Abstract
Parkinson’s disease is a neurodegenerative condition that affects motor function along with a wide range of cognitive domains, including executive function. The hallmark of the pathology is its significant loss of nigrostriatal dopamine, which is necessary for the cortico-striatal interactions that underlie executive control. Striatal dopamine reuptake is mediated by the SLC6A3 gene (formerly named DAT1) and its polymorphisms, which have been largely overlooked in Parkinson’s disease. Thirty patients (ages 53–68 years; 19 males, 11 females) at early stages of Parkinson’s disease, were genotyped according to a 9-repeat (9R) or 10-repeat (10R) allele on the SLC6A3/DAT1 gene. They underwent neuropsychological assessment and functional magnetic resonance imaging while performing a set-shifting task (a computerized Wisconsin Card Sorting Task) that relies on fronto-striatal interactions. Patients homozygous on the 10R allele performed significantly better on working memory tasks than 9R-carrier patients. Most importantly, patients carrying a 9R allele exhibited less activation than their 10R homozygous counterparts in the prefrontal cortex, premotor cortex and caudate nucleus, when planning and executing a set-shift. This pattern was exacerbated for conditions that usually recruit the striatum compared to those that do not. This is the first study indicating that the SLC6A3/DAT1 genotype has a significant effect on fronto-striatal activation and performance in Parkinson’s disease. This effect is stronger for conditions that engage the striatum. Longitudinal studies are warranted to assess this polymorphism’s effect on the clinical evolution of patients with Parkinson’s disease, especially with cognitive decline.
Keywords: polymorphism, SLC6A3/DAT1, dopamine, executive function, functional MRI
Introduction
Parkinson’s disease affects dopamine neurotransmission, with a 50% decrease of dopamine levels in the dorsal striatum 1–3 years after diagnosis, which degrades to 90% after 5 years (Kordower et al., 2013). Parkinson’s disease is typically diagnosed according to its motor symptoms, but also apparent, are cognitive deficits spanning one or more domains (Foltynie et al., 2004a), including executive function (Monchi et al., 2001, 2007; Nagano-Saito et al., 2008; Dirnberger and Jahanshahi, 2013). Executive function encompasses planning and set-shifting, which engage fronto-striatal brain networks that rely on dopaminergic function (Lange et al., 1992; Cools et al., 2002).
Dopamine availability in fronto-striatal circuits is regulated by proteins that undergo genetic variation. For example, a polymorphism of the catechol-O-methyltransferase (COMT) gene, Val158Met (valine to methionine mutation at position 158), affects frontal cortex dopamine expression and cognitive function (Diamond, 2007). In Parkinson’s disease, this polymorphism influences performance (Foltynie et al., 2004b) and frontal brain activity during planning (Williams-Gray et al., 2007, 2008). However, Parkinson’s disease affects cortico-striatal brain activity (Monchi et al., 2004, 2007), and dopamine availability in striatal systems is linked to variation in the dopamine transporter gene SLC6A3/DAT1 (Sesack et al., 1998). The transporter is involved in clearance of dopamine from the synaptic cleft (reuptake) in the striatum. Its gene, SLC6A3/DAT1, contains a variable number of tandem repeats (VNTR) (Vandenbergh et al., 1992); the most common being the 10- (10R) and 9-repeat (9R) alleles, with frequencies of ∼70% (10R) and 28% (9R) in North American European populations (proportions vary in other populations; Kang et al., 1999; Mitchell et al., 2000). These repeats are linked to dopamine transporter activity, where 9R carriers (9/9 and 9/10) differ from non-9R carriers (10/10) (Heinz et al., 2000; Fuke et al., 2001; Faraone et al., 2014). Accordingly, this polymorphism is associated with performance on various tasks of executive function, including task novelty (Garcia-Garcia et al., 2010), cognitive flexibility (van Holstein et al., 2011), working memory (Bertolino et al., 2009), and attention (Fossella et al., 2002) in healthy populations. Genetic dependence of dopamine neurotransmission in prefrontal-striatal pathways is critical to these executive function tasks, and nigrostriatal dopamine degeneration is central to the pathophysiology of Parkinson’s disease. However, the effect of dopamine transporter VNTR profile on the brain activity underlying executive function has not yet been evaluated in Parkinson’s disease.
In previous work, we assessed cortio-striatal functional MRI activity patterns in Parkinson’s disease and age-matched controls while performing the Wisconsin Card Sorting Task (Monchi et al., 2001, 2004, 2007; Nagano-Saito et al., 2008; Jubault et al., 2009). In controls, a ‘cognitive’ cortico-striatal loop involving prefrontal cortex and the caudate nucleus was solicited when planning a set-shift, whereas a ‘motor’ cortico-striatal loop encompassing the premotor cortex and putamen displayed increased activation during shift execution. Here, the same protocol was used to evaluate the role of SLC6A3/DAT1 genetic variation in Parkinson’s disease, on brain activation patterns during cognitive set-shifting. We hypothesized that patterns would differ between Parkinson’s disease patients homozygous for the 10R allele and those carrying a 9R allele, especially in conditions that rely heavily on the striatum. In addition, to ascertain that any differences were related to striatal dopamine and not cortical dopamine, we assessed variation of the COMT gene’s Val158Met polymorphism in our DAT groups.
Materials and methods
Subjects
Thirty patients with Parkinson’s disease at stages I and II of Hoehn and Yahr (mean age ± SD, 60.4 ± 5.2 years; range, 53–68; 19 males and 11 females) were diagnosed by neurologists expert in movement disorders and met the UK Brain Bank criteria for idiopathic Parkinson’s disease (Hughes et al., 1992). Participants underwent cognitive assessment and functional MRI in separate sessions on different days. Participants were asked to stop taking any dopaminergic medication at least 12 h prior to testing, and a blood sample was drawn for genotyping. Participants provided informed consent, and the protocol was approved by the Research Ethics Committee of the Regroupement Neuroimagerie Québec. Patients were separated into two groups according to the VNTR polymorphism of the SLC6A3/DAT1 gene: those homozygous for the 10/10 alleles (10R/10R, n = 14) and those with 9/9- or 9/10-alleles (n = 16, including n = 3 homozygous 9R/9R). Group demographics are shown in Table 1.
Table 1.
Demographics of patients with Parkinson’s disease according to SLC6A3/DAT1 polymorphism on the VNTR
| Parkinson’s disease SLC6A3 VNTR 10R/10R | Parkinson’s disease SLC6A3 VNTR 9R/9R or 9R/10R | Difference T stat, P-value | |
|---|---|---|---|
| n | 16 | 14 | |
| Age | 60.4 ± 4.39 | 59.8 ± 6.01 | 0.30, P = 0.37 |
| Male/female | 9m / 7f | 10m / 4f | 0.84, P = 0.20 |
| Years since diagnosis | 5.11 ± 3.10 | 4.16 ± 3.20 | 0.82, P = 0.21 |
| MoCA off | 27.4 ± 2.42 | 27.4 ± 1.87 | 0.07, P = 0.47 |
| UPDRS off | 28.8 ± 10.2 | 27.8 ± 7.10 | 0.32, P = 0.46 |
| BDI | 8.50 ± 5.22 | 11.42 ± 6.5 | 1.36, P = 0.09 |
| COMT genotype | |||
| number/group (% per group) | 0.48, P = 0.31 | ||
| Met/Met | 5 (31%) | 3 (21%) | |
| Val/Met or Met/Val | 7 (44%) | 7 (50%) | |
| Val/Val | 4 (25%) | 4 (29%) | |
| Anti-parkinsonian medication | |||
| l-DOPA daily intake (mg) | 450.0 ± 208.2 | 511.5 ± 326.7 | 0.57, P = 0.28 |
| n/group (% per group) | |||
| Dopamine agonist | 8 (50%) | 8 (57%) | |
| MAOB inhibitor | 8 (50%) | 6 (43%) | |
| COMT inhibitor | 9 (56%) | 7 (50%) | |
| Dopamine decarboxylase inhibitor | 13 (81%) | 13 (93%) | |
| Neuropsychological tests with significant differences (mean ±1 SEM) | |||
| Digit Span raw | 15.7 ± 0.87 | 12.6 ± 1.11 | 2.26, P = 0.016* |
| Digit Span scaled | 10.1 ± 0.68 | 7.13 ± 1.05 | 2.47, P = 0.010* |
| Tower of London corrected | 109.0 ± 4.91 | 119.2 ± 3.18 | 1.75, P = 0.047* |
Values presented as group means ± standard deviation. OFF indicates scores while patients were OFF dopaminergic medication for at least 12 h. Asterisks (*) indicate significant difference between groups, with an alpha level of 0.05.
BDI = Beck Depression Inventory; MoCA = Montreal Cognitive Assessment; UPDRS = Unified Parkinson's Disease Rating Scale.
Genotyping
SLC6A3: dopamine transporter, striatal dopamine
Genomic DNA was extracted from blood using the Puregene® DNA kit, (Gentra System). We were interested in the VNTR polymorphism of the dopamine transporter gene SLC6A3/DAT1 (solute carrier family 6 neurotransmitter transporter dopamine member 3 or dopamine transporter 1 gene), located in its 3′ untranslated region. The SLC6A3/DAT1 gene, located on chromosome 5p15, contains 15 exons spanning 60 kb, and in the 3′ untranslated region (UTR), a 40 bp VNTR ranging between 3 and 11 (Vandenbergh et al., 1992). A PCR with defined oligonucleotides primers was performed. We used a previously described procedure for fluorescent dye labelling of PCR fragments with an M13 tail (Schuelke, 2000). After appropriate PCR amplification, different PCR products were generated according to the number of repeats found for each allele. PCR product length was determined by loading products on a 3730 DNA Analyzer (Applied Biosystems) with a laser detection system. Allele calling was conducted using GeneMapperV4. To ensure validity and reproducibility of the method, three different CEPH control DNA (CEPH1331-1, CEPH1332-12, and CEPH1347-1) were genotyped, and the experiment was repeated to confirm individual results.
COMT (Val158Met, cortical dopamine)
Genotyping of the COMT polymorphism rs4680 (Val158Met) was conducted using PCR, followed by sequencing. A set of primers was designed to allow specific amplification of the amplicon: foward primer 5′-AGG GTG GGC AGA GGA GG-3′ and reverse primer 5′-GCC TGG TGA TAG TGG GTT TTC-3′. After appropriate PCR amplification, the PCR product was sequenced at the Genome Quebec Innovation Centre using a 3730XL DNA Analyzer (Applied Biosystems), and mutation detection analysis, with a Mutation surveyor (v.3.10, SoftGenetics).
Neuropsychological assessment
A screening test, the Montreal Cognitive Assessment (Nasreddine et al., 2005) was administered before the first scanning session. A comprehensive neuropsychological evaluation (OFF medication) targeted four main cognitive domains: executive function and attention, verbal learning and, memory, language, and visuo-perceptual abilities (Table 2).
Table 2.
Neuropsychological test battery according to cognitive domain
| Cognitive domain | Test | Reference |
|---|---|---|
| Executive function and attention | Digit Span | Wechsler, 1997 |
| Trail Making part B | Reitan and Wolfson, 1985 | |
| Stroop Color and Word | Golden and Freshwater, 1998 | |
| Tower of London | Culbertson and Zillmer, 2005 | |
| Brixton | Burgess and Shallice, 1997 | |
| Verbal Fluency-Orthographic Criteria subtest of the protocole Montréal d’Evaluation de la Communication (MEC) | Joanette et al., 2004 | |
| Verbal learning and memory | Logical Memory subtest of the Wechsler Memory Scale (WMS-III) | Wechsler, 1997 |
| Rey Auditory Verbal Learning Test (RAVLT) | Schmidt, 1996 | |
| Language | Boston Naming | Kaplan et al., 1983 |
| Verbal Fluency-Semantic Criteria sub-test of the MEC | Joanette et al., 2004 | |
| Vocabulary sub-test of the Wechsler Abbreviated Scale of Intelligence | Wechsler, 1999 | |
| Visuo-perceptual | Hooper Visual Organization and | Hooper, 1958 |
| Clock-drawing sub-test of the Montreal Cognitive Assessment (MoCA) | Nasreddine et al., 2005 | |
| Rey-Osterrieth Figure copy | Osterrieth, 1944 |
Neuropsychological test battery used for cognitive evaluation of patients with Parkinson’s disease.
Cognitive task during functional MRI
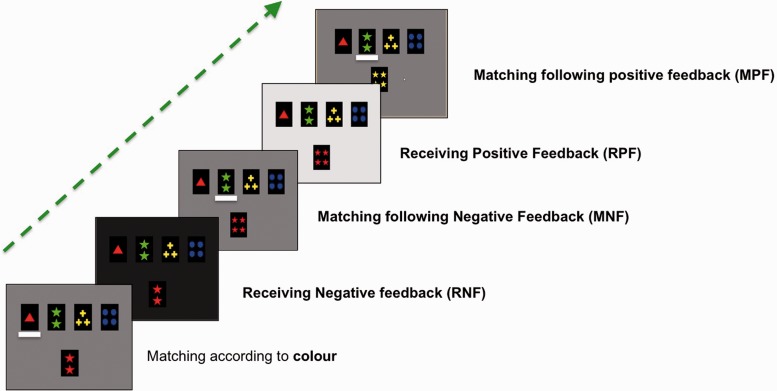
A computerized version of the Wisconsin Card Sorting Task (Monchi et al., 2001, 2004) was administered using custom presentation software. Throughout the task, four fixed reference cards were presented in a row at the top of the screen: the first displayed a red rectangle, the second two green stars, the third three yellow crosses, and the fourth four blue circles. In each trial, a new test card appeared in the middle of the screen below the reference cards (Fig. 1). Subjects were to match the test card to one of the reference cards according to colour, shape or number. Participants indicated their selection on a two-button response box: the index button pointed the cursor to their choice and the middle finger confirmed selection. Participants had to find the classification rule through the feedback (positive or negative) that was provided after each trial and continue to apply it as long as positive feedback followed. A change in screen brightness indicated a correct (bright screen) or incorrect (dark screen) response. Control trials consisted of a test card that was identical to one of the four reference cards, and participants had to select the twin reference card.
Figure 1.
Example of a negative, followed by a positive in the Wisconsin Card Sorting Task. Four fixed reference cards are shown in a row at the top of the screen, while a test card appears centred below. In this example, a participant is shown two red stars (first rectangle starting from the bottom). The subject matches according to colour, selecting the reference card with the single red triangle. However, this is not the current required rule, so the screen darkens (second rectangle), and the participant should now seek a new rule. This corresponds to receiving negative feedback (RNF). Then, a new card is shown (four red stars in this example, third rectangle), and the participant tries to match according to a different feature, now shape, by selecting the card with two green stars. This corresponds to matching after negative feedback (MNF). This happens to be the correct rule, which is indicated to the participant by the screen brightening (fourth square), and corresponds to receiving positive feedback (RPF). Another card is then shown (four yellow stars) and the participant continues with shape as the rule (by selecting the reference card with two green stars fifth square), which corresponds to matching after positive feedback (MPF).
To evaluate the pattern of activation during the different stages of the Wisconsin Card Sorting Task, four experimental and two control time periods were defined (Fig. 1): (i) receiving negative feedback: screen darkens (incorrect response), a set-shift is required and must be planned; (ii) matching after negative feedback: execution of the set-shift; (iii) receiving positive feedback: screen brightens, current matching criterion must continue; (iv) matching after positive feedback: selection using the same classification rule as the previous trial; (v) receiving control feedback: original screen brightness maintained; and (vi) control matching: selection of a reference card identical to the test card. Each feedback period lasted 2.3 s. The length of each matching period depended on participant response time.
Each functional MRI run contained blocks of each of the four trial types (colour, shape, number, and control) presented in pseudo-random order. For the experimental Wisconsin Card Sorting Task trial blocks, six consecutive correct-match responses were required before a change in dimension could occur. Control blocks contained eight trials.
Functional MRI scanning
Subjects were scanned with the Institut Universitaire de Gériatrie de Montréal’s 3 T Siemens TIM MRI scanner. Sessions began with T1-weighted 3D volume acquisition for anatomical localization (1 mm3 voxel size), followed by echoplanar T2*-weighted image acquisitions with blood oxygenation level-dependent contrast (echo time 30 ms; flip angle 90°). Functional images were acquired over five runs in a single session. Volumes were acquired continuously every 2.5 s, for a total 155 volumes within runs, and contained 36 slices (matrix size, 64 × 64 pixels; voxel size, 3.7 × 3.7 × 3.7 mm3).
Functional MRI data analysis
Data analysis, using fmristat (Worsley et al., 2002; Worsley, 2005), was similar to our previous studies (Monchi et al., 2001, 2004; Nagano-Saito et al., 2008; Jubault et al., 2009). Images from each run were realigned to the third frame of the first run and smoothed using a 6 mm full-width half-maximum kernel. Statistical analysis was based on a linear model with correlated errors, where the design matrix was first convolved with a haemodynamic response timed to coincide with each slice (Glover, 1999). Temporal drift was removed by adding a cubic spline in the frame timed to the design matrix, and spatial drift, by adding a covariate to the whole volume average. The linear model was then re-estimated using least squares on the whitened data to produce estimates of effects and their standard deviations at each voxel, for the following contrasts: receiving negative feedback versus receiving positive feedback for planning a set-shift; matching after negative feedback versus matching after positive feedback for executing the set-shift; receiving positive feedback versus receiving control feedback for maintaining set; and matching after positive feedback versus control matching for matching according to the same rule. Resulting effects and standard deviation images were non-linearly transformed into standard proportional stereotaxic space (ICBM152 template) using anatomical MRI to template transformation parameters, using a feature-matching algorithm (Collins et al., 1994; Zijdenbos et al., 2002). In the second step, runs and subjects were combined using a mixed-effects linear model that was performed by first, estimating the ratio of random-effects variance to fixed-effects variance, and then regularizing this ratio by spatial smoothing with a Gaussian filter. Both intra-group (9/9 + 9/10 versus 10/10) and intergroup comparisons were generated. Statistical maps were thresholded at P < 0.05 correcting for multiple comparisons using the minimum between a Bonferroni correction and random field theory, yielding a threshold of t > 4.82 for a single voxel with a cluster size > 40 mm3 for significance, assessed on the spatial extent of contiguous voxels. Predicted peaks reaching P < 0.0001 (t > 3.86) with a cluster size ≥40 mm3 assessed on the spatial extent of contiguous voxels, are reported and identified with an asterisk in the tables. A region was predicted if it was identified in our previous studies using this task (Monchi et al., 2001, 2004; Nagano-Saito et al., 2008; Jubault et al., 2009). For group comparisons, predicted peaks reaching P < 0.001 (t > 3.17) with a cluster size ≥ 40 mm3 are also reported with two asterisks in the tables.
Results
COMT distribution
The distribution on the COMT Val158Met polymorphism did not differ between groups (t = 0.48, P = 0.31, see demographics in Table 1).
Neuropsychological assessment
Parkinson’s disease groups were compared on each neuropsychological test using t-tests. Their results were similar, except for significant differences on the Digit Span (t = 2.26, P = 0.016) and Tower of London (t = 1.75, P = 0.047; Table 1), where the 10R group performed better than the 9R group (homozygous and heterozygous combined).
Behavioural results during functional MRI
In patients with 10R/10R alleles, mean reaction times for trials requiring matching after negative feedback were 3.63 s ± 1.14 s, after positive feedback 2.99 s ± 0.73 s, and with control feedback 2.87 s ± 1.17s. For patients with a 9R allele, mean reaction times for matching after negative feedback, positive feedback, and control feedback were 3.27 s ± 0.66 s, 2.84 s ± 0.73 s and 2.50 s ± 0.73 s, respectively. A mixed design repeated measures ANOVA revealed a significant difference in reaction times for the various matching trials matching after negative feedback, positive feedback, and control feedback (F = 26.070; P < 0.001), but no group difference (F = 1.778; P = 0.193), and no interaction between group and task (F = 0.424; P = 0.644). Perseverative error rates were similar in both groups (2.5% ± 1.5% for 10/10 and 2.2% ± 2.5% for 9R, P = 0.664).
Mean error rates on the control condition were 2.8% ± 3.5% (10R/10R) and 3.9% ± 6.7% (9R), and on the Wisconsin Card Sorting Task, 17.2% ± 9.1% (10R/10R) and 17.1% ± 12.6% (9R). A mixed design repeated measures ANOVA revealed a main effect of task (F = 88.308; P < 0.001), but no group differences (10R/10R versus 9R: F = 0.026; P = 0.678) and no interaction between group and task (F = 0.177; P = 0.678).
Imaging analysis
Planning the set-shift
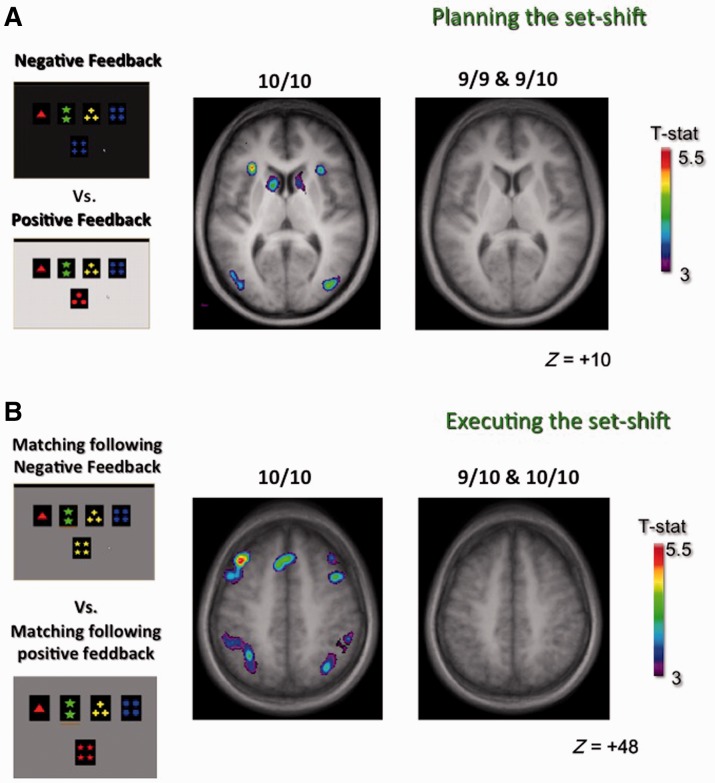
When receiving negative feedback was compared with receiving positive feedback (Table 3 and Fig. 2A), the 10R/10R group displayed significant bilateral activity in ventrolateral prefrontal cortex (prefrontal cortex; area 47/12), medial prefrontal cortex (area 8, 32), posterior parietal cortex (areas 7, 40), and occipital visual regions. Significant activation also appeared in the right dorsolateral prefrontal cortex (areas 46, 9/46), left posterior prefrontal cortex (areas 6, 8), and in the left cerebellum. The active areas overlapped those observed in healthy subjects from our previous studies (Monchi et al., 2001, 2004; Nagano-Saito et al., 2008; Jubault et al., 2009). For the 9R group, activation reached significance in the right lateral premotor cortex, the right occipital areas, and the left precuneus only.
Table 3.
Planning the Set-Shift: Receiving Negative Feedback (RNF) − Receiving Positive Feedback (RPF) for patients with Parkinson’s disease according to SLC6A3/DAT1 VNTR polymorphism
| Anatomical area | 10R/10R |
9R/9R or 9R/10R |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t-value | cluster size (mm3) | x | y | z | t-value | cluster size (mm3) | |
| Mid-dorsolateral prefrontal cortex (area 46, 9/46) | ||||||||||
| Left | ||||||||||
| Right | 40 | 28 | 24 | 5.36 | 2616 | 42 | 30 | 26 | 4.44* | 112 |
| Mid-ventolateral prefrontal cortex (area 47/12) | ||||||||||
| Left | −30 | 26 | 8 | 5.17 | 656 | |||||
| Right | 32 | 24 | 10 | 4.31* | 128 | |||||
| Anterior cingulate cortex/medial prefrontal cortex (area 32/6/8) | ||||||||||
| Left | −6 | 24 | 44 | 5.15 | 840 | |||||
| Right | 6 | 26 | 46 | 5.19 | 408 | |||||
| Lateral premotor cortex (area 6) | ||||||||||
| Left | ||||||||||
| Right | 34 | 14 | 32 | 4.44* | 208 | |||||
| Posterior prefrontal cortex (junction of areas 6, 8, 44) | ||||||||||
| Left | −42 | 6 | 30 | 5.47 | 1744 | |||||
| Right | ||||||||||
| Parietal cortex (area 40, 7) | ||||||||||
| Left | −30 | −68 | 34 | 6.08 | 5896 | |||||
| Right | 32 | −72 | 30 | 7.10 | 6808 | |||||
| Precuneus (area 7) | ||||||||||
| Left | −6 | −72 | 54 | 4.44* | 312 | |||||
| Right | ||||||||||
| Striate/extrastriate cortex (area 17,18,19) | ||||||||||
| Left | −28 | −66 | −6 | 6.15 | 2768 | |||||
| −50 | −60 | −6 | 4.92 | 1104 | ||||||
| Right | 24 | −70 | −10 | 5.38 | 3848 | |||||
| 40 | −82 | 20 | 4.69* | 472 | ||||||
| Caudate nucleus | ||||||||||
| Left | −12 | 10 | 8 | 4.29* | 192 | |||||
| Right | 12 | 12 | 4 | 4.10* | 40 | |||||
| Cerebellum | ||||||||||
| Left | −10 | −80 | −32 | 4.82 | 944 | |||||
| Right | −30 | −66 | −30 | 4.41* | 192 | |||||
| 10R/10R > 9R/10R + 9R/9R | 10R/10R < 9R/10R + 9R/9R | |||||||||
| NONE | ||||||||||
| Anterior cingulate cortex/medial prefrontal cortex (area 32/6/8) | ||||||||||
| Left | −4 | 26 | 40 | 3.26** | 48 | |||||
| Precuneus | ||||||||||
| Left | −2 | −64 | 24 | 3.32** | 48 | |||||
| Parietal cortex (area 40, 7) | ||||||||||
| Left | −24 | −70 | 30 | 3.38** | 96 | |||||
| Right | 44 | −66 | 28 | 3.66** | 96 | |||||
| Putamen | ||||||||||
| Left | −26 | 6 | 10 | 3.76* | 656 | |||||
| Right | 22 | 6 | 12 | 4.21* | 776 | |||||
Findings significant at P < 0.05 corrected, single asterisk (*) indicates predicted peaks of ≥ 40 mm3 at P < 0.0001 uncorrected, and double asterisks (**) at P < 0.001 uncorrected.
Figure 2.
Pattern of fronto-striatal activation for patients with Parkinson’s disease with and without a 9R allele in the SLC6A3/DAT1 VNTR polymorphism. (A) Planning the set-shift. Left: Appearance of the computer monitor, which darkens when ‘receiving’ negative feedback (top left) and brightens with positive feedback (bottom left). Right: Horizontal section (z = +10) in 10/10R patients with Parkinson’s disease (middle) and in 9R-carrying patients with Parkinson’s disease (right). The 10/10R group exhibited patterns of activation similar to healthy individuals from our previous studies; specifically, significant activation in ventrolateral prefrontal cortex and the caudate nucleus, which form the ‘cognitive’ cortico-striatal loop that is active during planning a set-shift. On the other hand, the 9R-carrying group had no significant activations in these regions. (B) Executing the set-shift. Left: Appearance of the display with brightness maintained during matching after negative feedback (top) and during matching following positive feedback (bottom). Right: Horizontal section (z = +48) for 10/10R patients with Parkinson’s disease (middle) and 9R-carrier patients with (right). The 10/10R group revealed significant activity in the premotor cortex and the posterior prefrontal cortex but not the putamen, whereas 9R-carriers displayed no significant activity in any of these regions. Our previous studies indicate that both premotor cortex and the posterior prefrontal cortex, together with putamen (‘motor’ cortico-striatal loop), display significant activity in healthy individuals. Furthermore, there was significant activation in the anterior cingulate cortex and the posterior parietal cortex in the 10/10R group (middle) but not in the 9R-carriers (right).
Group comparisons confirmed these differences: there was significantly more activation in the 10R/10R than the 9R group bilaterally in the left posterior parietal cortex, left medial prefrontal cortex, the left precuneus, and in the putamen bilaterally. No greater activation was observed for the 9R over the 10/10R group for these analyses.
Executing the set-shift
Comparing matching after negative feedback versus matching after positive feedback (Table 4 and Fig. 2B) in the 10/10R group revealed significant activation of bilateral posterior parietal cortex and occipital visual areas, of left anterior and medial prefrontal cortex, left precuneus, right mid-dorsolateral, ventrolateral and posterior prefrontal cortex, and lateral premotor cortex. These activation patterns overlapped those observed in control individuals from our previous studies using the same protocol except for the putamen (Monchi et al., 2001, 2004; Nagano-Saito et al., 2008; Jubault et al., 2009). In the 9R group, however, significant activation was only observed in the cerebellum bilaterally. A group comparison confirmed these differences with significantly greater activations in the 10/10R than in the 9R group in the posterior parietal cortex and occipital visual areas, and in the right posterior prefrontal cortex and the left precuneus.
Table 4.
Executing the Set-Shift: Matching after Negative Feedback (MNF) − Matching after Positive Feedback (MPF) for patients with Parkinson's disease according to SLC6A3/DAT1 VNTR polymorphism
| Anatomical area | 10R/10R |
9R/9R or 9R/10R |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t-value | cluster size (mm3) | x | y | z | t-value | cluster size (mm3) | |
| Mid-dorsolateral prefrontal cortex (area 46, 9/46) | ||||||||||
| Left | ||||||||||
| Right | 44 | 30 | 40 | 4.04* | 64 | |||||
| Mid-ventolateral prefrontal cortex (area 47/12) | ||||||||||
| Left | ||||||||||
| Right | 34 | 20 | 2 | 4.15* | 48 | |||||
| Anterior cingulate cortex/medial prefrontal cortex (area 32/6/8) | ||||||||||
| Left | −8 | 22 | 50 | 4.60* | 768 | |||||
| Right | ||||||||||
| Lateral premotor cortex (area 6) | ||||||||||
| Left | ||||||||||
| Right | 24 | 18 | 60 | 4.11* | 48 | |||||
| Posterior prefrontal cortex (junction of areas 6, 8, 44) | ||||||||||
| Left | ||||||||||
| Right | 42 | 12 | 48 | 4.35* | 120 | |||||
| Anterior prefrontal cortex (area 10) | ||||||||||
| Left | −38 | 52 | 12 | 4.28* | 264 | |||||
| Right | ||||||||||
| Parietal cortex (area 40, 7) | ||||||||||
| Left | −34 | −58 | 42 | 4.80 | 632 | |||||
| Right | 34 | −68 | 44 | 4.45* | 384 | |||||
| Precuneus (area 7) | ||||||||||
| Left | −8 | −86 | −16 | 4.88 | 4560 | |||||
| Right | ||||||||||
| Striate/Extrastriate cortex (area 17,18,19) | ||||||||||
| Left | −28 | −76 | −14 | 5.23 | 4560 | |||||
| Right | 14 | −84 | −18 | 4.77 | 1128 | |||||
| 40 | −88 | 6 | 5.36 | 1032 | ||||||
| Cerebellum | ||||||||||
| Left | 34 | −64 | −28 | 4.44* | 336 | |||||
| Right | 14 | −76 | −26 | 4.18* | 96 | |||||
| 10R/10R > 9R/10R + 9R/9R | M10R/10R < 9R/10R + 9R/9R | |||||||||
| Posterior prefrontal cortex (junction of areas 6, 8, 44) | NONE | |||||||||
| Left | ||||||||||
| Right | 40 | 10 | 48 | 3.97* | 288 | |||||
| Parietal cortex (area 40, 7) | ||||||||||
| Left | −56 | −34 | 46 | 3.51** | 184 | |||||
| Right | 56 | −38 | 46 | 3.45** | 136 | |||||
| 34 | −32 | 66 | 3.25** | 64 | ||||||
| Precuneus (area 7) | ||||||||||
| Left | −4 | −64 | 42 | 3.50** | 112 | |||||
| Right | ||||||||||
| Striate/extrastriate cortex (area 17,18,19) | ||||||||||
| Left | −18 | −88 | −18 | 3.99* | 282 | |||||
| Right | 26 | −90 | −20 | 3.38** | 40 | |||||
Findings significant at P < 0.05 corrected, single asterisk (*) indicates predicted peaks of ≥ 40 mm3 at P < 0.0001 uncorrected, and double asterisks (**) at P < 0.001 uncorrected.
Maintaining-set
Comparison of receiving positive feedback and receiving control feedback (Table 5) in the 10/10R group, revealed significant bilateral activation in occipital visual regions and in right precuneus. Activation was also observed in the left caudate nucleus. 9R patients exhibited significant activation in bilateral occipital visual areas, in left posterior parietal cortex, and the right cerebellum. For the 10/10R to 9R comparison, activation was significantly greater in the left occipital visual cortex.
Table 5.
Maintaining set: receiving positive feedback (RPF) − receiving control feedback (RCF) for patients with Parkinson’s disease according to SLC6A3/DAT1 VNTR polymorphism
| Anatomical area | 10R/10R |
9R/9R or 9R/10R |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t-value | cluster size (mm3) | x | y | z | t-value | cluster size (mm3) | |
| Parietal cortex (area 40, 7) | ||||||||||
| Left | −4 | −84 | 28 | 4.89 | 720 | |||||
| Right | ||||||||||
| Precuneus (area 7) | ||||||||||
| Left | ||||||||||
| Right | 10 | −76 | 40 | 4.12* | 136 | |||||
| Striate/extrastriate cortex (area 17,18,19) | ||||||||||
| Left | −14 | −72 | 10 | 6.42 | 33376 | −14 | −92 | −2 | 4.65* | 1640 |
| Right | 28 | −78 | −14 | 4.20* | 224 | 2 | −86 | −2 | 4.26* | 1640 |
| 14 | −62 | 8 | 6.77 | 33376 | 14 | −80 | 12 | 5.06 | 1560 | |
| Caudate tail | ||||||||||
| Left | −22 | −24 | 20 | 4.18* | 168 | |||||
| Cerebellum | ||||||||||
| Left | ||||||||||
| Right | 46 | −60 | −26 | 4.76 | 896 | |||||
| 10R/10R > 9R/10R + 9R/9R | 10R/10R < 9R/10R + 9R/9R | |||||||||
| Striate/extrastriate cortex (area 17,18,19) | NONE | |||||||||
| Left | −52 | −64 | −28 | 3.53 | 40 | |||||
| Right | ||||||||||
Findings significant at P < 0.05 corrected, single asterisk (*) indicates predicted peaks of ≥ 40 mm3 at P < 0.0001 uncorrected, and double asterisks (**) at P < 0.001 uncorrected.
Matching according to the same rule
In the 10/10R group, comparison of matching after positive feedback and control matching (Table 6) revealed significant activation in bilateral occipital visual areas, the left posterior parietal cortex, and the midline precuneus. In the 9R group, activation was observed in the right lateral premotor cortex, the left parietal cortex, and occipital visual areas. In the 10/10R versus 9R comparison, activation was greater in left occipital visual areas.
Table 6.
Matching according to the same rule. Matching following positive feedback − control matching for patients with Parkinson’s disease according to SLC6A3/DAT1 VNTR polymorphism
| Anatomical area | 10R/10R |
9R/10R or 9R/9R |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| x | y | z | t-value | cluster size (mm3) | x | y | z | t-value | cluster size (mm3) | |
| Lateral premotor cortex (area 6) | ||||||||||
| Right | 28 | −6 | 50 | 4.28* | 104 | |||||
| Parietal cortex (area 40, 7) | ||||||||||
| Left | −32 | −74 | 26 | 4.18* | 360 | −26 | −62 | 64 | 4.25* | 48 |
| Precuneus (area 7) | ||||||||||
| Middle | 0 | −72 | 16 | 4.35* | 192 | |||||
| Striate/extrastriate cortex (area 17,18,19) | ||||||||||
| Left | −18 | −74 | −14 | 4.91 | 520 | −10 | −80 | 12 | 3.93* | 40 |
| −6 | −76 | −10 | 4.21* | 40 | −8 | −74 | 16 | 4.28* | 480 | |
| −20 | −56 | −6 | 4.95 | 1208 | −32 | −74 | 22 | 4.11* | 136 | |
| Right | 20 | −44 | −18 | 4.39* | 104 | |||||
| 24 | −64 | −18 | 4.42* | 528 | ||||||
| 10R/10R > 9R/10R + 9R/9R | 10R/10R < 9R/10R + 9R/9R | |||||||||
| Striate/extrastriate cortex (area 17,18,19) | NONE | |||||||||
| Left | −26 | −76 | −20 | 3.45** | 48 | |||||
| −20 | −74 | −16 | 3.50** | 48 | ||||||
| −28 | −64 | −6 | 3.22** | 40 | ||||||
Findings significant at P < 0.05 corrected, single asterisk (*) indicates predicted peaks of ≥40 mm3 at P < 0.0001 uncorrected, and double asterisks (**) at P < 0.001 uncorrected.
Discussion
In Parkinson’s disease, genetic variation of the dopamine transporter gene (SLC6A3/DAT1; striatal dopamine levels) and its impact on brain activity underlying cognitive function, has been overlooked. Our results indicate a strong effect of SLC6A3/DAT1 polymorphism on cortico-striatal brain activation patterns. We found decreased activation in both the cognitive and motor cortico-striatal loops while planning and executing a set-shift, respectively, in patients with Parkinson’s disease carrying a 9R allele. In contrast, individuals homozygous on the 10-repeat allele show patterns similar to controls from our previous studies (Monchi et al., 2001, 2004; Nagano-Saito et al., 2008). In addition, neuropsychological testing revealed that patients with a 9R allele (homozygous or heterozygous) performed worse than those homozygous for the 10R, on tasks linked to executive function and working memory, including the Tower of London and Digit Span. Finally, the COMT Val158Met polymorphism, which modulates frontal cortical dopamine levels and therefore, could affect performance and brain activity, is an unlikely factor here, as our SLC6A3/DAT1 groups (striatal dopamine) had similar distributions of COMT variation.
Interestingly, the weaker brain activation of the 9R Parkinson’s disease patient group was exacerbated for Wisconsin Card Sorting Task conditions that usually recruit the striatum compared to those that do not (Monchi et al., 2001, 2004; Nagano-Saito et al., 2008). This is consistent with the dopamine transporter gene’s (SLC6A3/DAT1) involvement in dopamine reuptake, or clearance, from the synaptic cleft in the striatum. In non-Parkinson’s disease individuals, data on dopamine availability according to the presence or absence of a 9R allele have yielded mixed results (Heinz et al., 2000; Fuke et al., 2001; Faraone et al., 2014), although a recent meta-analysis suggests stronger dopamine transporter binding (so less dopamine at the synaptic cleft) in 9R carriers. This is consistent with our findings of weaker activation and worse performance in patients with Parkinson’s disease carrying a 9R.
Behavioural data show a different pattern, however. In healthy individuals 10R homozygosity is linked to weaker executive function (Fossella et al., 2002; Bellgrove et al., 2007; Garcia-Garcia et al., 2010; van Holstein et al., 2011) and to attention deficit hyperactivity disorder (Cornish et al., 2005; Yang et al., 2007). In Parkinson’s disease patients, we find the opposite: those homozygous on the 10R display better performance and stronger brain activation than 9R carriers. This difference in activation patterns could be interpreted as the 9R-carriers being more efficient at performing the task than homozygous-10R patients. However, the weaker activation in 9R-carriers is observed in the subtraction analyses used to contrast task conditions. This suggests that in 9R-carriers, neuronal resource allocation differs less across conditions and therefore, indicates that task specificity is diminished in 9R-carriers.
Another possibility is that the effect of l-DOPA differs with DAT1 polymorphism. Work on healthy individuals indicates that the SLC6A3/DAT1 polymorphism affects cognitive flexibility and dopamine agonist response: individuals homozygous on the 10R allele performed worse than 9R carriers, but benefited from the dopamine agonist bromocriptine, whereas the 9R did not (van Holstein et al., 2011). The same pattern could arise in our patients with Parkinson’s disease, where those homozygous on the 10R benefit further from their dopamine agonist treatment than do 9R carriers. Even though our patients were asked to withdraw from dopamine medication 12 h before testing, residual effects may persist, or long-term changes in dopamine regulation could have taken place. For example, it has been suggested that the dopamine transporter serves to maintain stable levels of synaptic dopamine (Sossi et al., 2007), which could be less steady in 9R carriers. Accordingly, one study has reported a higher proportion of psychosis and/or dyskinesia in patients with Parkinson’s disease with a 9R allele, compared to those homozygous on the 10R allele (Kaiser et al., 2003). This interaction, however, is unlikely to explain our global findings, as Unified Parkinson's Disease Rating Scale (UPDRS) scores are similar across groups.
A final possibility is related to the inverted-U hypothesis linking frontal dopamine levels and genotype to fronto-striatal function in early Parkinson’s disease: depending on position along this curve, an increase in dopamine could lead to a decline as performance shifts down the right-hand side of the inverted-U curve (Williams-Gray et al., 2008). When comparing COMT Val158Met polymorphisms (affecting frontal dopamine levels) in patients with Parkinson’s disease, cognitive performance as a function of genotype appear opposite to those of controls, but is explained by this positional continuum along the inverted-U curve (Fallon et al., 2013). Analogously, an inverted-U shaped curve between performance or brain activation patterns and dopamine levels might exist for striatal function, with positional shift along the curve depending on SLC6A3/DAT1 genotype and disease progression. Furthermore, in early Parkinson’s disease, striatal dopamine loss can be tied to increased frontal dopamine (Rakshi et al., 1999). Therefore, parkinsonian 9R-carriers, with putatively less striatal dopamine, could exhibit larger increases in frontal dopamine than 10R homozygotes, which in turn leads to weaker fronto-striatal activity regardless of COMT genotype (no COMT differences between our SLC6A3/DAT1 groups). In the future larger scale studies, evaluating multiple genotype interactions, will be performed.
Longitudinal studies are needed to address whether carrying a 9R allele eventually affects cognitive function across multiple domains. Anecdotally, our patients with Parkinson’s disease homozygous on the 9R allele (three patients) had mild cognitive impairment at the time of study, and two became demented 18 months after. However, patients with Parkinson’s disease homozygous on the 9R allele are not driving the differences reported in the present work, because removing them from the analyses did not change our results (not shown). Without the 9R/9R individuals, our main findings hold: patients with Parkinson’s disease carrying a 9R allele display decreased activation of the cognitive cortico-striatal loop while planning a set-shift, and of the motor loop while executing the shift, compared to those without a 9R (10/10R).
In conclusion, we find that genetic variation of the dopamine transporter gene SLC6A3 (formerly named DAT1) in Parkinson’s disease affects patterns of cortico-striatal brain activity and may influence the cognitive profile and evolution of patients with Parkinson’s disease.
Funding
This work was supported by a Canadian Institutes of Health Research operating grant (MOP-126017) to O.M., and a Parkinson Society Canada pilot grant to O.M. and G.A.R. A.N. received a Doctoral Award - Frederick Banting and Charles Best Graduate Scholarship by the (Canadian Institutes of Health Research). G.A.R. holds the Canada Research Chair and a Jeanne-et-J-Louis-Levesque Chair for the Genetics of Brain Diseases.
Glossary
Abbreviation
- VNTR
variable number of tandem repeats
References
- Bellgrove MA, Chambers CD, Johnson KA, Daibhis A, Daly M, Hawi Z, et al. Dopaminergic genotype biases spatial attention in healthy children. Mol Psychiatry. 2007;12:786–92. doi: 10.1038/sj.mp.4002022. [DOI] [PubMed] [Google Scholar]
- Bertolino A, Fazio L, Di Giorgio A, Blasi G, Romano R, Taurisano P, et al. Genetically determined interaction between the dopamine transporter and the D2 receptor on prefronto-striatal activity and volume in humans. J Neurosci. 2009;29:1224–34. doi: 10.1523/JNEUROSCI.4858-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burgess P, Shallice T. The Hayling and Brixton Tests. Test manual. Bury St, Edmunds: Thames Valley Test Company; 1997. [Google Scholar]
- Collins DL, Neelin P, Peters TM, Evans AC. Automatic 3D intersubject registration of MR volumetric data in standardized Talairach space. J Comput Assist Tomogr. 1994;18:192–205. [PubMed] [Google Scholar]
- Cools R, Stefanova E, Barker RA, Robbins TW, Owen AM. Dopaminergic modulation of high-level cognition in Parkinson's disease: the role of the prefrontal cortex revealed by PET. Brain. 2002;125(Pt 3):584–94. doi: 10.1093/brain/awf052. [DOI] [PubMed] [Google Scholar]
- Cornish KM, Manly T, Savage R, Swanson J, Morisano D, Butler N, et al. Association of the dopamine transporter (DAT1) 10/10-repeat genotype with ADHD symptoms and response inhibition in a general population sample. Mol Psychiatry. 2005;10:686–98. doi: 10.1038/sj.mp.4001641. [DOI] [PubMed] [Google Scholar]
- Culbertson WC, Zillmer EA. Tower of London Drexel University. Chicago, IL: Multi-Health Systems; 2005. [Google Scholar]
- Diamond A. Consequences of variations in genes that affect dopamine in prefrontal cortex. Cereb Cortex. 2007;17(Suppl 1):i161–70. doi: 10.1093/cercor/bhm082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dirnberger G, Jahanshahi M. Executive dysfunction in Parkinson's disease: a review. J Neuropsychol. 2013;7:193–224. doi: 10.1111/jnp.12028. [DOI] [PubMed] [Google Scholar]
- Fallon SJ, Williams-Gray CH, Barker RA, Owen AM, Hampshire A. Prefrontal dopamine levels determine the balance between cognitive stability and flexibility. Cereb Cortex. 2013;23:361–9. doi: 10.1093/cercor/bhs025. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Spencer TJ, Madras BK, Zhang-James Y, Biederman J. Functional effects of dopamine transporter gene genotypes on in vivo dopamine transporter functioning: a meta-analysis. Mol Psychiatry. 2014;19:880–9. doi: 10.1038/mp.2013.126. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Brayne CE, Robbins TW, Barker RA. The cognitive ability of an incident cohort of Parkinson's patients in the UK. The CamPaIGN study. Brain. 2004a;127(Pt 3):550–60. doi: 10.1093/brain/awh067. [DOI] [PubMed] [Google Scholar]
- Foltynie T, Goldberg TE, Lewis SG, Blackwell AD, Kolachana BS, Weinberger DR, et al. Planning ability in Parkinson's disease is influenced by the COMT val158met polymorphism. Mov Disord. 2004b;19:885–91. doi: 10.1002/mds.20118. [DOI] [PubMed] [Google Scholar]
- Fossella J, Sommer T, Fan J, Wu Y, Swanson JM, Pfaff DW, et al. Assessing the molecular genetics of attention networks. BMC Neurosci. 2002;3:14. doi: 10.1186/1471-2202-3-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuke S, Suo S, Takahashi N, Koike H, Sasagawa N, Ishiura S. The VNTR polymorphism of the human dopamine transporter (DAT1) gene affects gene expression. Pharmacogenomics J. 2001;1:152–6. doi: 10.1038/sj.tpj.6500026. [DOI] [PubMed] [Google Scholar]
- Garcia-Garcia M, Barcelo F, Clemente IC, Escera C. The role of DAT1 gene on the rapid detection of task novelty. Neuropsychologia. 2010;48:4136–41. doi: 10.1016/j.neuropsychologia.2010.10.005. [DOI] [PubMed] [Google Scholar]
- Glover GH. Deconvolution of impulse response in event-related BOLD fMRI. Neuroimage. 1999;9:416–29. doi: 10.1006/nimg.1998.0419. [DOI] [PubMed] [Google Scholar]
- Golden CJ, Freshwater SM. The stroop color and word test: a manual for clinical and experimental uses. Wood Dale, IL: Stoelting Co.; 1998. [Google Scholar]
- Heinz A, Goldman D, Jones DW, Palmour R, Hommer D, Gorey JG, et al. Genotype influences in vivo dopamine transporter availability in human striatum. Neuropsychopharmacology. 2000;22:133–9. doi: 10.1016/S0893-133X(99)00099-8. [DOI] [PubMed] [Google Scholar]
- Hooper HE. The hooper visual organization test manual. Los Angles, CA: Western Psychological Services; 1958. [Google Scholar]
- Hughes AJ, Daniel SE, Kilford L, Lees AJ. Accuracy of clinical diagnosis of idiopathic Parkinson's disease: a clinico-pathological study of 100 cases. J Neurol Neurosurg Psychiatry. 1992;55:181–4. doi: 10.1136/jnnp.55.3.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanette Y, Ska B, Coté H. France: 2004. Protocole MEC - Protocole Montréal d’Évaluation de la Communication: Ortho Édition. [PubMed] [Google Scholar]
- Jubault T, Monetta L, Strafella AP, Lafontaine AL, Monchi O. L-dopa medication in Parkinson's disease restores activity in the motor cortico-striatal loop but does not modify the cognitive network. PLoS One. 2009;4:e6154. doi: 10.1371/journal.pone.0006154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser R, Hofer A, Grapengiesser A, Gasser T, Kupsch A, Roots I, et al. L-dopa-induced adverse effects in PD and dopamine transporter gene polymorphism. Neurology. 2003;60:1750–5. doi: 10.1212/01.wnl.0000068009.32067.a1. [DOI] [PubMed] [Google Scholar]
- Kang AM, Palmatier MA, Kidd KK. Global variation of a 40-bp VNTR in the 3'-untranslated region of the dopamine transporter gene (SLC6A3) Biol Psychiatry. 1999;46:151–60. doi: 10.1016/s0006-3223(99)00101-8. [DOI] [PubMed] [Google Scholar]
- Kaplan E, Googlass H, Weintrab S. Philadelphia: Lea and Febiger; 1983. Boston naming test. [Google Scholar]
- Kordower JH, Olanow CW, Dodiya HB, Chu Y, Beach TG, Adler CH, et al. Disease duration and the integrity of the nigrostriatal system in Parkinson's disease. Brain. 2013;136(Pt 8):2419–31. doi: 10.1093/brain/awt192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange KW, Robbins TW, Marsden CD, James M, Owen AM, Paul GM. L-dopa withdrawal in Parkinson's disease selectively impairs cognitive performance in tests sensitive to frontal lobe dysfunction. Psychopharmacology. 1992;107:394–404. doi: 10.1007/BF02245167. [DOI] [PubMed] [Google Scholar]
- Mitchell RJ, Howlett S, Earl L, White NG, McComb J, Schanfield MS, et al. Distribution of the 3' VNTR polymorphism in the human dopamine transporter gene in world populations. Hum Biol. 2000;72:295–304. [PubMed] [Google Scholar]
- Monchi O, Petrides M, Doyon J, Postuma RB, Worsley K, Dagher A. Neural bases of set-shifting deficits in Parkinson's disease. J Neurosci. 2004;24:702–10. doi: 10.1523/JNEUROSCI.4860-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Mejia-Constain B, Strafella AP. Cortical activity in Parkinson's disease during executive processing depends on striatal involvement. Brain. 2007;130(Pt 1):233–44. doi: 10.1093/brain/awl326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monchi O, Petrides M, Petre V, Worsley K, Dagher A. Wisconsin Card Sorting revisited: distinct neural circuits participating in different stages of the task identified by event-related functional magnetic resonance imaging. J Neurosci. 2001;21:7733–41. doi: 10.1523/JNEUROSCI.21-19-07733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. J Neurosci. 2008;28:3697–706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–9. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- Osterrieth PA. Filetest de copie d'une figure complex: contribution a l'etude de la perception et de la memoire. Arch de Psychologie. 1944;30:286–356. [Google Scholar]
- Rakshi JS, Uema T, Ito K, Bailey DL, Morrish PK, Ashburner J, et al. Frontal, midbrain and striatal dopaminergic function in early and advanced Parkinson's disease A 3D [(18)F]dopa-PET study. Brain. 1999;122(Pt 9):1637–50. doi: 10.1093/brain/122.9.1637. [DOI] [PubMed] [Google Scholar]
- Reitan RM, Wolfson D. The halstead-reitan neuropsychological test battery. Tucson: Neuropsychology Press; 1985. [Google Scholar]
- Schmidt M. Psychological Assessments. California: WPS publishing; 1996. Rey auditory verbal learning test (RAVLT) [Google Scholar]
- Schuelke M. An economic method for the fluorescent labeling of PCR fragments. Nat Biotechnol. 2000;18:233–4. doi: 10.1038/72708. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Hawrylak VA, Matus C, Guido MA, Levey AI. Dopamine axon varicosities in the prelimbic division of the rat prefrontal cortex exhibit sparse immunoreactivity for the dopamine transporter. J Neurosci. 1998;18:2697–708. doi: 10.1523/JNEUROSCI.18-07-02697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossi V, de la Fuente-Fernandez R, Schulzer M, Troiano AR, Ruth TJ, Stoessl AJ. Dopamine transporter relation to dopamine turnover in Parkinson's disease: a positron emission tomography study. Ann Neurol. 2007;62:468–74. doi: 10.1002/ana.21204. [DOI] [PubMed] [Google Scholar]
- van Holstein M, Aarts E, van der Schaaf ME, Geurts DE, Verkes RJ, Franke B, et al. Human cognitive flexibility depends on dopamine D2 receptor signaling. Psychopharmacology. 2011;218:567–78. doi: 10.1007/s00213-011-2340-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenbergh DJ, Persico AM, Hawkins AL, Griffin CA, Li X, Jabs EW, et al. Human dopamine transporter gene (DAT1) maps to chromosome 5p15.3 and displays a VNTR. Genomics. 1992;14:1104–6. doi: 10.1016/s0888-7543(05)80138-7. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler’s memory scale (WMS-III) New York: Psychological Corporation; 1997. [Google Scholar]
- Wechsler D. Abbreviated Scale of Intelligence™ (WASI™) New York: Psychological Corporation; 1999. [Google Scholar]
- Williams-Gray CH, Hampshire A, Barker RA, Owen AM. Attentional control in Parkinson's disease is dependent on COMT val 158 met genotype. Brain. 2008;131(Pt 2):397–408. doi: 10.1093/brain/awm313. [DOI] [PubMed] [Google Scholar]
- Williams-Gray CH, Hampshire A, Robbins TW, Owen AM, Barker RA. Catechol O-methyltransferase Val158Met genotype influences frontoparietal activity during planning in patients with Parkinson's disease. J Neurosci. 2007;27:4832–8. doi: 10.1523/JNEUROSCI.0774-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worsley KJ. Spatial smoothing of autocorrelations to control the degrees of freedom in fMRI analysis. Neuroimage. 2005;26:635–41. doi: 10.1016/j.neuroimage.2005.02.007. [DOI] [PubMed] [Google Scholar]
- Worsley KJ, Liao CH, Aston J, Petre V, Duncan GH, Morales F, et al. A general statistical analysis for fMRI data. Neuroimage. 2002;15:1–15. doi: 10.1006/nimg.2001.0933. [DOI] [PubMed] [Google Scholar]
- Yang B, Chan RC, Jing J, Li T, Sham P, Chen RY. A meta-analysis of association studies between the 10-repeat allele of a VNTR polymorphism in the 3'-UTR of dopamine transporter gene and attention deficit hyperactivity disorder. Am J Med Genet. 2007;144B:541–50. doi: 10.1002/ajmg.b.30453. [DOI] [PubMed] [Google Scholar]
- Zijdenbos AP, Forghani R, Evans AC. Automatic “pipeline” analysis of 3-D MRI data for clinical trials: application to multiple sclerosis. IEEE Trans Med Imaging. 2002;21:1280–91. doi: 10.1109/TMI.2002.806283. [DOI] [PubMed] [Google Scholar]