right inferior longitudinal fasciculus, which also showed decreased fractional anisotropy (Fig. 3). No white matter tracts exhibited significantly different axial diffusivity across groups. Moreover, there were no white matter tracts showing either increased fractional anisotropy, decreased mean diffusivity or decreased axial diffusivity in the concussed group. Interestingly, not a single map revealed diffusion anomalies in the fornices, or in midbrain, pontine, cerebellar or medullar fibres of the brainstem of concussed individuals. The general pattern of abnormality could be characterized as mainly fronto-parietal, and to some extent temporal on the right side. The affected sections of the main inter hemispheric fibre tract (i.e. corpus callosum) were consistent with a fronto-parietal pattern of white matter anomalies (Figs 1–3).
Regression analysis of white matter integrity and cognition
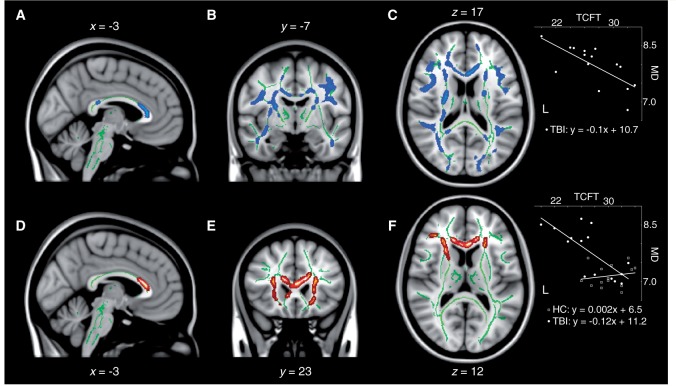
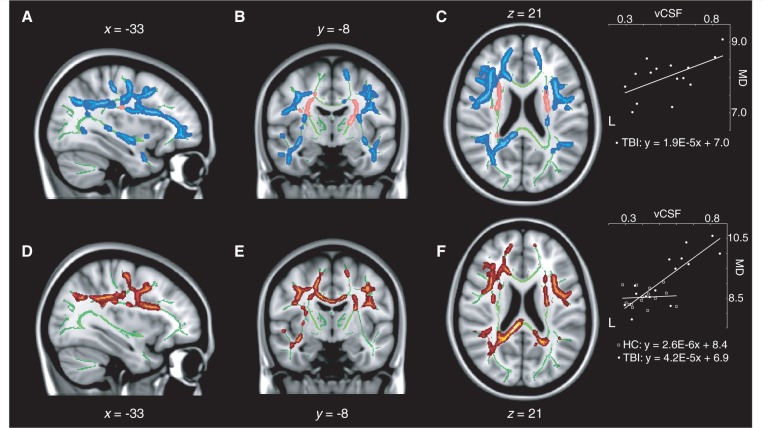
Numerous correlations were found between measures of cognitive function and the various white matter integrity indices. First, visual episodic memory function was found to be correlated to mean diffusivity, axial diffusivity and radial diffusivity in the concussed group, such that increases in those diffusivity measures predicted memory decline on the delayed recall of the Taylor Complex Figure Test. In the cases of mean diffusivity and radial diffusivity maps, for which significant group differences were uncovered, clusters of significant association were dispersed across the entire fronto-parietal network described in the previous section, in addition to both associated inferior longitudinal fasciculi (Fig. 4A–C). The integrity of the anterior body and genu of the corpus callosum was also predictive of episodic memory function. With regards to the axial diffusivity map, for which no group differences were found, small bilateral clusters in the frontal poles at the rostral extremity of the forceps minor were the only observations of significant associations. No relationship was found between measures of fractional anisotropy and visual episodic memory performance on this test in concussed individuals. Moreover, none of these structure-function relationships were present in the control group. Interaction analyses revealed a significant group interaction on the relationship between mean diffusivity measurements and memory function, whereby elevated mean diffusivity in concussed participants was predictive of reduced performance on the Taylor Complex Figure Test, while no such relationship was present in control participants (Fig. 4D–F). Voxels of significant interaction were mainly located in the anterior body and genu of the corpus callosum, the forceps minor and anterior corona radiata extending in the bilateral prefrontal lobes.
Figure 4.
Decreases in visual memory correlate with increases in mean diffusivity (MD) in concussed participants. Sagittal (A), coronal (B), and axial (C) slices of the regression analysis between delayed recall at the Taylor Complex Figure Test (TCFT) and mean diffusivity in concussed participants. (D, E and F) Group interaction analysis on the relationship between delayed recall at the Taylor Complex Figure Test and mean diffusivity. The results are thresholded at P < 0.05, corrected for multiple comparisons. Insets illustrate linear relationships at one example voxel.
Regression analysis of white matter integrity and motor learning
The integrity of various white matter tracts correlated with sequential motor learning in healthy controls. The radial diffusivity of the left corona radiata, including the left corticospinal tract in its central aspect, as well as its frontal radiations into primary motor and premotor cortices, was predictive of good motor learning (Fig. 5A–C). This result is expected as participants completed the motor task with their contralateral limb (i.e. right hand). Measures of mean diffusivity in the left frontal lobe also correlated negatively with motor learning in healthy controls, albeit to a spatial extent restricted to the dorso-rostral radiations of the left corona radiata feeding into the left superior frontal gyrus. No measures of fractional anisotropy or axial diffusivity explained any variance in motor learning efficiency in controls. Interestingly, not a single anatomical-functional relationship uncovered in controls was replicated in the concussed group. In fact, a significant group interaction was observed over the radial diffusivity of many major left hemisphere tracts (Fig. 5D–F). More specifically, the relationship between radial diffusivity in left hemisphere tracts and sequential motor learning was significantly different in the concussed group across the entire left corona radiata including the left corticospinal tract, as well as the left superior longitudinal fasciculus. Importantly, the area of significant interaction covered all tracts that were previously found to be correlated with the extent of motor learning in healthy controls (Fig. 5). In summary, the normal negative relationship between radial diffusivity in the left corona radiata and sequential motor learning with the right hand was disrupted in remotely concussed individuals.
Figure 5.
Abnormal relationship between sequential motor learning and radial diffusivity in concussed participants. Sagittal (A), coronal (B), and axial (C) slices of the regression analysis between sequential motor learning at the Serial Reaction Time Task (SRTT) and radial diffusivity (RD) in control (HC) participants. (D–F) Group interaction analysis on the relationship between sequential motor learning at the Serial Reaction Time Task and radial diffusivity. The results are thresholded at P < 0.05, corrected for multiple comparisons. Insets illustrate linear relationships at one example voxel. L = left.
Regression analysis of white matter integrity and ventricular system
As previously stated, the concussed group presented significantly enlarged lateral ventricles compared to the control group. The extent of this ventricular enlargement was found to be correlated with multiple indices of white matter integrity. Axial, radial and mean diffusivity were all found to be positively correlated with ventricular enlargement in the concussed group across a wide array of white matter tracts. In particular, mean diffusivity across the entire network of fronto-parietal connections that was previously shown to be significantly different across groups was found to be positively related to the volume of the lateral ventricles in concussed individuals (Fig. 6A–C). In addition, this relationship involved both temporal lobes and inferior longitudinal fasciculi. Maps of radial diffusivity exhibited a very similar spatial pattern of relationship. Of particular interest, mean and radial diffusivity did not correlate with ventricular enlargement in most major white matter tracts immediately adjacent to the lateral ventricles, including the anterior and rostral posterior limbs of the internal capsule, the medial aspect of the corpus callosum, as well as ventromedial parts of the corona radiata (Fig. 6A–C; blue traces). However, the axial diffusivity of these periventricular white matter tracts was positively correlated with ventricular enlargement, with the exception of the medial aspect of the corpus callosum (Fig. 6A–C; pink traces). In the control group, none of the preceding observations were replicated, leading to significant group interactions. First, the relationship between ventricular volume and mean diffusivity was significantly different in concussed compared to control participants over the entire span of white matter tracts that showed a significant positive correlation in the concussed group (Fig. 6D–F). Radial diffusivity maps of the interaction showed a very similar pattern, covering most of the tracts that correlated significantly with ventricular enlargement in concussed participants, with the exception of the temporal part of the right superior longitudinal fasciculus and the right inferior longitudinal fasciculus. Finally, maps of group interaction on axial diffusivity measures showed a diffuse array of significant clusters involving all periventricular tracts that were previously shown to be positively correlated with ventricular volume in concussed participants. Therefore, although variations in lateral ventricular volume were not predictive of white matter anomalies in healthy controls, it was a significant predictor in the case of concussed individuals, whose ventricles were abnormally enlarged relative to controls.
Figure 6.
Ventricular enlargement in concussed participants correlate with increases in mean diffusivity and in periventricular axial diffusivity. Sagittal (A), coronal (B), and axial (C) slices of the regression analysis between lateral ventriclar volume (vCSF) and mean diffusivity (MD, blue) or axial diffusivity (pink) in concussed participants. (D, E and F) Group interaction analysis on the relationship between ventricular volume and mean diffusivity. The results are thresholded at P < 0.05, corrected for multiple comparisons. Insets illustrate linear relationships at one example voxel.
Discussion
The present study is the first to demonstrate the presence of diffuse white matter abnormalities in the brains of aged retired athletes with concussions who are otherwise clinically normal. The current findings in concussed individuals cannot be attributed to other factors such as depression, MCI, APOE genotype, dementia, drug or alcohol abuse, or any other confounding variables that are known to impact cognitive or motor systems with advancing age. To our knowledge, this is the only attempt to disentangle the specific contributions of ageing and sports concussions from other risk factors that are currently confusing the picture of the persistent effects of concussion on brain health (Mccrory et al., 2013). Further, multiple neurocognitive and brain integrity variables were correlated with the uncovered white matter abnormalities, which further emphasizes the clinical pertinence of the current findings.
Relation to traumatic brain injury of higher severity
Our results show that ageing with a history of prior sports-related concussions induces a diffuse pattern of white matter anomalies affecting many major inter-hemispheric, intra-hemispheric as well as projection fibre tracts. Namely, fractional anisotropy maps revealed abnormally low anisotropy throughout a distributed fronto-parietal network of fibres. Low fractional anisotropy has been linked to the presence of immunohistologically-confirmed traumatic axonal injury (Mac Donald et al., 2007a, b), a hallmark of TBIs of higher severity (Povlishock and Christman, 1995). In animal models of TBI, low fractional anisotropy is detected in the acute phase of the injury and persists throughout the subacute and chronic phases, reflecting mostly a secondary axonal injury implicating Wallerian degeneration, axonal collapse, demyelination and/or gliosis (Mac Donald et al., 2007a). In human cases of chronic TBI, reduced fractional anisotropy with increased mean diffusivity are consistently reported (Inglese et al., 2005; Salmond et al., 2006; Benson et al., 2007; Kraus et al., 2007; Xu et al., 2007; Niogi et al., 2008; Sidaros et al., 2008; Kennedy et al., 2009; Kinnunen et al., 2011; Mac Donald et al., 2011). Across the TBI spectrum, whole brain voxel-wise techniques such as TBSS typically reveal a diffuse pattern of anomalies affecting various major fibre tracts. However, in mild TBI, it seems that the anterior aspect of the corpus callosum as well as the internal capsule consistently and preferentially exhibit either reduced fractional anisotropy, increased mean diffusivity, or a combination of both (Shenton et al., 2012). In our sample of concussed retired athletes, the anterior corpus callosum as well as the internal capsule showed a combination of decreased fractional anisotropy and increased mean diffusivity, which is consistent with findings in the chronic post-injury phase in younger patients with mild TBI.
White matter changes in young versus older concussed athletes
In young concussed athletes, the chronic phase seems to be characterized by a heterogeneous pattern of anomalies, although studies documenting this population are sparse (Gardner et al., 2012). Findings are rather inconsistent, oscillating between reduced fractional anisotropy with increased mean diffusivity and increased fractional anisotropy with reduced mean diffusivity. We know from animal models of TBI that the acute phase of injury includes cytotoxic oedema and inflammatory responses, which compress white matter tissue leading to an acute decrease in mean diffusivity (Albensi et al., 2000; Van Putten et al., 2005). Later stages of injury are thought to implicate secondary degenerative mechanisms that reverse this pattern of observation (Mac Donald et al., 2007a). More studies are needed to bridge the acute white matter findings with the persistent anomalies uncovered in an ageing population of concussed athletes. With regard to anatomical locations of damage, the corticospinal tract is often cited as being especially susceptible to the effects of concussive injury, although this result might be biased by region of interest approaches that specifically target the corticospinal tract based on previous findings of motor system abnormalities in young asymptomatic concussed athletes (De Beaumont et al., 2007; Henry et al., 2011; Chamard et al., 2013; but see Pearce et al., 2014; Tremblay et al., 2014). Whole-brain analyses of white matter tract integrity have revealed anomalies including, but not restricted to the corticospinal tract in concussed athletes well into the chronic post-injury phase (Zhang et al., 2006; Cubon et al., 2011; Chamard et al., 2013). It is difficult to surmise how the changes observed in younger athletes in the subacute and chronic post-injury phases might inform long-term consequences such as those observed in the current study. If a single supposition can be made based on the extant literature it may be that changes to white matter, once manifest, do not resolve with time (Gardner et al., 2012).
Comparisons with the normal ageing process
Perhaps the most difficult aspect of understanding concussions is that they occur on a dynamic substrate. That is to say, in addition to any changes that the injury itself may visit upon the brain, the brain itself is also changing secondary to several factors, chief among them being ageing which also leads to significant changes in white matter integrity. In order to understand the net result of the interaction between the chronic effects of concussions and ageing, it is necessary to compare the current pattern of anomalies with those documented in normal and pathological ageing. In normal ageing, significant white matter volume loss is documented in individuals in their 70s and 80s, mostly within the frontal and temporal regions (Fjell et al., 2013). It is thought that grey matter volume loss might start early and is progressive, while white matter loss would appear later but in a more precipitous way. In addition, white matter hyperintensities on T2-weighted images become apparent mostly in periventricular white matter and are thought to suggest white matter damage of probable ischaemic origin (Malloy et al., 2007). These observations often reflect rarefaction of myelin, vessel endothelium damage and microvascular disease. Demyelination, redundant myelination, and abnormal myelination including the formation of cavities within the myelin sheet are noticeable observations on post-mortem neuropathological examinations of older individuals (Gunning-Dixon et al., 2009). These histological manifestations of normal ageing lead to specific observations using DTI. Most notably, an anterior-posterior gradient of fractional anisotropy reduction is observed using DTI in healthy older individuals, with the frontal regions showing the most important variations with age in contrast to more posterior regions (Salat et al., 2005; Ardekani et al., 2007; Grieve et al., 2007). This gradient is also apparent within the corpus callosum, with the genu exhibiting significantly more fractional anisotropy reduction with advancing age compared to the splenium (Sullivan et al., 2006). This reduction in fractional anisotropy across the brain is thought to be partly explained by an increase in radial diffusivity (Davis et al., 2009; Bennett et al., 2010; Burzynska et al., 2010), which reflects myelin pathology (Song et al., 2002).
When interpreting our current results in concussed retired athletes, many similarities to the normal ageing effects are readily apparent as evidenced by DTI metrics. First, we found a fronto-parietal network of anomalies sparing most posterior parietal and occipital white matter, consistent with the anterior-posterior gradient documented in healthy ageing. Reduced fractional anisotropy affected mainly frontal regions that are subject to age-related decline in white matter integrity. Likewise, the corpus callosum showed a similar gradient of injury, with the genu exhibiting significantly more anomalies than the splenium or posterior body. Importantly, most regions exhibiting decreased fractional anisotropy in combination with increased mean diffusivity in our sample also showed a significant increase in radial diffusivity in the absence of differences in axial diffusivity, which suggests that the major contributor to DTI anomalies in our sample may be myelin-related pathology (Song et al., 2002). Such an interpretation would also be in-line with the documented effects of normal ageing on myelin integrity, which is known to decrease with advancing age (Peters, 2002). One notable difference with the ageing process, however, has to do with the absence of group differences on the occurrence of age-related white matter hyperintensities in our current sample. This could be indicative of differential pathomechanisms between normal ageing and ageing with a history of concussions, whereby vasogenic factors might play a minor role in the latter population. This may also be reflective of our sample where physical activity and body mass index, factors known to reduce cardiovascular pathology, were accounted for, thus minimizing or eliminating any group differences. Nevertheless, DTI metrics that are sensitive to small alterations in white matter microstructure allow important parallels to be drawn between normal ageing and ageing with a history of concussions. The currently reported group differences between older retired athletes with or without concussions match a pattern of anomalies that is consistent with the effects of normal ageing. We believe these observations suggest that the concussed brain is more vulnerable to the pathological effects of normal ageing. This interpretation would be in line with findings of exacerbated cortical thinning and ventricular expansion with advancing age in the same sample of former concussed athletes (Tremblay et al., 2013). Overall, these results provide support for the notion that structural injury from TBI, even if not grossly apparent, might reduce the resilience of the brain and expedite the degenerative effects of ageing (Moretti et al., 2012). The consequence of this in practical terms may be that those individuals who have been concussed may experience age-related cognitive alterations earlier in life.
Comparisons with pathological ageing
It is also important to consider the possibility that the current pattern of anomalies might be associated with pathological ageing rather than normal ageing. As previously stated, numerous studies have drawn a parallel between a history of TBI and the later development of Alzheimer’s disease (Sivanandam and Thakur, 2012). Moreover, epidemiological studies have established links between a history of concussions and the onset of MCI, a condition often referred to as a prodromal state of Alzheimer’s disease (Petersen et al., 1999; Guskiewicz et al., 2005). However, studies investigating the white matter microstructure of patients with MCI using DTI have typically found a different pattern of anomalies of that found in the current sample. White matter anomalies in MCI patients are evident in the posterior regions of the brain as well as in the temporal lobes adjacent to the entorhinal and parahippocampal gyri (Chua et al., 2008; Liu et al., 2013; Nir et al., 2013; Stricker et al., 2013). The splenium of the corpus callosum and the fornix are also affected (Zhang et al., 2007, 2013; Wang et al., 2014). Moreover, significant increases in axial diffusivity are uncovered throughout the brain (Nir et al., 2013). This pattern is sharply distinct from the one documented here in older retired athletes with concussions, where anomalies are mostly frontal, sparing the splenium of the corpus callosum and fornix, with no observed differences in axial diffusivity. This contrast between MCI and concussion + ageing white matter anomaly profiles might be important in establishing a differential diagnosis when an elderly patient with a history of concussion presents with memory decline that does not impair daily living activities.
Ventricular system expansion and white matter tissue compression
In a previous study by our group, we were puzzled by the absence of noticeable grey or white matter atrophy in the presence of lateral ventricles enlargement in aged concussed athletes (Tremblay et al., 2013). We hypothesized from those observations that traumatic axonal injury might be responsible for a subtle white matter loss that would be undetectable using a whole-brain white matter volume analysis. The present study confirms that white matter anomalies are present in the brains of older concussed athletes, and shows that these anomalies are correlated with the extent of ventricular expansion in that population. However, the spatial pattern of anomalies is rather heterogeneous. Specifically, we found that expanded lateral ventricles were correlated with an increase in axial diffusivity in the white matter immediately adjacent to the lateral ventricles. Elsewhere in the brain, ventricular enlargement was correlated with increases in mean and radial diffusivity, the usual markers of axonal and myelin damage. This pattern of results implies that the white matter adjacent to the ventricular space may be slightly compressed under the influence of ventricular enlargement, potentially leading to a reduction in radial diffusivity and an increase in axial diffusivity (Mac Donald et al., 2007a). This compression would decrease as a function of distance from the ventricles, potentially making the periventricular white matter more vulnerable to age-related degeneration. While this could be a pathomechanism specific to the concussed group, it is important to remember that, on average, older concussed athletes exhibit significant fractional anisotropy reduction in periventricular white matter compared to controls. Since we found no study looking at the relationship between ventricular volume and DTI metrics in normal or pathological ageing, this interpretation should be considered highly speculative.
Motor system abnormalities
Our group has documented sequential motor learning deficits in both young and older concussed athletes (De Beaumont et al., 2012b, 2013). Here, we investigated the relationship between sequential motor learning and white matter tract integrity across the entire brain. We found that in controls, white matter integrity of left frontal motor areas was correlated with sequential motor learning performance. This finding is consistent with the only other study documenting the relationship between sequential motor learning and DTI-based metrics of white matter integrity (Bennett et al., 2011). In retired concussed athletes, we found no such relationship, leading to a significant group interaction over the same set of tracts. To help understand this effect, we need to consider that, compared to controls, aged concussed athletes exhibit reduced motor learning and reduced fractional anisotropy with increased mean diffusivity and radial diffusivity in corresponding tracts. From this observation, we can only speculate that the relationship between sequential motor learning and the integrity of the implicated fibre tracts in concussed athletes follows a non-linear relationship. In the normal range of motor function, this relationship might appear linear, while in the range at which concussed individuals operate, the relationship might flatten. Since motor system anomalies are observable in concussed athletes from their early adulthood (De Beaumont et al., 2007, 2011, 2012b), adaptive plasticity might be responsible for a reorganization of motor circuitry operating throughout the lifespan that would alter the relationship between motor function and their usual microstructural correlates. This adaptive plasticity would not, however, lead to complete restoration of pre-trauma function.
Conclusion
Overall, we provide the first description of diffuse white matter anomalies in the brains of older but clinically normal, retired athletes with concussion history. We find these anomalies to be related to multiple cognitive and brain structure variables, which, in addition to previous functional and structural characterizations, begin to reveal a clinical profile that can be contrasted with other clinical entities. We suggest that the current characterization fits better with the pattern of changes that is typically observed in normal ageing. From this interpretation, we propose that the nature of the interaction between ageing and a history of concussions involves a latent microstructural injury that leaves the brain more vulnerable to the deleterious effects of ageing. That is to say, our results suggest that while ageing exerts its own effects on the brain, concussions act synergistically with the normal ageing process, resulting in detectable decline in both structure and function.
Funding
This work was supported by grants to H.T., M.L., and L.D.B. from the Canadian Institutes of Health Research (CIHR) and the Natural Sciences and Engineering Research Council of Canada (NSERC). The NSERC Alexander Graham Bell Canada Graduate Scholarship supported S.T.
Supplementary material
Supplementary material is available at Brain online.
Glossary
Abbreviations
- DTI
diffusion tensor imaging
- MCI
mild cognitive impairment
- TBI
traumatic brain injury
- TBSS
tract-based spatial statistics
References
- Albensi BC, Knoblach SM, Chew BG, O'Reilly MP, Faden AI, Pekar JJ. Diffusion and high resolution MRI of traumatic brain injury in rats: time course and correlation with histology. Exp Neurol. 2000;162:61–72. doi: 10.1006/exnr.2000.7256. [DOI] [PubMed] [Google Scholar]
- Ardekani S, Kumar A, Bartzokis G, Sinha U. Exploratory voxel-based analysis of diffusion indices and hemispheric asymmetry in normal aging. Magn Reson Imaging. 2007;25:154–67. doi: 10.1016/j.mri.2006.09.045. [DOI] [PubMed] [Google Scholar]
- Assaf Y, Pasternak O. Diffusion tensor imaging (DTI)-based white matter mapping in brain research: a review. J Mol Neurosci. 2008;34:51–61. doi: 10.1007/s12031-007-0029-0. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Jones DK. Diffusion-tensor MRI: theory, experimental design and data analysis - a technical review. NMR Biomed. 2002;15:456–67. doi: 10.1002/nbm.783. [DOI] [PubMed] [Google Scholar]
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI 1996. J Magn Reson. 2011;213:560–70. doi: 10.1016/j.jmr.2011.09.022. [DOI] [PubMed] [Google Scholar]
- Bazarian JJ, Zhu T, Blyth B, Borrino A, Zhong J. Subject-specific changes in brain white matter on diffusion tensor imaging after sports-related concussion. Magn Reson Imaging. 2012;30:171–80. doi: 10.1016/j.mri.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard DV, Howard JH. Age-related differences in multiple measures of white matter integrity: a diffusion tensor imaging study of healthy aging. Hum Brain Mapp. 2010;31:378–90. doi: 10.1002/hbm.20872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett IJ, Madden DJ, Vaidya CJ, Howard JH, Howard DV. White matter integrity correlates of implicit sequence learning in healthy aging. Neurobiol Aging. 2011;32 doi: 10.1016/j.neurobiolaging.2010.03.017. 2317.e1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson RR, Meda SA, Vasudevan S, Kou Z, Govindarajan KA, Hanks RA, et al. Global white matter analysis of diffusion tensor images is predictive of injury severity in traumatic brain injury. J Neurotrauma. 2007;24:446–59. doi: 10.1089/neu.2006.0153. [DOI] [PubMed] [Google Scholar]
- Broglio SP, Eckner JT, Paulson HL, Kutcher JS. Cognitive decline and aging: the role of concussive and subconcussive impacts. Exerc Sport Sci Rev. 2012;40:138–44. doi: 10.1097/JES.0b013e3182524273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burzynska AZ, Preuschhof C, Bäckman L, Nyberg L, Li S-C, Lindenberger U, et al. Age-related differences in white matter microstructure: region-specific patterns of diffusivity. Neuroimage. 2010;49:2104–12. doi: 10.1016/j.neuroimage.2009.09.041. [DOI] [PubMed] [Google Scholar]
- Chamard E, Lassonde M, Henry L, Tremblay J, Boulanger Y, De Beaumont L, et al. Neurometabolic and microstructural alterations following a sports-related concussion in female athletes. Brain Inj. 2013;27:1038–46. doi: 10.3109/02699052.2013.794968. [DOI] [PubMed] [Google Scholar]
- Chappell MH, Uluğ AM, Zhang L, Heitger MH, Jordan BD, Zimmerman RD, et al. Distribution of microstructural damage in the brains of professional boxers: a diffusion MRI study. J Magn Reson Imaging. 2006;24:537–42. doi: 10.1002/jmri.20656. [DOI] [PubMed] [Google Scholar]
- Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer's disease: a review. Curr Opin Neurol. 2008;21:83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- Collins MW, Lovell MR, Iverson GL, Cantu RC, Maroon JC, Field M. Cumulative effects of concussion in high school athletes. Neurosurgery. 2002;51:1175–81. doi: 10.1097/00006123-200211000-00011. [DOI] [PubMed] [Google Scholar]
- Cubon VA, Putukian M, Boyer C, Dettwiler A. A diffusion tensor imaging study on the white matter skeleton in individuals with sports-related concussion. J Neurotrauma. 2011;28:189–201. doi: 10.1089/neu.2010.1430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SW, Dennis NA, Buchler NG, White LE, Madden DJ, Cabeza R. Assessing the effects of age on long white matter tracts using diffusion tensor tractography. Neuroimage. 2009;46:530–41. doi: 10.1016/j.neuroimage.2009.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaumont L, Henry LC, Gosselin N. Long-term functional alterations in sports concussion. Neurosurg Focus. 2012a;33:E8. doi: 10.3171/2012.9.FOCUS12278. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Lassonde M, Leclerc S, Théoret H. Long-term and cumulative effects of sports concussion on motor cortex inhibition. Neurosurgery. 2007;61:329–36. doi: 10.1227/01.NEU.0000280000.03578.B6. discussion 336–7. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Mongeon D, Tremblay S, Messier J, Prince F, Leclerc S, et al. Persistent motor system abnormalities in formerly concussed athletes. J Athl Train. 2011;46:234–40. doi: 10.4085/1062-6050-46.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Beaumont L, Théoret H, Mongeon D, Messier J, Leclerc S, Tremblay S, et al. Brain function decline in healthy retired athletes who sustained their last sports concussion in early adulthood. Brain. 2009;132:695–708. doi: 10.1093/brain/awn347. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Tremblay S, Poirier J, Lassonde M, Théoret H. Altered bidirectional plasticity and reduced implicit motor learning in concussed athletes. Cerebral Cortex. 2012b;22:112–21. doi: 10.1093/cercor/bhr096. [DOI] [PubMed] [Google Scholar]
- De Beaumont L, Tremblay SB, Henry LC, Poirier J, Lassonde M, Oret HT. Motor system alterations in retired former athletes: the role of aging and concussion history. BMC Neurol. 2013;13:109. doi: 10.1186/1471-2377-13-109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Didehbani N, Munro Cullum C, Mansinghani S, Conover H, Hart J. Depressive symptoms and concussions in aging retired NFL Players. Arch Clin Neuropsychol. 2013;28:418–24. doi: 10.1093/arclin/act028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Amlien I, Espeseth T, Reinvang I, Raz N, et al. High consistency of regional cortical thinning in aging across multiple samples. Cerebral Cortex. 2009;19:2001–12. doi: 10.1093/cercor/bhn232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fjell AM, Westlye LT, Grydeland H, Amlien I, Espeseth T, Reinvang I, et al. Critical ages in the life course of the adult brain: nonlinear subcortical aging. Neurobiol Aging. 2013;34:2239–47. doi: 10.1016/j.neurobiolaging.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gardner A, Kay-Lambkin F, Stanwell P, Donnelly J, Williams WH, Hiles A, et al. A systematic review of diffusion tensor imaging findings in sports-related concussion. J Neurotrauma. 2012;29:2521–38. doi: 10.1089/neu.2012.2628. [DOI] [PubMed] [Google Scholar]
- Giza CC, Kutcher JS, Ashwal S, Barth J, Getchius TSD, Gioia GA, et al. Summary of evidence-based guideline update: evaluation and management of concussion in sports: report of the Guideline Development Subcommittee of the American Academy of Neurology. Neurology. 2013;80:2250–7. doi: 10.1212/WNL.0b013e31828d57dd. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude IS, Ashburner J, Henson RN, Friston KJ, Frackowiak RS. A voxel-based morphometric study of ageing in 465 normal adult human brains. Neuroimage. 2001;14:21–36. doi: 10.1006/nimg.2001.0786. [DOI] [PubMed] [Google Scholar]
- Grieve SM, Williams LM, Paul RH, Clark CR, Gordon E. Cognitive aging, executive function, and fractional anisotropy: a diffusion tensor MR imaging study. AJNR Am J Neuroradiol. 2007;28:226–35. [PMC free article] [PubMed] [Google Scholar]
- Gunning-Dixon FM, Brickman AM, Cheng JC, Alexopoulos GS. Aging of cerebral white matter: a review of MRI findings. Int J Geriatr Psychiatry. 2009;24:109–17. doi: 10.1002/gps.2087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guskiewicz KM, Marshall SW, Bailes J, Mccrea M, Cantu RC, Randolph C, et al. Association between recurrent concussion and late-life cognitive impairment in retired professional football players. Neurosurgery. 2005;57:719–26. doi: 10.1093/neurosurgery/57.4.719. [DOI] [PubMed] [Google Scholar]
- Hart J, Kraut MA, Womack KB, Strain J, Didehbani N, Bartz E, et al. Neuroimaging of cognitive dysfunction and depression in aging retired national football league players. JAMA Neurol. 2013;70:326. doi: 10.1001/2013.jamaneurol.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry LC, Tremblay J, Tremblay S, Lee A, Brun C, Lepore N, et al. Acute and chronic changes in diffusivity measures after sports concussion. J Neurotrauma. 2011;28:2049–59. doi: 10.1089/neu.2011.1836. [DOI] [PubMed] [Google Scholar]
- Inglese M, Makani S, Johnson G, Cohen BA, Silver JA, Gonen O, et al. Diffuse axonal injury in mild traumatic brain injury: a diffusion tensor imaging study. J Neurosurg. 2005;103:298–303. doi: 10.3171/jns.2005.103.2.0298. [DOI] [PubMed] [Google Scholar]
- Kennedy MRT, Wozniak JR, Muetzel RL, Mueller BA, Chiou H-H, Pantekoek K, et al. White matter and neurocognitive changes in adults with chronic traumatic brain injury. J Int Neuropsychol Soc. 2009;15:130–6. doi: 10.1017/S1355617708090024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinnunen KM, Greenwood R, Powell JH, Leech R, Hawkins PC, Bonnelle V, et al. White matter damage and cognitive impairment after traumatic brain injury. Brain. 2011;134:449–63. doi: 10.1093/brain/awq347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein M, Houx PJ, Jolles J. Long-term persisting cognitive sequelae of traumatic brain injury and the effect of age. J Nerv Ment Dis. 1996;184:459–67. doi: 10.1097/00005053-199608000-00002. [DOI] [PubMed] [Google Scholar]
- Koerte IK, Kaufmann D, Hartl E, Bouix S, Pasternak O, Kubicki M, et al. A prospective study of physician-observed concussion during a varsity university hockey season: white matter integrity in ice hockey players. Part 3 of 4. Neurosurg Focus. 2012;33:E3. doi: 10.3171/2012.10.FOCUS12303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–19. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- Langlois JA, Rutland-Brown W, Wald MM. The epidemiology and impact of traumatic brain injury: a brief overview. J Head Trauma Rehabil. 2006;21:375–8. doi: 10.1097/00001199-200609000-00001. [DOI] [PubMed] [Google Scholar]
- Lindsay J, Laurin D, Verreault R, Hébert R, Helliwell B, Hill GB, et al. Risk factors for Alzheimer's disease: a prospective analysis from the Canadian study of health and aging. Am J Epidemiol. 2002;156:445–53. doi: 10.1093/aje/kwf074. [DOI] [PubMed] [Google Scholar]
- Liu J, Yin C, Xia S, Jia L, Guo Y, Zhao Z, et al. White matter changes in patients with amnestic mild cognitive impairment detected by diffusion tensor imaging. PLoS One. 2013;8:e59440. doi: 10.1371/journal.pone.0059440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyttelton O, Boucher M, Robbins S, Evans A. An unbiased iterative group registration template for cortical surface analysis. Neuroimage. 2007;34:1535–44. doi: 10.1016/j.neuroimage.2006.10.041. [DOI] [PubMed] [Google Scholar]
- Mac Donald CL, Dikranian K, Bayly P, Holtzman D, Brody D. Diffusion tensor imaging reliably detects experimental traumatic axonal injury and indicates approximate time of injury. J Neurosci. 2007a;27:11869–76. doi: 10.1523/JNEUROSCI.3647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Dikranian K, Song SK, Bayly PV, Holtzman DM, Brody DL. Detection of traumatic axonal injury with diffusion tensor imaging in a mouse model of traumatic brain injury. Exp Neurol. 2007b;205:116–31. doi: 10.1016/j.expneurol.2007.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mac Donald CL, Johnson AM, Cooper D, Nelson EC, Werner NJ, Shimony JS, et al. Detection of blast-related traumatic brain injury in U.S. military personnel. N Engl J Med. 2011;364:2091–100. doi: 10.1056/NEJMoa1008069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden DJ, Bennett IJ, Song AW. Cerebral white matter integrity and cognitive aging: contributions from diffusion tensor imaging. Neuropsychol Rev. 2009;19:415–35. doi: 10.1007/s11065-009-9113-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malloy P, Correia S, Stebbins G, Laidlaw DH. Neuroimaging of white matter in aging and dementia. Clin Neuropsychol. 2007;21:73–109. doi: 10.1080/13854040500263583. [DOI] [PubMed] [Google Scholar]
- Marchi N, Bazarian JJ, Puvenna V, Janigro M, Ghosh C, Zhong J, et al. Consequences of repeated blood-brain barrier disruption in football players. PLoS One. 2013;8:e56805. doi: 10.1371/journal.pone.0056805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marner L, Nyengaard JR, Tang Y, Pakkenberg B. Marked loss of myelinated nerve fibers in the human brain with age. J Comp Neurol. 2003;462:144–52. doi: 10.1002/cne.10714. [DOI] [PubMed] [Google Scholar]
- Masliah E, Crews L, Hansen L. Synaptic remodeling during aging and in Alzheimer's disease. J Alzheimers Dis. 2006;9:91–9. doi: 10.3233/jad-2006-9s311. [DOI] [PubMed] [Google Scholar]
- Mccrory P, Meeuwisse W, Johnston K, Dvorak J, Aubry M, Molloy M, et al. Consensus statement on concussion in sport: the 3rd international conference on concussion in sport held in Zurich, November 2008. Br J Sports Med. 2009;43:i76–84. doi: 10.1136/bjsm.2009.058248. [DOI] [PubMed] [Google Scholar]
- Mccrory P, Meeuwisse WH, Kutcher JS, Jordan BD, Gardner A. What is the evidence for chronic concussion-related changes in retired athletes: behavioural, pathological and clinical outcomes? Br J Sports Med. 2013;47:327–30. doi: 10.1136/bjsports-2013-092248. [DOI] [PubMed] [Google Scholar]
- McKee AC, Cantu RC, Nowinski CJ, Hedley-Whyte ET, Gavett BE, Budson AE, et al. Chronic traumatic encephalopathy in athletes: progressive tauopathy after repetitive head injury. J Neuropathol Exp Neurol. 2009;68:709–35. doi: 10.1097/NEN.0b013e3181a9d503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee AC, Stern RA, Nowinski CJ, Stein TD, Alvarez VE, Daneshvar DH, et al. The spectrum of disease in chronic traumatic encephalopathy. Brain. 2013;136:43–64. doi: 10.1093/brain/aws307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti JM, Voss MW, Pence A, McAuley E, Kramer AF, Cohen NJ. History of mild traumatic brain injury is associated with deficits in relational memory, reduced hippocampal volume, and less neural activity later in life. Front Aging Neurosci. 2013;5:41. doi: 10.3389/fnagi.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moretti L, Cristofori I, Weaver SM, Chau A, Portelli JN, Grafman J. Cognitive decline in older adults with a history of traumatic brain injury. Lancet Neurol. 2012;11:1103–12. doi: 10.1016/S1474-4422(12)70226-0. [DOI] [PubMed] [Google Scholar]
- Niogi SN, Mukherjee P, Ghajar J, Johnson C, Kolster RA, Sarkar R, et al. Extent of microstructural white matter injury in postconcussive syndrome correlates with impaired cognitive reaction time: a 3T diffusion tensor imaging study of mild traumatic brain injury. AJNR Am J Neuroradiol. 2008;29:967–73. doi: 10.3174/ajnr.A0970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nir TM, Jahanshad N, Villalon-Reina JE, Toga AW, Jack CR, Weiner MW, et al. Effectiveness of regional DTI measures in distinguishing Alzheimer's disease, MCI, and normal aging. Neuroimage Clin. 2013;3:180–95. doi: 10.1016/j.nicl.2013.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patenaude B, Smith SM, Kennedy DN, Jenkinson M. A Bayesian model of shape and appearance for subcortical brain segmentation. Neuroimage. 2011;56:907–22. doi: 10.1016/j.neuroimage.2011.02.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearce AJ, Hoy KE, Rogers MA, Corp DT, Maller JJ, Drury HGK, et al. The long-term effects of sports concussion on retired Australian football players: a study using transcranial magnetic stimulation. J Neurotrauma. 2014;31:1139–45. doi: 10.1089/neu.2013.3219. [DOI] [PubMed] [Google Scholar]
- Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol. 2002;31:581–93. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–8. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC, Thomas RG, Grundman M, Bennett D, Doody R, Ferris S, et al. Vitamin E and donepezil for the treatment of mild cognitive impairment. N Engl J Med. 2005;352:2379–88. doi: 10.1056/NEJMoa050151. [DOI] [PubMed] [Google Scholar]
- Povlishock JT, Christman CW. The pathobiology of traumatically induced axonal injury in animals and humans: a review of current thoughts. J Neurotrauma. 1995;12:555–64. doi: 10.1089/neu.1995.12.555. [DOI] [PubMed] [Google Scholar]
- Raz N, Gunning FM, Head D, Dupuis JH, McQuain J, Briggs SD, et al. Selective aging of the human cerebral cortex observed in vivo: differential vulnerability of the prefrontal gray matter. Cereb Cortex. 1997;7:268–82. doi: 10.1093/cercor/7.3.268. [DOI] [PubMed] [Google Scholar]
- Raz N, Rodrigue KM. Differential aging of the brain: patterns, cognitive correlates and modifiers. Neurosci Biobehav Rev. 2006;30:730–48. doi: 10.1016/j.neubiorev.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salat DH, Kaye JA, Janowsky JS. Prefrontal gray and white matter volumes in healthy aging and Alzheimer disease. Arch Neurol. 1999;56:338–44. doi: 10.1001/archneur.56.3.338. [DOI] [PubMed] [Google Scholar]
- Salat DH, Tuch DS, Greve DN, van der Kouwe AJW, Hevelone ND, Zaleta AK, et al. Age-related alterations in white matter microstructure measured by diffusion tensor imaging. Neurobiol Aging. 2005;26:1215–27. doi: 10.1016/j.neurobiolaging.2004.09.017. [DOI] [PubMed] [Google Scholar]
- Salmond CH, Menon DK, Chatfield DA, Williams GB, Pena A, Sahakian BJ, et al. Diffusion tensor imaging in chronic head injury survivors: correlations with learning and memory indices. Neuroimage. 2006;29:117–24. doi: 10.1016/j.neuroimage.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Shenton ME, Hamoda HM, Schneiderman JS, Bouix S, Pasternak O, Rathi Y, et al. A review of magnetic resonance imaging and diffusion tensor imaging findings in mild traumatic brain injury. Brain Imaging Behav. 2012;6:137–92. doi: 10.1007/s11682-012-9156-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidaros A, Engberg AW, Sidaros K, Liptrot MG, Herning M, Petersen P, et al. Diffusion tensor imaging during recovery from severe traumatic brain injury and relation to clinical outcome: a longitudinal study. Brain. 2008;131:559–72. doi: 10.1093/brain/awm294. [DOI] [PubMed] [Google Scholar]
- Sivanandam TM, Thakur MK. Traumatic brain injury: a risk factor for Alzheimer's disease. Neurosci Biobehav Rev. 2012;36:1376–81. doi: 10.1016/j.neubiorev.2012.02.013. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31:1487–505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, et al. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 2004; 23 (Suppl 1): S208-19. [DOI] [PubMed]
- Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, et al. Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. Neuroimage. 2002;17:479–89. doi: 10.1006/nimg.2002.1040. [DOI] [PubMed] [Google Scholar]
- Song S-K, Sun S-W, Ramsbottom MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but Unchanged Axial) diffusion of water. Neuroimage. 2002;17:1429–36. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- Strain J, Didehbani N, Cullum CM, Mansinghani S, Conover H, Kraut MA, et al. Depressive symptoms and white matter dysfunction in retired NFL players with concussion history. Neurology. 2013;81:25–32. doi: 10.1212/WNL.0b013e318299ccf8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stricker NH, Salat DH, Foley JM, Zink TA, Kellison IL, McFarland CP, et al. Decreased white matter integrity in neuropsychologically defined mild cognitive impairment is independent of cortical thinning. J Int Neuropsychol Soc. 2013;19:925–37. doi: 10.1017/S1355617713000660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan EV, Adalsteinsson E, Pfefferbaum A. Selective age-related degradation of anterior callosal fiber bundles quantified in vivo with fiber tracking. Cereb Cortex. 2006;16:1030–9. doi: 10.1093/cercor/bhj045. [DOI] [PubMed] [Google Scholar]
- Sun SW, Liang HF, Le TQ, Armstrong RC, Cross AH, Song SK. Differential sensitivity of in vivo and ex vivo diffusion tensor imaging to evolving optic nerve injury in mice with retinal ischemia. Neuroimage. 2006;32:1195–204. doi: 10.1016/j.neuroimage.2006.04.212. [DOI] [PubMed] [Google Scholar]
- Tisserand DJ, Pruessner JC, Sanz Arigita EJ, van Boxtel MPJ, Evans AC, Jolles J, et al. Regional frontal cortical volumes decrease differentially in aging: an MRI study to compare volumetric approaches and voxel-based morphometry. Neuroimage. 2002;17:657–69. [PubMed] [Google Scholar]
- Tremblay S, Beaulé V, Proulx S, Tremblay S, Marjańska M, Doyon J, et al. Multimodal assessment of primary motor cortex integrity following sport concussion in asymptomatic athletes. Clin Neurophysiol. 2014;125:1371–9. doi: 10.1016/j.clinph.2013.11.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay S, De Beaumont L, Henry LC, Boulanger Y, Evans AC, Bourgouin P, et al. Sports concussions and aging: a neuroimaging investigation. Cerebral Cortex. 2013;23:1159–66. doi: 10.1093/cercor/bhs102. [DOI] [PubMed] [Google Scholar]
- Van Putten HP, Bouwhuis MG, Muizelaar JP, Lyeth BG, Berman RF. Diffusion-weighted imaging of edema following traumatic brain injury in rats: effects of secondary hypoxia. J Neurotrauma. 2005;22:857–72. doi: 10.1089/neu.2005.22.857. [DOI] [PubMed] [Google Scholar]
- Virji-Babul N, Borich MR, Makan N, Moore T, Frew K, Emery CA, et al. Diffusion tensor imaging of sports-related concussion in adolescents. Pediatr Neurol. 2013;48:24–9. doi: 10.1016/j.pediatrneurol.2012.09.005. [DOI] [PubMed] [Google Scholar]
- Wang PN, Chou KH, Chang NJ, Lin KN, Chen WT, Lan GY, et al. Callosal degeneration topographically correlated with cognitive function in amnestic mild cognitive impairment and alzheimer's disease dementia. Hum Brain Mapp. 2014;35:1529–43. doi: 10.1002/hbm.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiest-Daesslé N, Prima S, Coupé P, Morrissey SP, Barillot C. Rician noise removal by non-Local Means filtering for low signal-to-noise ratio MRI: applications to DT-MRI. Med Image Comput Comput Assist Interv. 2008;11:171–9. doi: 10.1007/978-3-540-85990-1_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Medical Association. World medical association declaration of helsinki: ethical principles for medical research involving human subjects. JAMA. 2013;310:2191–4. doi: 10.1001/jama.2013.281053. [DOI] [PubMed] [Google Scholar]
- Xu J, Rasmussen IA, Lagopoulos J, Håberg A. Diffuse axonal injury in severe traumatic brain injury visualized using high-resolution diffusion tensor imaging. J Neurotrauma. 2007;24:753–65. doi: 10.1089/neu.2006.0208. [DOI] [PubMed] [Google Scholar]
- Zhang K, Johnson B, Pennell D, Ray W, Sebastianelli W, Slobounov S. Are functional deficits in concussed individuals consistent with white matter structural alterations: combined FMRI & DTI study. Exp Brain Res. 2010;204:57–70. doi: 10.1007/s00221-010-2294-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Heier LA, Zimmerman RD, Jordan B, Ulug AM. Diffusion anisotropy changes in the brains of professional boxers. AJNR Am J Neuroradiol. 2006;27:2000–4. [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Ravdin LD, Relkin N, Zimmerman RD, Jordan B, Lathan WE, et al. Increased diffusion in the brain of professional boxers: a preclinical sign of traumatic brain injury? AJNR Am J Neuroradiol. 2003;24:52–7. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Camacho M, Chao LL, Fletcher TP, Yaffe K, et al. MRI markers for mild cognitive impairment: comparisons between white matter integrity and gray matter volume measurements. PLoS One. 2013;8:e66367. doi: 10.1371/journal.pone.0066367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Schuff N, Jahng G-H, Bayne W, Mori S, Schad L, et al. Diffusion tensor imaging of cingulum fibers in mild cognitive impairment and Alzheimer disease. Neurology. 2007;68:13–19. doi: 10.1212/01.wnl.0000250326.77323.01. [DOI] [PMC free article] [PubMed] [Google Scholar]