Abstract
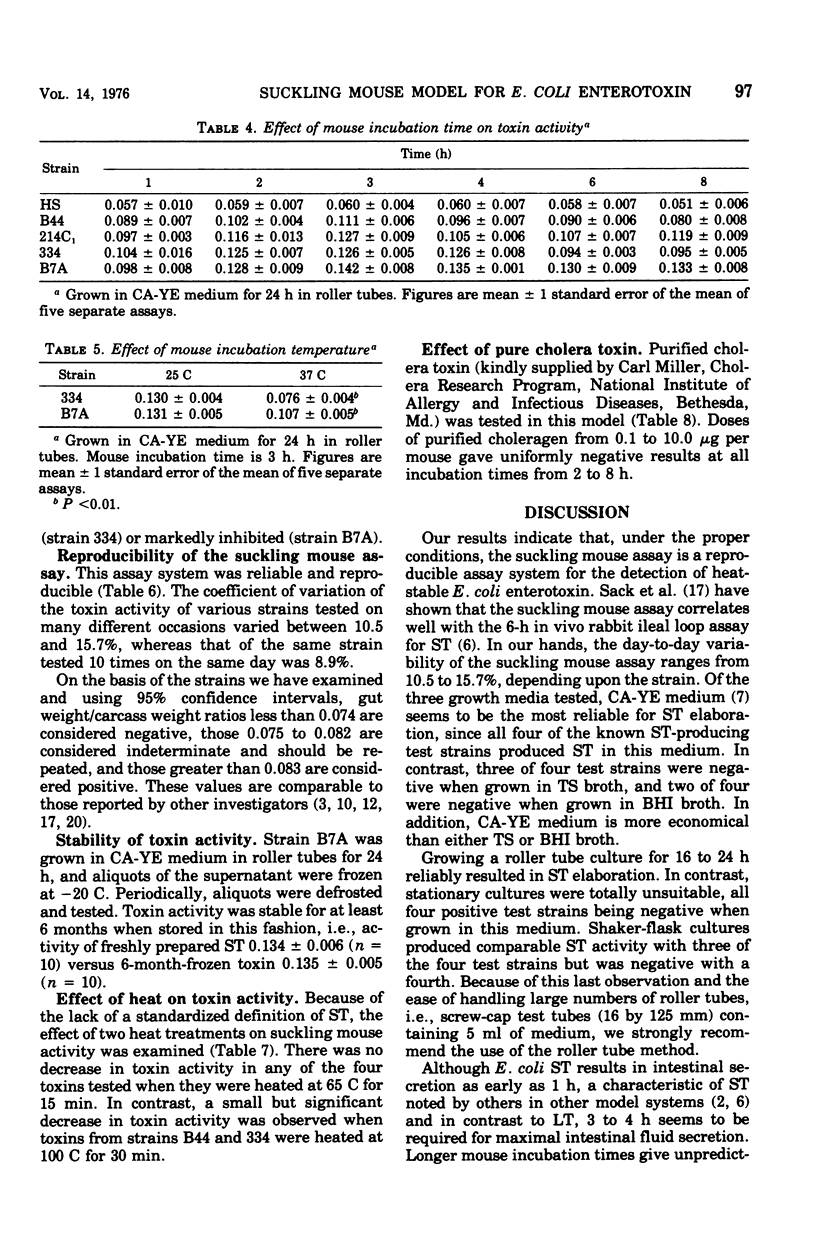
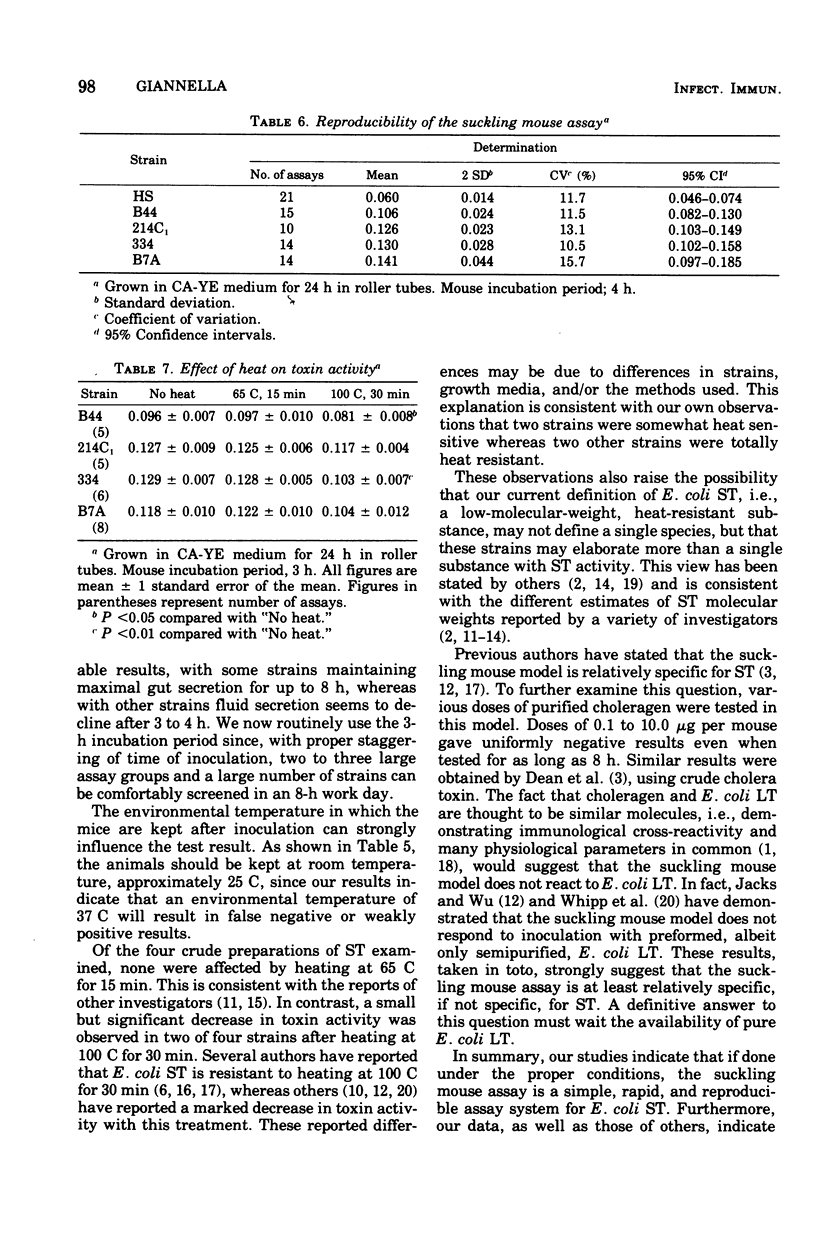
Although the suckling mouse assay is widely used for the detection of heat-stable Escherichia coli enterotoxin (ST), few data have been published concerning the reproducibility, optimal growth, and test conditions of this assay. Four strains of toxigenic E. coli known to elaborate both heat-labile enterotoxin and ST or ST alone were used to study these parameters. ST activity after heat treatment and the effect of purified choleragen were also examined. ST production was optimal in Casamino Acids-yeast extract media, but both Trypticase soy and brain heart infusion broths resulted in several false negative reactions. Growing cultures in roller tubes was the most reliable method of ST production. Shaking-flask cultures and stationary-grown cultures resulted in suboptimal ST production in several strains. Optimal mouse incubation time was 3 h, and fluid secretion did not rise thereafter. Adequate toxin production occurred after 16 to 24 h of incubation. The coefficient of variation of various toxins tested on many occasions varied between 10.5 and 15.7%. Toxin activity was stable for 6 months when frozen at - 20 C. There was no decrease in ST activity when heated at 65 C for 15 min, but a small decrease was observed in two of four strains after heating at 100 C for 30 min. Choleragen, tested at various doses and at multiple times, gave uniformly negative results. These studies indicate that when done under the proper conditions, the suckling mouse assay is a simple, rapid, and reproducible assay for E. coli ST.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banwell J. G., Sherr H. Effect of bacterial enterotoxins on the gastrointestinal tract. Gastroenterology. 1973 Sep;65(3):467–497. [PubMed] [Google Scholar]
- Bywater R. J. Dialysis and ultrafiltration of heat-stable enterotoxin from Escherichia coli. J Med Microbiol. 1972 Aug;5(3):337–343. doi: 10.1099/00222615-5-3-337. [DOI] [PubMed] [Google Scholar]
- Dean A. G., Ching Y. C., Williams R. G., Harden L. B. Test for Escherichia coli enterotoxin using infant mice: application in a study of diarrhea in children in Honolulu. J Infect Dis. 1972 Apr;125(4):407–411. doi: 10.1093/infdis/125.4.407. [DOI] [PubMed] [Google Scholar]
- Donta S. T., Moon H. W., Whipp S. C. Detection of heat-labile Escherichia coli enterotoxin with the use of adrenal cells in tissue culture. Science. 1974 Jan 25;183(4122):334–336. doi: 10.1126/science.183.4122.334. [DOI] [PubMed] [Google Scholar]
- Evans D. G., Evans D. J., Jr, Pierce N. F. Differences in the response of rabbit small intestine to heat-labile and heat-stable enterotoxins of Escherichia coli. Infect Immun. 1973 Jun;7(6):873–880. doi: 10.1128/iai.7.6.873-880.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans D. J., Jr, Evans D. G., Gorbach S. L. Production of vascular permeability factor by enterotoxigenic Escherichia coli isolated from man. Infect Immun. 1973 Nov;8(5):725–730. doi: 10.1128/iai.8.5.725-730.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grady G. F., Keusch G. T. Pathogenesis of bacterial diarrheas. I. N Engl J Med. 1971 Oct 7;285(15):831–841. doi: 10.1056/NEJM197110072851505. [DOI] [PubMed] [Google Scholar]
- Guerrant R. L., Brunton L. L., Schnaitman T. C., Rebhun L. I., Gilman A. G. Cyclic adenosine monophosphate and alteration of Chinese hamster ovary cell morphology: a rapid, sensitive in vitro assay for the enterotoxins of Vibrio cholerae and Escherichia coli. Infect Immun. 1974 Aug;10(2):320–327. doi: 10.1128/iai.10.2.320-327.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerrant R. L., Moore R. A., Kirschenfeld P. M., Sande M. A. Role of toxigenic and invasive bacteria in acute diarrhea of childhood. N Engl J Med. 1975 Sep 18;293(12):567–572. doi: 10.1056/NEJM197509182931201. [DOI] [PubMed] [Google Scholar]
- Jacks T. M., Wu B. J. Biochemical properties of Escherichia coli low-molecular-weight, heat-stable enterotoxin. Infect Immun. 1974 Feb;9(2):342–347. doi: 10.1128/iai.9.2.342-347.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell I. D., Tame M. J., Kenworthy R. Separation and purification of enterotoxins from a strain Escherichia coli pathogenic for pigs. J Med Microbiol. 1974 Nov;7(4):439–450. doi: 10.1099/00222615-7-4-439. [DOI] [PubMed] [Google Scholar]
- Moon H. W., Whipp S. C., Engstrom G. W., Baetz A. L. Response of the rabbit ileal loop to cell-free products from Escherichia coli enteropathogenic for swine. J Infect Dis. 1970 Feb;121(2):182–187. doi: 10.1093/infdis/121.2.182. [DOI] [PubMed] [Google Scholar]
- Pierce N. F., Wallace C. K. Stimulation of jejunal secretion by a crude Escherichia coli enterotixin. Gastroenterology. 1972 Sep;63(6):439–448. [PubMed] [Google Scholar]
- Sack D. A., Merson M. H., Wells J. G., Sack R. B., Morris G. K. Diarrhoea associated with heat-stable enterotoxin-producing strains of Escherichia coli. Lancet. 1975 Aug 9;2(7928):239–241. doi: 10.1016/s0140-6736(75)90958-7. [DOI] [PubMed] [Google Scholar]
- Sack R. B. Human diarrheal disease caused by enterotoxigenic Escherichia coli. Annu Rev Microbiol. 1975;29:333–353. doi: 10.1146/annurev.mi.29.100175.002001. [DOI] [PubMed] [Google Scholar]
- Truszczyński M., Ciosek D. The influence of the contents of different K antigens and of physical factors on the toxicity of enterotoxins and water extracts of Escherichia coli. Res Vet Sci. 1972 May;13(3):212–218. [PubMed] [Google Scholar]
- Whipp S. C., Moon H. W., Lyon N. C. Heat-stable Escherichia coli enterotoxin production in vivo. Infect Immun. 1975 Aug;12(2):240–244. doi: 10.1128/iai.12.2.240-244.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]



