Abstract
Lung diseases remain a significant and devastating cause of morbidity and mortality worldwide. In contrast to many other major diseases, lung diseases notably chronic obstructive pulmonary diseases (COPD), including both asthma and emphysema, are increasing in prevalence and COPD is expected to become the 3rd leading cause of disease mortality worldwide by 2020. New therapeutic options are desperately needed. A rapidly growing number of investigations of stem cells and cell therapies in lung biology and diseases as well as in ex vivo lung bioengineering have offered exciting new avenues for advancing knowledge of lung biology as well as providing novel potential therapeutic approaches for lung diseases. These initial observations have led to a growing exploration of endothelial progenitor cells and mesenchymal stem (stromal) cells in clinical trials of pulmonary hypertension and chronic obstructive pulmonary disease (COPD) with other clinical investigations planned. Ex vivo bioengineering of the trachea, larynx, diaphragm, and the lung itself with both biosynthetic constructs as well as decellularized tissues have been utilized to explore engineering both airway and vascular systems of the lung. Lung is thus a ripe organ for a variety of cell therapy and regenerative medicine approaches. Current state-of-the-art progress for each of the above areas will be presented as will discussion of current considerations for cell therapy based clinical trials in lung diseases.
Keywords: Lung, lung regeneration, lung diseases, endogenous progenitor cell, mesenchymal stem cell, endothelial progenitor cell, embryonic stem cell, induced pluripotent stem cell, cell therapy, bioengineering
Introduction
Development of cell therapies and bioengineering approaches for lung diseases has rapidly progressed over the past approximate 10 years. Initial focus on structural engraftment following administration of exogenous stem or progenitor cells has been largely supplanted by study and application of immunomodulatory and paracrine actions of mesenchymal stem (stromal) cells (MSCs) and endothelial progenitor cells (EPCs) and by the rapidly growing field of ex vivo lung bioengineering. This includes a cautious initial but growing exploration of clinical investigations of cell therapies in lung diseases. Better understanding of the identity and function of endogenous lung progenitor cells and increased sophistication in techniques for inducing development of functional lung cells from both embryonic (ESCs) and induced pluripotent (iPS) stem cells offers further promise. A concise review of each of these areas is presented and an overview schematic is presented in Figure 1. Representative references are provided and readers are referred to relevant indicated review articles for further details and the wider range of published articles in each area.
Figure 1. Schematic illustrating various stem cell, cell therapy and ex vivo bioengineering approaches for lung diseases.
Abbreviations: AFSC amniotic fluid stem cell; BM-MNC bone marrow-derived mononuclear cells; EPC endothelial progenitor cell; ESC embryonic stem cell; iPSC induced pluripotent stem cell; MSC mesenchymal stem (stromal) cell;.
Structural Engraftment of Circulating or Exogenously Administered Stem or Progenitor Cells
A number of early reports initially suggested that bone marrow-derived cells, including hematopoietic stem cells (HSCs), MSCs, EPCs, and other populations could structurally engraft as mature differentiated airway and alveolar epithelial cells or as pulmonary vascular or interstitial cells (reviewed in 1,2). A smaller body of literature in clinical bone marrow and lung transplantation also suggested varying degrees of apparent chimerism in lungs of the transplant recipients (1,2). However, although bone marrow or adipose-derived MSCs can be induced in vitro to express phenotypic markers of alveolar or airway epithelial cells (3), a number of technical issues contributed to misinterpretation of results in these reports. With more sophisticated approaches, some recent reports continue to suggest that engraftment of donor-derived airway and/or alveolar epithelium with several different types of bone marrow-derived cells can occur (3-7). Nonetheless, engraftment of lung epithelium, vasculature, or interstitium by circulating or exogenously administered stem or progenitor cells of bone marrow or other non-lung origins is currently felt to be a rare phenomenon of unlikely physiologic or clinical significance (1,8). Whether engraftment can be achieved by intratracheal or systemic administration of endogenous lung progenitor cells has not yet been well explored.
Ex Vivo Derivation of Lung Epithelial Cells from Embryonic Stem Cells or Induced Pluripotent Stem Cells (iPS)
Early findings from several laboratories demonstrated that both mouse and human ESCs could be induced in culture to express surfactant proteins and lamellar bodies and even form pseudoglandular structures suggestive of type 2 alveolar epithelial (ATII) cell phenotype (8-10). Other early studies suggested development of cells with phenotypic markers of airway epithelial cells following culture of the ESCs under air-liquid interface conditions (11,12). However, these studies were limited by focus on generally one or two immunophenotypic markers, for example expression of surfactant protein, and it has never been clear that the derived cells acquired appropriate functions of airway or alveolar cells. More recent protocols incorporating more sophisticated understanding and application of cell signaling pathways guiding embryologic lung development and development of definitive endoderm, as well as newly developed lineage tracing tools such as Nkx2.1-GFP expressing mice, have yielded more robust in vitro derivation of cells with phenotypic characteristics of airway cells and of both type 2 (ATII) and type 1 (ATI) alveolar epithelial cells from murine and human ESCs as well as from iPS cells, including those derived from iPS cells obtained from patients with CF (13-17). These derived cells can re-populate decellularized whole lung scaffolds but other functional properties have yet to be elucidated (15). The generation of disease specific human ESC cells from patients with CF and of iPS cell lines from patients with both genetic and acquired lung diseases including CF, alpha 1 anti-trypsin deficiency, sickle cell, and scleroderma provides further opportunity to utilize iPS for study of lung diseases (18,19). As such, there is expectation of further rapid advances in use of ESCs and iPS cells to further understand injury and repair process in the lung. However, the current knowledge base does not yet support clinical use of either ESCs or iPS cells for treatment of lung diseases.
Endogenous Lung Stem and Progenitor Cells
The lung is a complex organ containing many distinct epithelial cell types that are distributed in several different regional microenvironments along the pulmonary tract, depicted in schematic form in Figure 2. Consequently, while identification of cells in the lung that can proliferate under steady state or injury conditions has been relatively straightforward, characterization and classification of putative endogenous lung stem and progenitor epithelial cells into a hierarchy has been challenging. This has been complicated by the terminology and nomenclature utilized as the terms “stem” and “progenitor” are often used interchangeably and inconsistently (reviewed in 1, 20-22). Further, it remains unclear whether paradigms and hierarchies described for endogenous stem and progenitor cells in organs such as the intestine and skin also apply to the lung, particularly the lung epithelium (20-22).
Figure 2. Lung epithelial stem and progenitor cell candidates.
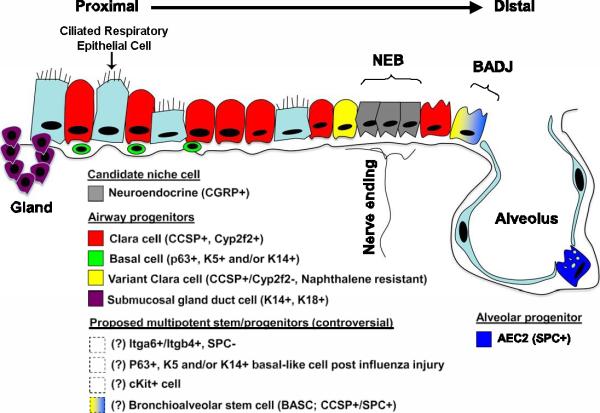
Shown is a schematic of proposed lung epithelial candidate stem or progenitor cells and their niches in the proximal conducting airways and distal alveoli. Cells whose localization or existence is not yet clear or accepted are indicated with dashed boxes and/or question marks. AEC2 = type 2 alveolar epithelial cell; BADJ = bronchoalveolar duct junction; Gland = submucosal gland duct; NEB = neuroepithelial body.Marker abbreviations used for each cell subtype include the following: CCSP = Clara cell secretory protein; CGFP = calcitonin gene– related peptide; Itg = integrin; K = cytokeratin; SPC = surfactant protein C. Modified with permission from Kotton D. Next Generation Research: The hope and hype of lung stem cell research. Am J. Resp Crit Care Med, 198:125501260, 2012 (116).
A large body of evidence in mouse models and a smaller literature in human lungs describes putative populations of adult endogenous airway and alveolar epithelial stem and progenitor cells (20-42). A growing literature, predominantly in mouse models, also describes endogenous adult stem or progenitor cells that function to replace or repair damaged lung stroma or pulmonary vasculature (20-27). Notably, there seems to be regional specificity in mouse lungs with different epithelial stem or progenitor populations described for proximal airways, distal airways, and alveoli (Figure 2).
However, some of the published studies have generated controversy and there is not yet uniform agreement on the identity and/or function of endogenous lung epithelial stem or progenitor cells in either mouse or human lungs (20-22, 28,29). In significant part, this reflects a relatively limited set of lineage markers and other tools with which to isolate and characterize the different putative stem and progenitor populations and their niches. There are also differences in use of tools, particularly flow cytometry and fluorescence active cell sorting (FACS), between different laboratories. As such, different putative cell populations described by different laboratories may in fact represent similar cells characterized by different techniques.
Other considerations specific to the lung include a low constitutive epithelial turnover rate. As such, lung injury models specific to particular regions of lung epithelium, for example sulfur dioxide, ozone, or nitrogen dioxide inhalation (trachea, large airways), naphthalene administration (non-ciliated club cells (formerly known as Clara cells) in the bronchiolar epithelium), or bleomycin (alveolar epithelium) have been used in mice and other adult animal models to identify stem/progenitor cells by inducing cellular proliferation and repopulation of the lung epithelium (20-32). For example, lineage tracing studies in mice have suggesteded that basal cells can give rise to club cells and ciliated cells in the proximal airways during homeostasis as well as after sulfur dioxide injury in mice (33-34).
Cell signaling pathways including β-catenin, Notch, and tissue factor appear to regulate function and fate of the basal epithelial cells and other putative epithelial progenitor populations. Similar conclusions have been derived using human proximal airway basal epithelial cells in ex vivo or in vitro culture systems (26,38).
However, the situation is complex and there may be subpopulations of basal epithelial cells that have more restricted lineages or specific roles. In distal mouse lung airways, differentiated club epithelial cells, although exhibiting a low steady-state proliferative index, can both self-renew and also function to replenish ciliated cells in both the trachea as well as in the distal airways during normal homeostasis and also during injury (36,37).
A subpopulation of toxin-resistant club cells, termed variant club cells (vCE), can function as bronchiolar stem cells located within two discrete cell niches: the neuroepithelial body (NEB) and the bronchoalveolar duct junction (BADJ) (32,38) (Figure 1). Other putative distal airway progenitor cells identified in adult mice include bronchoalveolar stem cells (BASCs), CD45negCD31negEpCAMhiCD49fposCD104posCD24low, integrin α6β4pos SP-Cneg cells, and CK5pos p63pos cells (30,39-42). These cells can both have different localizations in the airway tree and may function differently in repair from experimentally induced lung injury (37,42). However, the situation remains complicated and there is no overall consensus on the identity and functional role of any of the putative endogenous lung epithelial progenitor cells described.
Other tools, particularly, functional assays that recapitulate the in vivo environment, for example re-population of decellularized lung scaffolds, will add further insight. Increased collaboration and cross fertilization to share and compare methods is essential as several of these distal airway epithelial progenitor populations may represent the same cells or phenotypic variants of the same cell population characterized in different ways in different laboratories.
Further, it has become apparent that putative airway progenitor cells may be quiescent in response to less severe injuries and may not play any significant role in normal airway epithelial homeostasis (37). As such, endogenous airway progenitor cells may serve as a reserve population that can function in either normal maintenance or more relevantly following depletion of the facultative progenitor pool. Further, although it is attractive to speculate that lung diseases may in part be a consequence of endogenous airway stem cell failure to regenerate damaged tissue, this is not yet clear from currently available data. A smaller but growing literature also describes endogenous progenitor cells that serve to potentially repair or replenish the vasculature and interstitial components of the lung (43).
Alveolar epithelial repair and regeneration remains centered on the type 2 (ATII) alveolar epithelial cell and the long held concept that ATII cells are precursors for type 1 alveolar epithelial (ATI) cells (29,44). However, recent data suggest that several populations of distal airway epithelial and other progenitor cells in adult mice, including BASCs and CK5+/p63+ cells can differentiate into ATII and ATII cells in vitro and conceivably might contribute to repair of damaged alveoli in in vivo models (30,41,).
There is also interest in the possible roles of endogenous or alveolar airway stem or progenitor cells as lung cancer stem or tumor initiating cells (30,45,46). Further, MSCs, EPCs, and fibrocytes may contribute to development of primary and metastatic lung carcinoma and other malignancies in mouse models, in part, by providing a supportive stroma for the cancers and/or by participating in tumor vascularization (47,48). In contrast, MSCs and EPCs have been demonstrated to home to areas of tumor development and EPCs and MSCs, engineered to express anti-tumor substances, including the tumor necrosis factor-related apoptosis inducing ligand (TRAIL) or IFNβ, have been utilized to suppress tumor growth in mouse tumor models of primary lung cancers, metastatic lung cancers, and of other cancers metastatic to the lung (49-52). As such, a growing yet cautious investigation of MSCs and perhaps other cell types in treatment of lung cancers is anticipated to occur over the next several years.
Circulating Fibrocytes
Circulating fibrocytes have been implicated in the pathogenesis of several lung diseases including both mouse and clinical models of pulmonary fibrosis, pulmonary hypertension, the sub-epithelial fibrosis that can develop in severe asthma, sickle cell lung disease, and in clinical bronchiolitis obliterans in lung and bone marrow transplant patients (53,54). Further, elevated levels of circulating or bronchoalveolar lavage (BAL) fluid fibrocyte levels have been suggested to indicate worse prognosis in acute lung injury (394), IPF (55,56), pulmonary hypertension (57), and development of bronchiolitis obliterans after lung transplant (58). The mechanisms by which fibrocytes are recruited to lung induced to undergo phenotypic transformation into fibroblasts and myofibroblasts and contribute to fibrogenesis in lung are incompletely understood (54). Circulating fibrocytes may also be important in lung cancer development or metastasis (59). As such, although additional information on mechanisms of fibrocyte actions are required, inhibition of fibrocyte actions or alternatively their use as drug delivery vehicles may be potential therapeutic targets.
Endothelial Progenitor Cells
Increasing evidence demonstrates that EPCs play a role in the pathogenesis of a wide variety of lung diseases including pulmonary hypertension, pulmonary fibrosis, asthma, COPD, acute lung injury, lung cancer, bronchopulmonary dysplasia, and obstructive sleep apnea in children (48,60,61303). The number of circulating EPCs has been correlated with several clinical variables in different lung diseases, including lung cancers, demonstrating the potential utility of EPCs as biomarkers (62-67). An increasing number of studies demonstrate that systemic administration of EPCs can mitigate experimentally-induced lung injuries in pre-clinical rodent and dog models of pulmonary hypertension, endotoxin-induced acute lung injury, and bronchopulmonary dysplasia (68-70). Whether this includes structural contributions of the administered cells, paracrine stimulation of endogenous vascular progenitor cells, or other paracrine immunomodulatory actions remains unclear (70). A combination of all of these effects may occur in different disease states. Further, EPCs can be transduced to express proangiogenic factors such as endothelial nitric oxide synthetase (eNOS) or inhibitors of smooth muscle cell proliferation such as calcitonin gene related peptide and appear to home to sites of endothelial damage and lung injury (68,72).
EPCs also appear to home to metastatic tumors in lung and suggests that modification of EPCs to express suicide genes or other therapeutic molecules could be potentially utilized in cell-based therapy approaches for lung cancer (49,51). EPCs can also preferentially localize to areas of injured lung following systemic administration and may also have paracrine effects to decrease inflammation (71). As such, systemic administration of autologous EPCs in both adult and pediatric patients with primary pulmonary hypertension resulted in improved cardiopulmonary and symptomatic outcomes (73,74). Importantly, no short term (12 week) adverse effects of EPC administration were noted although long term follow-up is pending. A growing number of clinical investigations of EPCs for pulmonary hypertension, including use of autologous EPCs transduced to express eNOS are listed on clinicaltrials.gov.
Mesenchymal Stem (Stromal) Cells (MSCs) and Immunomodulation of Lung Diseases
MSCs of bone marrow, adipose, placental tissue, and other origins are been widely investigated for their immunomodulatory effects in a wide range of inflammatory and immune diseases (reviewed in 75,76). The mechanisms of MSC actions are only partly understood and in addition to paracrine actions of soluble peptide and other mediators, a growing body of data suggests that release of episomal or microsomal particles by MSCs can influence behavior of both surrounding structural cells and also surrounding inflammatory cells. MSCs can also act as antigen presenting cells and have recently been demonstrated to transfer mitochondria and likely other cytosolic components through connexin bridges (77). A recent report suggests that MSCs may also promote repair through activation of endogenous distal lung airway progenitor cell populations in mouse models (78). MSCs can be transduced through several transfection or transduction approaches and are also increasingly described as vehicles for delivery of therapeutic genes and proteins. In parallel, administration of non-HLA matched allogeneic MSCs appears to be feasible and safe in a growing number of clinical trials in a range of autoimmune and inflammatory diseases (79,80).
As such a rapidly growing number of studies demonstrate efficacy of either systemic or intratracheal MSC administration in a growing spectrum of lung injury models in rodents and other animal models, explanted human lungs, and in a slowly growing number of clinical investigations in lung diseases (reviewed in 1,81,82). This includes over 90 publications to date in rodent and other pre-clinical models of acute lung injury and bacterial lung infection, asthma, bronchiolitis obliterans, bronchopulmonary dysplasia (BPD), COPD, fibrosing pulmonary injury, pulmonary hypertension, pulmonary ischemia re-perfusion injury, obstructive sleep apnea, radiation-induced lung injury, sepsis and burns, and other critical illness or autoimmune-related lung injuries including hemorrhagic shock, pancreatitis, silicosis, and ventilator- induced lung injury (reviewed 1,81,82). Administration of MSCs of either bone marrow or placental origin has also been demonstrated to decrease injury and inflammation in endotoxin or bacterially-injured injured human lung explants (83).
However, many of these studies utilized different preparations of MSCs ranging from populations of heterogenous plastic adherent adipose stromal cells to purified well characterized bone marrow-derived MSCs obtained from core facilities such as the NCRR/NIH sponsored Texas (formerly Tulane) Center for Preparation and Distribution of Adult Stem Cells (MSCs) (http://medicine.tamhsc.edu/irm/msc-distribution.html). Further, few studies to date have directly compared different MSC preparations and differences between syngeneic, allogeneic, and xenogeneic MSC administration have been less well explored in pre-clinical lung injury models. A growing number of studies demonstrates efficacy of human MSCs in lung injury models in both immune-deficient as well as immune-competent mice (1,89). The different published studies also have varying degrees of rigor with respect to use of appropriate controls. Thus although the general trend has been amelioration of disease-specific endpoints in the different models, each study should be carefully scrutinized and much further work is required to understand the mechanisms of MSCs actions as well as optimizing dosing regimens for potential clinical application. Further, MSCs may not always ameliorate lung injury and available pre-clinical data suggests that MSCs may contribute to established lung fibrosis (84,85).
The mechanisms by which MSCs are acting in the different lung disease models are not fully understood and are likely to be different reflecting the different inflammatory and immune environments in each disease (Figure 3). Following systemic administration, a number of studies have demonstrated that MSCs initially localize in lung and that lung injury results in increased localization and/or retention of marrow-derived cells in lung. Retention in the lung may also trigger the cells to have functional effects. For example, embolization of systemically administered MSCs in lung was felt to result in secretion of an anti-inflammatory protein, TSG-6 (86). In contrast, a growing number of reports suggest that administration of conditioned media obtained from MSCs may mimic many of the ameliorating effects resulting from MSC administration in different lung injury models (87).
Figure 3. Schematic illustrating the range of in vitro immune-modulating effects described for mesenchymal stromal (stem) cells (MSCs).
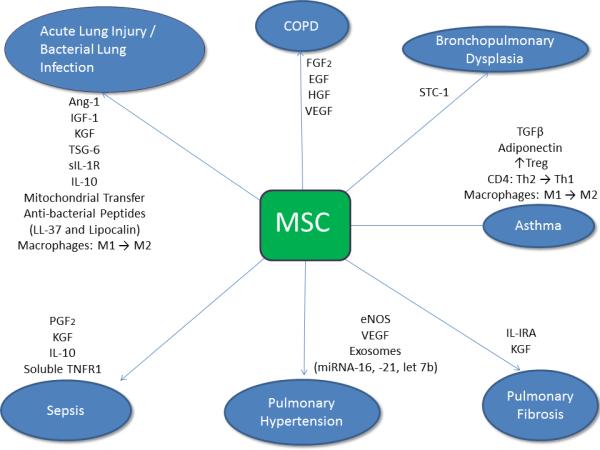
Ang 1 angiopoietin 1;EGF epidermal growth factor; eNOS endothelial nitric oxide synthase; FGF2 fibroblast growth factor 2; HGF = hepatocyte growth factor; IGF1 insulin-like growth factor; (s)IL -1RA = (soluble) interleukin-1 receptor antagonist; IL-10 interleukin 10; KGF keratinocyte growth factor; soluble TNFR1 = soluble tumor necrosis factor-α receptor antagonist; stanniocalcin 1; TGF-β1 = transforming growth factor- β1; TSG-6 Tumor necrosis factor-inducible gene 6 protein; VEGF = vascular endothelial growth factor. Adapted with permission from Weiss DJ and Rojas M. MSCs in Chronic Lung Diseases: COPD and Lung Fibrosis. In Stem Cell-Dependent Therapies (2013), Copyright DeGruyter Publisher GmbH Berlin, G. Gross G, T. Häupl, editors (117).
MSCs can also exert effects on lung inflammation and injury through primary interactions with the immune system rather than through direct actions in lung. For example, a growing body of evidence suggests that MSCs ameliorate allergic airways inflammation in mice by increasing T-regulatory cells or by promoting a Th1 phenotype in vivo in antigen-specific CD4 T cells and in circulating antigen-specific immunoglobulins as a means of abrogating Th2-mediated lung injury (88). As such, MSCs appear to be capable of a spectrum of effects in different lung injuries and also in critical illnesses such as sepsis. This is a critically important point as clinical use of MSCs must be tailored towards the specific disease process.
Other relevant factors about optimal cell preparations, storage and vehicle buffers, dosing, and route of administration (systemic vs direct airway) are poorly understood. Further, cell-based immunomodulation of lung diseases may not be restricted to MSCs as a populations of bone marrow-derived mononuclear cells, human amniotic fluid cells, and human amnion epithelial cells have each been recently described to decrease lung injury in several immune-competent mouse models (90,91). These are ripe areas for further study.
Clinical Trials of Cell-Based Therapies for Lung Diseases
A robust pre-clinical literature supports use of EPCs in pulmonary hypertension and MSCs in acute lung injury or inflammatory critical illnesses and in more chronic inflammatory and immune mediated conditions such as asthma, bronchiolitis obliterans, bronchopulmonary dysplasia and others. Nonetheless, as pre-clinical lung disease models do not necessarily fully mimic human disease pathogeneses or predict clinical behaviors (92), clinical investigations of cell-based therapies for lung diseases have been relatively slow to develop.
A recent multicenter, double-blind, placebo-controlled Phase II trial of systemic administration of a bone marrow-derived MSC preparation (PROCHYMALTM,Osiris Therapeutics Inc, Columbia MD) in patients with moderate-severe COPD in the United States demonstrated safety with no acute infusional toxicity and no attributable mortality or serious adverse events over a subsequent two year follow-up period (93). Although the study was not powered to assess efficacy, a significant early decrease in the systemic inflammatory marker C-reactive protein (CRP), occurred in a sub-population of MSC-treated patients with elevated CRP levels at study onset. This trial provides a firm basis for safety of MSC use, including multiple infusions, in patients with chronic lung diseases and also provides a potential mechanistic clue of in vivo MSC effects.
However, chronic persistent lung diseases with low level or smoldering inflammation, such as COPD, or diseases in which currently available pre-clinical data suggest that MSCs may worsen the disease process, such as IPF, may not be the best therapeutic targets for MSC intervention at present (94,95). More acute inflammatory lung or systemic diseases such as ARDS or sepsis/septic shock, or chronic immune-mediated lung diseases such as severe asthma, may be better targets (81). To this end, clinical trials of MSCs for ARDS and for septic shock are currently in development in the United States and in Canada, respectively.
A growing number of other sanctioned clinical investigations of MSCs and also of EPCs in lung diseases are listed on the Clinical Trials.gov website and demonstrate growing efforts towards carefully conducted closely regulated clinical trials of cell therapies for lung diseases in Europe, Brazil, and Australia as well as in the United States and Canada. However, a growing number of websites and other venues offer unsubstantiated claims of cell therapy efficacy in a range of lung diseases. Significant harm and even death may result in patients who undergo these treatments (96). The FDA has recently begun working with other governmental agencies to attempt to regulate or in some cases close websites making unsubstantiated claims (97). As such, prominent non-profit respiratory disease foundations including the American Thoracic Society, American Lung Association, Pulmonary Hypertension Association, and others have joined with prominent stem cell societies, notably the International Society for Stem Cell Research, in issuing strong statements against stem cell medical tourism on their respective websites.
Ex Vivo Lung Bioengineering
Many lung diseases including asthma, BPD, CF, COPD, and IPF have no cure apart from lung transplantation. However, a critical shortage of donor lungs and acute and chronic rejection necessitating lifelong immunosuppression and resulting in 50% five year mortality has stimulated effort towards ex vivo engineering of functional lung tissues that can be surgically implanted. Significant recent progress has been made utilizing both synthetic scaffolds as well as decellularized cadaveric or donor tissues for ex vivo generation of trachea and diaphragm resulting in growing clinical use of these engineered tissues (98,99). Comparable approaches using 3-dimensional scaffolds generated from synthetic or biomimetic materials scaffolds have been utilized to develop ex vivo lung parenchymal and vascular systems, including implantation of various scaffolds impregnated with stem or other cells in order to produce functioning lung tissue in animal models (100,101).
However, artificial scaffolds neither contain all the extracellular matrix (ECM) components essential for normal lung development and function nor fully replicate the complexity of the lung architecture. As such, decellularized whole lungs may provide more physiologic scaffolds for potential clinical use (102-113). Recent proof-of-concept studies in rodent models demonstrated short term gas exchange following surgical implantation of decellularized rodent lungs, seeded with mixtures of fetal rodent lung homogenates and other cells (105,106). While these have stimulated intense investigation, they have also illustrated a number of practical issues prior to use of decellularized human lungs for clinical transplantation. These include the source of the lung, the decellularization process utilized, types, combinations, and order of cell to be inoculated, potential immunogenicity of the scaffolds, use of bioreactor culture systems and other environmental considerations, implantation, ethics, and the overall practicality of this approach (107,111-113).
The challenges in developing complex 3-dimensional functional lung tissues ex vivo will be in recapitulating the normal dynamic integrated 3-D network of cells in the appropriate environment and architecture. Other approaches such as human epithelialcells and human capillary endothelial cells coated onto porous polydimethylsiloxane chips (“lung-on-a-chip”) can mimic alveolar architecture and can be utilized to study pathophysiologic processes and also high throughput drug screening (114,115).
Summary
Exciting progress in each of these areas provides further understanding of lung biology and repair after lung injury and further a sound scientific basis for therapeutic use of cell therapies and bioengineering approaches in treatment of lung diseases. However, many challenges remain including better understanding of the identity of endogenous lung airway and other progenitor cells in the adult lung, development of functional airway and alveolar epithelial cells from ESCs and iPS cells, and better understanding of the physiologic and pathophysiologic roles of EPCs and fibrocytes in lung diseases. Cautious progress in clinical investigations of EPCs and MSCs needs to be tempered with clear understanding of the potential actions of these cells in different clinical lung disease conditions. The rapidly growing field of ex vivo lung bioengineering offers further promise and has already yielded therapeutic promise for tracheal diseases. However, clinical use of either artificial engineered or decellularized scaffolds for treatment of lung diseases is likely to be a number of years in the future.
Table 1.
Abbreviations
| AEC2 | type 2 alveolar epithelial cell |
| AFSC | amniotic fluid stem cell |
| Ang 1 | angiopoietin 1 |
| ALI/ARDS | acute lung injury/adult respiratory distress syndrome |
| BADJ | bronchoalveolar duct junction |
| BM-MNC | bone marrow-derived mononuclear cells |
| BPD | bronchopulmonary dysplasia |
| CGFP | calcitonin gene– related peptide |
| COPD | chronic obstructive pulmonary disease |
| CCSP | club (Clara) cell secretory protein |
| EGF | epidermal growth factor |
| eNOS | endothelial nitric oxide synthase |
| EPC | endothelial progenitor cell |
| ESC | embryonic stem cell |
| FGF2 | fibroblast growth factor 2 |
| HGF | hepatocyte growth factor |
| IGF1 | insulin-like growth factor |
| IPF | idiopathic pulmonary fibrosis |
| iPSC | induced pluripotent stem cell |
| (s)IL -1RA | (soluble) interleukin-1 receptor antagonist |
| IL-10 | interleukin 10 |
| Itg | integrin |
| KGF | keratinocyte growth factor |
| MSC | mesenchymal stem (stromal) cell |
| NEB | neuroepithelial body |
| Pulm HTN | pulmonary hypertension. |
| soluble TNFR1 | soluble tumor necrosis factor-α receptor antagonist |
| STC-1 | stanniocalcin 1 |
| SPC | surfactant protein C |
| TGF-β1 | transforming growth factor- β1 |
| TSG-6 | tumor necrosis factor-inducible gene 6 protein |
| VEGF | vascular endothelial growth factor. |
Acknowledgments
Research Support: NIH ARRA RC4HL106625 (DJW), NHLBI R21HL108689 (DJW)
References
- 1.Weiss DJ, Bertoncello I, Borok Z, Kim C, Panoskaltsis-Mortari A, Reynolds S, Rojas M, Stripp B, Warburton D, Prockop DJ. Stem cells and cell therapies in lung biology and lung diseases. Proc Am Thorac Soc. 2011;8(3):223–72. doi: 10.1513/pats.201012-071DW. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kassmer SH, Krause DS. Detection of bone marrow-derived lung epithelial cells. Exp Hematol. 2010;38(7):564–73. doi: 10.1016/j.exphem.2010.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sueblinvong V, Loi R, Eisenhauer PL, Bernstein IM, Suratt BT, Spees JL, Weiss DJ. Derivation of lung epithelium from human cord blood-derived mesenchymal stem cells. Am J Respir Crit Care Med. 2007 doi: 10.1164/rccm.200706-859OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Loi R, Beckett T, Goncz KK, Suratt BT, Weiss DJ. Limited restoration of cystic fibrosis lung epithelium in vivo with adult bone marrow-derived cells. Am J Respir Crit Care Med. 2006;173(2):171–9. doi: 10.1164/rccm.200502-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kassmer SH, Bruscia EM, Zhang PX, Krause DS. Nonhematopoietic cells are the primary source of bone marrow-derived lung epithelial cells. Stem Cells. 2012;30(3):491–9. doi: 10.1002/stem.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.De Paepe ME, Mao Q, Ghanta S, Hovanesian V, Padbury JF. Alveolar epithelial cell therapy with human cord blood-derived hematopoietic progenitor cells. Am J Path. 2011;178(3):1329–39. doi: 10.1016/j.ajpath.2010.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wong AP, Keating A, Lu WY, Duchesneau P, Wang X, Sacher A, Hu J, Waddell TK. Identification of a bone marrow-derived epithelial-like population capable of repopulating injured mouse airway epithelium. J Clin Inv. 2009;119(2):336–48. doi: 10.1172/JCI36882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kotton DN, Fabian AJ, Mulligan RC. Failure of bone marrow to reconstitute lung epithelium. Am J Respir Cell Mol Biol. 2005;33(4):328–34. doi: 10.1165/rcmb.2005-0175RC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Samadikuchaksaraei A, Cohen S, Isaac K, Rippon HJ, Polak JM, Bielby RC, Bishop AE. Derivation of distal airway epithelium from human embryonic stem cells. Tissue Eng. 2006;12(4):867–75. doi: 10.1089/ten.2006.12.867. [DOI] [PubMed] [Google Scholar]
- 10.Wang D, Haviland DL, Burns AR, Zsigmond E, Wetsel RA. A pure population of lung alveolar epithelial type II cells derived from human embryonic stem cells. Proc Nat Acad Sci U S A. 2007;104(11):4449–54. doi: 10.1073/pnas.0700052104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coraux C, Nawrocki-Raby B, Hinnrasky J, Kileztky C, Gaillard D, Dani C, Puchelle E. Embryonic stem cells generate airway epithelial tissue. Am J Respir Cell Mol Biol. 2005;32(2):87–92. doi: 10.1165/rcmb.2004-0079RC. [DOI] [PubMed] [Google Scholar]
- 12.Van Haute L, De Block G, Liebaers I, Sermon K, De Rycke M. Generation of lung epithelial-like tissue from human embryonic stem cells. Resp Res. 2009;10:105. doi: 10.1186/1465-9921-10-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Roszell B, Mondrinos MJ, Seaton A, Simons DM, Koutzaki SH, Fong GH, Lelkes PI, Finck CM. Efficient derivation of alveolar type II cells from embryonic stem cells for in vivo application. Tiss Eng Part A. 2009;15(11):3351–65. doi: 10.1089/ten.tea.2008.0664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Green MD, Chen A, Nostro MC, d'Souza SL, Schaniel C, Lemischka IR, Gouon- Evans V, Keller G, Snoeck HW. Generation of anterior foregut endoderm from human embryonic and induced pluripotent stem cells. Nature Biotech. 2011;29(3):267–72. doi: 10.1038/nbt.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Longmire TA, Ikonomou L, Hawkins F, Christodoulou C, Cao Y, Jean JC, Kwok LW, Mou H, Rajagopal J, Shen SS, Dowton AA, Serra M, Weiss DJ, Green MD, Snoeck HW, Ramirez MI, Kotton DN. Efficient derivation of purified lung and thyroid progenitors from embryonic stem cells. Cell Stem Cell. 2012;10(4):398–411. doi: 10.1016/j.stem.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mou H, Zhao R, Sherwood R, Ahfeldt T, Lapey A, Wain J, Sicilian L, Izvolsky K, Musunuru K, Cowan C, Rajagopal J. Generation of multipotent lung and airway progenitors from mouse ESCs and patient-specific cystic fibrosis iPSCs. Cell Stem Cell. 2012;10(4):385–97. doi: 10.1016/j.stem.2012.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wong AP, Bear CE, Chin S, Pasceri P, Thompson TO, Huan LJ, Ratjen F, Ellis J, Rossant J. Directed differentiation of human pluripotent stem cells into mature airway epithelia expressing functional CFTR protein. Nat Biotech. 2012;30(9):876–82. doi: 10.1038/nbt.2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pickering SJ, Minger SL, Patel M, Taylor H, Black C, Burns CJ, Ekonomou A, Braude PR. Generation of a human embryonic stem cell line encoding the cystic fibrosis mutation deltaF508, using preimplantation genetic diagnosis. Reprod Biomed Online. 2005;10(3):390–7. doi: 10.1016/s1472-6483(10)61801-9. [DOI] [PubMed] [Google Scholar]
- 19.Somers A, Jean JC, Sommer CA, Omari A, Ford CC, Mills JA, Ying L, Sommer AG, Jean JM, Smith BW, Lafyatis R, Demierre MF, Weiss DJ, French DL, Gadue P, Murphy GJ, Mostoslavsky G, Kotton DN. Generation of transgene-free lung disease-specific human induced pluripotent stem cells using a single excisable lentiviral stem cell cassette. Stem Cells. 2010;28(10):1728–40. doi: 10.1002/stem.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rock JR, Hogan BL. Epithelial progenitor cells in lung development, maintenance, repair, and disease. Ann Rev Cell & Dev Biol. 2011;27:493–512. doi: 10.1146/annurev-cellbio-100109-104040. [DOI] [PubMed] [Google Scholar]
- 21.McQualter JL, Bertoncello I. Concise review: Deconstructing the lung to reveal its regenerative potential. Stem Cells. 2012;30(5):811–6. doi: 10.1002/stem.1055. [DOI] [PubMed] [Google Scholar]
- 22.Rackley CR, Stripp BR. Building and maintaining the epithelium of the lung. J Clin Inv. 2012;122(8):2724–30. doi: 10.1172/JCI60519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driscoll B, Kikuchi A, Lau AN, Lee J, Reddy R, Jesudason E, Kim CF, Warburton D. Isolation and characterization of distal lung progenitor cells. Methods in Mol Biol. 2012;879:109–22. doi: 10.1007/978-1-61779-815-3_7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds SJ, Brechbul HM, Smith KS, Smith RW, Ghosh M. Lung Epithelial Healing: A Modified Seed and Soil Concept. Proc Am Thorac Soc. 2012;9:27–37. doi: 10.1513/pats.201201-008MS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rock J, Konigshoff M. Endogenous Lung Regeneration: Potential and Limitations. Am J Respir Crit Care Med. 2012;186:1213–1219. doi: 10.1164/rccm.201207-1151PP. [DOI] [PubMed] [Google Scholar]
- 26.Rawlins EL, Hogan BL. Epithelial stem cells of the lung: privileged few or opportunities for many? Development. 2006;133:2455–65. doi: 10.1242/dev.02407. [DOI] [PubMed] [Google Scholar]
- 27.Borthwick DW, Shahbazian M, Krantz QT, Dorin JR, Randell SH. Evidence for stem-cell niches in the tracheal epithelium. Am J Respir Cell Mol Biol. 2001;24:662–70. doi: 10.1165/ajrcmb.24.6.4217. [DOI] [PubMed] [Google Scholar]
- 28.Anversa P, Kajstura J, Leri A, Loscalzo J. Tissue-specific adult stem cells in the human lung. Nat Med. 2011;17(9):1038–9. doi: 10.1038/nm.2463. [DOI] [PubMed] [Google Scholar]
- 29.Dobbs LG, Johnson MD, Vanderbilt J, Allen L, Gonzalez R. The great big alveolar TI cell: evolving concepts and paradigms. Cell Physiol Biochem. 2010;25:55–62. doi: 10.1159/000272063. [DOI] [PubMed] [Google Scholar]
- 30.Kim CF, Jackson EL, Woolfenden AE, Lawrence S, Babar I, Vogel S, Crowley D, Bronson RT, Jacks T. Identification of bronchioalveolar stem cells in normal lung and lung cancer. Cell. 2005;121:823–35. doi: 10.1016/j.cell.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 31.Cole BB, Smith RW, Jenkins KM, Graham BB, Reynolds PR, Reynolds SD. Tracheal basal cells: a facultative progenitor cell pool. Am J Pathol. 2010;177(1):362–76. doi: 10.2353/ajpath.2010.090870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hong KU, Reynolds SD, Giangreco A, Hurley CM, Stripp BR. Clara cell secretory protein-expressing cells of the airway neuroepithelial body microenvironment include a label-retaining subset and are critical for epithelial renewal after progenitor cell depletion. Am J Respir Cell Mol Biol. 2001;24:671–81. doi: 10.1165/ajrcmb.24.6.4498. [DOI] [PubMed] [Google Scholar]
- 33.Rock JR, Randell SH, Hogan BL. Airway basal stem cells: a perspective on their roles in epithelial homeostasis and remodeling. Dis Models & Mech. 2010;3(9-10):545–56. doi: 10.1242/dmm.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ghosh M, Brechbuhl HM, Smith RW, Li B, Hicks DA, Titchner T, et al. Context-dependent differentiation of multipotential keratin 14-expressing tracheal basal cells. Am J Respir Cell Mol Biol. 2011;45:403–10. doi: 10.1165/rcmb.2010-0283OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hegab AE, Ha VL, Darmawan DO, Gilbert JL, Ooi AT, Attiga YS, Bisht B, Nickerson DW, Gomperts BN. Isolation and in vitro characterization of basal and submucosal gland duct stem/progenitor cells from human proximal airways. Stem Cells Trans Med. 2012;1(10):719–24. doi: 10.5966/sctm.2012-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perl AK, Wert SE, Loudy DE, Shan Z, Blair PA, Whitsett JA. Conditional recombination reveals distinct subsets of epithelial cells in trachea, bronchi, and alveoli. Am J Respir Cell Mol Biol. 2005;33:455–62. doi: 10.1165/rcmb.2005-0180OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Giangreco A, Arwert EN, Rosewell IR, Snyder J, Watt FM, Stripp BR. Stem cells are dispensable for lung homeostasis but restore airways after injury. Proc Natl Acad Sci U S A. 2009;106:9286–91. doi: 10.1073/pnas.0900668106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Giangreco A, Reynolds SD, Stripp BR. Terminal bronchioles harbor a unique airway stem cell population that localizes to the bronchoalveolar duct junction. Am J Pathol. 2002;161:173–82. doi: 10.1016/S0002-9440(10)64169-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Teisanu RM, Chen H, Matsumoto K, McQualter JL, Pott E, Foster WM, et al. Functional analysis of two distinct bronchiolar progenitors during lung injury and repair. Am J Respir Cell Mol Biol. 2011;44(6):794–803. doi: 10.1165/rcmb.2010-0098OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chapman HA, Li X, Alexander JP, Brumwell A, Lorizio W, Tan K, et al. Integrin β6α4 identifies an adult distal lung epithelial population with regenerative potential in mice. J Clin Invest. 2011;121:2855–62. doi: 10.1172/JCI57673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumar PA, Hu Y, Yamamoto Y, Hoe NB, Wei TS, Mu D, Sun Y, Joo LS, Dagher R, Zielonka EM, Wang de Y, Lim B, Chow VT, Crum CP, Xian W, McKeon F. Distal airway stem cells yield alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;47(3):525–38. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen H, Matsumoto K, Brockway BL, Rackley CR, Liang J, Lee JH, Jiang D, Noble PW, Randell SH, Kim CF, Stripp BR. Airway epithelial progenitors are region specific and show differential responses to bleomycin-induced lung injury. Stem Cells. 2012;30(9):1948–60. doi: 10.1002/stem.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Volckaert T, Dill E, Campbell A, Tiozzo C, Majka S, Bellusci S, De Langhe SP. Parabronchial smooth muscle constitutes an airway epithelial stem cell niche in the mouse lung after injury. J Clin Inv. 2011;121(11):4409–19. doi: 10.1172/JCI58097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buckley S, Shi W, Carraro G, Sedrakyan S, Da Sacco S, Driscoll BA, Perin L, De Filippo RE, Warburton D. The milieu of damaged alveolar epithelial type 2 cells stimulates alveolar wound repair by endogenous and exogenous progenitors. Am J Physiol Lung Cell Mol Physiol. 2011;45(6):1212–21. doi: 10.1165/rcmb.2010-0325OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Giangreco A, Groot KR, Janes SM. Lung cancer and lung stem cells: strange bedfellows? Am J Respir Crit Care Med. 2007;175(6):547–53. doi: 10.1164/rccm.200607-984PP. [DOI] [PubMed] [Google Scholar]
- 46.Alison MR, Lebrenne AC, Islam S. Stem cells and lung cancer: future therapeutic targets? Expert Opinion on Biological Therapy. 2009;9(9):1127–41. doi: 10.1517/14712590903103803. [DOI] [PubMed] [Google Scholar]
- 47.Direkze NC, Hodivala-Dilke K, Jeffery R, Hunt T, Poulsom R, Oukrif D, Alison MR, Wright NA. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64(23):8492–5. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 48.Hilbe W, Dirnhofer S, Oberwasserlechner F, Schmid T, Gunsilius E, Hilbe G, Woll E, Kahler CM. CD133 positive endothelial progenitor cells contribute to the tumour vasculature in non-small cell lung cancer. J Clin Pathol. 2004;57(9):965–9. doi: 10.1136/jcp.2004.016444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Arap W, Pasqualini R. Engineered embryonic endothelial progenitor cells as therapeutic Trojan horses. Cancer Cell. 2004;5(5):406–8. doi: 10.1016/s1535-6108(04)00121-7. [DOI] [PubMed] [Google Scholar]
- 50.Loebinger MR, Janes SM. Stem cells as vectors for antitumour therapy. Thorax. 2010;65(4):362–9. doi: 10.1136/thx.2009.128025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wei J, Blum S, Unger M, Jarmy G, Lamparter M, Geishauser A, Vlastos GA, Chan G, Fischer KD, Rattat D, Debatin KM, Hatzopoulos AK, Beltinger C. Embryonic endothelial progenitor cells armed with a suicide gene target hypoxic lung metastases after intravenous delivery. Cancer Cell. 2004;5(5):477–88. doi: 10.1016/s1535-6108(04)00116-3. [DOI] [PubMed] [Google Scholar]
- 52.Matsuzuka T, Rachakatla RS, Doi C, Maurya DK, Ohta N, Kawabata A, Pyle MM, Pickel L, Reischman J, Marini F, Troyer D, Tamura M. Human umbilical cord matrix-derived stem cells expressing interferon-beta gene significantly attenuate bronchioloalveolar carcinoma xenografts in SCID mice. Lung Cancer. 2010;70(1):28–36. doi: 10.1016/j.lungcan.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gomperts BN, Strieter RM. Fibrocytes in lung disease. J Leukoc Biol. 2007;82(3):449–56. doi: 10.1189/jlb.0906587. [DOI] [PubMed] [Google Scholar]
- 54.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol. 2006;35(2):175–81. doi: 10.1165/rcmb.2005-0239OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Fujiwara A, Kobayashi H, Masuya M, Maruyama M, Nakamura S, Ibata H, Fujimoto H, Ohnishi M, Urawa M, Naito M, Takagi T, Kobayashi T, Gabazza EC, Takei Y, Taguchi O. Correlation between circulating fibrocytes, and activity and progression of interstitial lung diseases. Respirology. 2012;17(4):693–8. doi: 10.1111/j.1440-1843.2012.02167.x. [DOI] [PubMed] [Google Scholar]
- 56.Quesnel C, Piednoir P, Gelly J, Nardelli L, Garnier M, Lecon V, Lasocki S, Bouadma L, Philip I, Elbim C, Mentre F, Soler P, Crestani B, Dehoux M. Alveolar fibrocyte percentage is an independent predictor of poor outcome in patients with acute lung injury. Crit Care Med. 2012;40(1):21–8. doi: 10.1097/CCM.0b013e31822d718b. [DOI] [PubMed] [Google Scholar]
- 57.Yeager ME, Nguyen CM, Belchenko DD, Colvin KL, Takatsuki S, Ivy DD, Stenmark KR. Circulating fibrocytes are increased in children and young adults with pulmonary hypertension. Eur Resp J. 2012;39(1):104–11. doi: 10.1183/09031936.00072311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.LaPar DJ, Burdick MD, Emaminia A, Harris DA, Strieter BA, Liu L, Robbins M, Kron IL, Strieter RM, Lau CL. Circulating fibrocytes correlate with bronchiolitis obliterans syndrome development after lung transplantation: a novel clinical biomarker. Annals of Thoracic Surg. 2011;92(2):470–7. doi: 10.1016/j.athoracsur.2011.04.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ishii G, Ito TK, Aoyagi K, Fujimoto H, Chiba H, Hasebe T, Fujii S, Nagai K, Sasaki H, Ochiai A. Presence of human circulating progenitor cells for cancer stromal fibroblasts in the blood of lung cancer patients. Stem Cells. 2007;25(6):1469–77. doi: 10.1634/stemcells.2006-0449. [DOI] [PubMed] [Google Scholar]
- 60.Asosingh K, Swaidani S, Aronica M, Erzurum SC. Th1-and Th2-dependent endothelial progenitor cell recruitment and angiogenic switch in asthma. J Immunol. 2007;178(10):6482–94. doi: 10.4049/jimmunol.178.10.6482. [DOI] [PubMed] [Google Scholar]
- 61.Fadini GP, Schiavon M, Avogaro A, Agostini C. The emerging role of endothelial progenitor cells in pulmonary hypertension and diffuse lung diseases. Sarc Vascul & Diffuse Lung Dis. 2007;24(2):85–93. [PubMed] [Google Scholar]
- 62.Burnham EL, Taylor WR, Quyyumi AA, Rojas M, Brigham KL, Moss M. Increased circulating endothelial progenitor cells are associated with survival in acute lung injury. Am J Respir Crit Care Med. 2005;172(7):854–60. doi: 10.1164/rccm.200410-1325OC. [DOI] [PubMed] [Google Scholar]
- 63.Palange P, Testa U, Huertas A, Calabro L, Antonucci R, Petrucci E, Pelosi E, Pasquini L, Satta A, Morici G, Vignola MA, Bonsignore MR. Circulating haemopoietic and endothelial progenitor cells are decreased in COPD. Eur Respir J. 2006;27(3):529–41. doi: 10.1183/09031936.06.00120604. [DOI] [PubMed] [Google Scholar]
- 64.Dome B, Timar J, Dobos J, Meszaros L, Raso E, Paku S, Kenessey I, Ostoros G, Magyar M, Ladanyi A, Bogos K, Tovari J. Identification and clinical significance of circulating endothelial progenitor cells in human non-small cell lung cancer. Cancer Res. 2006;66(14):7341–7. doi: 10.1158/0008-5472.CAN-05-4654. [DOI] [PubMed] [Google Scholar]
- 65.Borghese, et al. Circulating endothelial progenitor cells in pre-term infants with bronchopulmonary dysplasia. Am J Resp Crit Care Med. 2009;180:540–546. doi: 10.1164/rccm.200812-1949OC. [DOI] [PubMed] [Google Scholar]
- 66.Cribbs SK, Sutcliffe DJ, Taylor WR, Rojas M, Easley KA, Tang L, et al. Circulating endothelial progenitor cells inversely associate with organ dysfunction in sepsis. Intensive Care Medicine. 2012;38(3):429–36. doi: 10.1007/s00134-012-2480-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huertas A, Palange P. Circulating endothelial progenitor cells and chronic pulmonary diseases. European Respiratory Journal. 2011;37(2):426–31. doi: 10.1183/09031936.00034810. [DOI] [PubMed] [Google Scholar]
- 68.Zhao YD, Courtman DW, Deng Y, Kugathasan L, Zhang Q, Stewart DJ. Rescue of monocrotaline-induced pulmonary arterial hypertension using bone marrow-derived endothelial-like progenitor cells: efficacy of combined cell and eNOS gene therapy in established disease. Circ Res. 2005;96(4):442–50. doi: 10.1161/01.RES.0000157672.70560.7b. [DOI] [PubMed] [Google Scholar]
- 69.Lam C-F, Roan J-N, Lee C-H, Chang P-J, Huang C-C, Liu Y-C, et al. Transplantation of Endothelial Progenitor Cells Improves Pulmonary Endothelial Function and Gas Exchange in Rabbits with Endotoxin-Induced Acute Lung Injury. Anesthesia & Analgesia. 2011;112(3):620–27. doi: 10.1213/ANE.0b013e3182075da4. [DOI] [PubMed] [Google Scholar]
- 70.Balasubramaniam V, Ryan SL, Seedorf GJ, Roth EV, Heumann TR, Yoder MC, Ingram DA, Hogan CJ, Markham NE, Abman SH. Bone marrow-derived angiogenic cells restore lung alveolar and vascular structure after neonatal hyperoxia in infant mice. Am J Physiol -Lung Cell & Mol Physiol. 2010;298(3):L315–23. doi: 10.1152/ajplung.00089.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stewart DJ, Mei SH. Cell-based therapies for lung vascular diseases: lessons for the future. Proc Am Thorac Soc. 2011;8(6):535–40. doi: 10.1513/pats.201105-035MW. [DOI] [PubMed] [Google Scholar]
- 72.Lavoie JR, Stewart DJ. Genetically modified endothelial progenitor cells in the therapy of cardiovascular disease and pulmonary hypertension. Curr Vasc Pharmacol. 2012;10(3):289–99. doi: 10.2174/157016112799959413. [DOI] [PubMed] [Google Scholar]
- 73.Wang XX, Zhang FR, Shang YP, Zhu JH, Xie XD, Tao QM, Zhu JH, Chen JZ. Transplantation of autologous endothelial progenitor cells may be beneficial in patients with idiopathic pulmonary arterial hypertension: a p54. Pilot randomized controlled trial. J Am Coll Cardiol. 2007;49(14):1566–71. doi: 10.1016/j.jacc.2006.12.037. [DOI] [PubMed] [Google Scholar]
- 74.Zhu JH, Wang XX, Zhang FR, Shang YP, Tao QM, Zhu JH, Chen JZ. Safety and efficacy of autologous endothelial progenitor cells transplantation in children with idiopathic pulmonary arterial hypertension: open-label pilot study. Ped Transp. 2008;12(6):650–5. doi: 10.1111/j.1399-3046.2007.00863.x. [DOI] [PubMed] [Google Scholar]
- 75.Keating A. Mesenchymal stromal cells: new directions. Cell Stem Cell. 2012;10(6):709–16. doi: 10.1016/j.stem.2012.05.015. [DOI] [PubMed] [Google Scholar]
- 76.Prockop DJ, Oh JY. Medical therapies with adult stem/progenitor cells(MSCs): a backward journey from dramatic results in vivo to the cellular and molecular explanations. J Cell Biochem. 2012;113(5):1460–9. doi: 10.1002/jcb.24046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Islam MN, Das SR, Emin MT, Wei M, Sun L, Westphalen K, Rowlands DJ, Quadri SK, Bhattacharya S, Bhattacharya J. Mitochondrial transfer from bone-marrow-derived stromal cells to pulmonary alveoli protects against acute lung injury. Nature Medicine. 2012;8(5):759–65. doi: 10.1038/nm.2736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tropea KA, Leder E, Aslam M, Lau AN, Raiser DM, Lee JH, Balasubramaniam V, Fredenburgh LE, Mitsialis A, Kourembanas S, Kim CF. Bronchioalveolar stem cells increase after mesenchymal stromal cell treatment in a mouse model of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2012;302:L829–L837. doi: 10.1152/ajplung.00347.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ. Canadian Critical Care Trials Group. Safety of cell therapy with mesenchymal stromal cells(SafeCell): a systematic review and meta-analysis of clinical trials. PLoS ONE [Electronic Resource] 2012;7(10):e47559. doi: 10.1371/journal.pone.0047559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Von Bahr L, Batsis I, Moll G, Szako A, Sundberg B, Uzunel M, Ringden O, LeBlanc K. Analysis of tissues following mesenchymal stromal cell therapy in humans indicates limited long-term engraftment and no ectopic tissue formation. Stem Cells. 2012;30:1575–78. doi: 10.1002/stem.1118. [DOI] [PubMed] [Google Scholar]
- 81.Matthay MA, Taylor Thompson B, Read EJ, McKenna DH, Jr, Liu KD, Calfee CS, Lee JW. Therapeutic potential of mesenchymal stem cells for severe acute lung injury. Chest. 2010;138:965–972. doi: 10.1378/chest.10-0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O'Reilly M, Thebaud B. Cell-based strategies to reconstitute lung function in infants with severe bronchopulmonary dysplasia. Clinics in Perinatology. 2012;39(3):703–25. doi: 10.1016/j.clp.2012.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee JW, Krasnodembskaya A, McKenna DH, Song Y, Abbott J, Matthay MA. Therapeutic effects of human mesenchymal stem cells in ex vivo human lungs injured with live bacteria. Am J Respir Crit Care Med. doi: 10.1164/rccm.201206-0990OC. rccm.201206-0990OC First published online January 4, 2013 as doi:10.1164/rccm.201206-0990OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Epperly MW, Guo H, Gretton JE, Greenberger JS. Bone marrow origin of myofibroblasts in irradiation pulmonary fibrosis. Am J Respir Cell Mol Biol. 2003;29(2):213–24. doi: 10.1165/rcmb.2002-0069OC. [DOI] [PubMed] [Google Scholar]
- 85.Yan X, Liu Y, Han Q, Jia M, Liao L, Qi M, Zhao RC. Injured microenvironment directly guides the differentiation of engrafted Flk-1(+) mesenchymal stem cell in lung. Exp Hematol. 2007;35(9):1466–75. doi: 10.1016/j.exphem.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 86.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5(1):54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ionescu L, Byrne RN, van Haaftern T, Vadivel A, Alphonse RS, Rey-Parra GJ, Weissmann G, Hall A, Eaton F, Thebaud B. Stem cell conditioned medium improves acute lung injury in mice: in vivo evidence for stem cell paracrine action. Am J Physiol Lung Cell Mol Physiol. 2012;303:L967–L977. doi: 10.1152/ajplung.00144.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goodwin M, Sueblinvong V, Eisenhauer P, Ziats NP, Leclair L, Poynter ME, Steele C, Rincon M, Weiss DJ. Bone marrow derived mesenchymal stromal cells inhibit th2-Mediated allergic airways inflammation in mice. Stem Cells. 2011;29:1137–48. doi: 10.1002/stem.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kim ES, Chang YS, Choi SJ, Kim JK, Yoo HS, Ahn SY, Sung DK, Kim SY, Park YR, Park WS. Intratracheal transplantation of human umbilical cord blood-derived mesenchymal stem cells attenuates Escherichia coli-induced acute lung injury in mice. Resp Res. 2011;12:108. doi: 10.1186/1465-9921-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Araujo IM, Abreu SC, Maron-Gutierrez T, Cruz F, Fujisaki L, Carreira H, Jr, Ornellas F, Ornellas D, Vieira-de-Abreu A, Castro-Faria-Neto HC, Muxfeldt Ab'Saber A, Teodoro WR, Diaz BL, Peres Dacosta C, Capelozzi VL, Pelosi P, Morales MM, Rocco PR. Bone marrow-derived mononuclear cell therapy in experimental pulmonary and extrapulmonary acute lung injury. Crit Care Med. 2010;38(8):1733–41. doi: 10.1097/CCM.0b013e3181e796d2. [DOI] [PubMed] [Google Scholar]
- 91.Hodges RJ, Lim R, Jenkin G, Wallace EM. Amnion epithelial cells as a candidate therapy for acute and chronic lung injury. Stem Cells Int. 2012;709:63. doi: 10.1155/2012/709763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Matute-Bello G, Downey G, Moore BB, Groshong SD, Matthay MA, Slutsky AS, Kuebler WM. Acute Lung Injury in Animals Study Group. An official American Thoracic Society workshop report: features and measurements of experimental acute lung injury in animals. Am J Resp Cell & Mol Biol. 2011;44(5):725–38. doi: 10.1165/rcmb.2009-0210ST. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Weiss DJ, Casaburi R, Flannery R, LeRoux-Williams M, Tashkin DP. A placebo-controlled randomized trial of mesenchymal stem cells in chronic obstructive pulmonary disease. Chest. 2013 doi: 10.1378/chest.12-2094. [in press] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Toonkel RL, Hare JM, Matthay MA, Glassberg MK. Mesenchymal Stem Cells and Idiopathic Pulmonary Fibrosis: Potential for Clinical Testing. Am J Respir Crit Care Med. doi: 10.1164/rccm.201207-1204PP. First published online January 9, 2013 as doi:10.1164/rccm.201207-1204PP. [DOI] [PubMed] [Google Scholar]
- 95.Weiss DJ, Ortiz LA. Cell Therapy Trials for Lung Diseases: Progress and Cautions. Am J Resp Crit Care Med. 2013 doi: 10.1164/rccm.201302-0351ED. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Master Z, Resnik DB. Stem cell tourism and scientific responsibility. EMBO Reports. 2011;12:992–995. doi: 10.1038/embor.2011.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Qiu J. Trading on hope. Nat Biotech. 2010;27:790–92. doi: 10.1038/nbt0909-790. [DOI] [PubMed] [Google Scholar]
- 97.Zarzeczny A, Rachul C, Nisbet M. Caulfield. Stem cell clinics in the news. Nat Biotech. 2012;28:1243–46. doi: 10.1038/nbt1210-1243b. [DOI] [PubMed] [Google Scholar]
- 98.Badylak SF, Weiss DJ, Caplan A, Macchiarini P. Engineered whole organs and complex tissues. Lancet. 2012;379(9819):943–52. doi: 10.1016/S0140-6736(12)60073-7. [DOI] [PubMed] [Google Scholar]
- 99.Fishman JM, De Coppi P, Elliott MJ, Atala A, Birchall MA, Macchiarini P. Airway tissue engineering. Exp Op Bio Ther. 2011;11(12):1623–35. doi: 10.1517/14712598.2011.623696. [DOI] [PubMed] [Google Scholar]
- 100.Mondrinos MJ, Koutzaki S, Jiwanmall E, Li M, Dechadarevian JP, Lelkes PI, Finck CM. Engineering three-dimensional pulmonary tissue constructs. Tissue Eng. 2006;12(4):717–28. doi: 10.1089/ten.2006.12.717. [DOI] [PubMed] [Google Scholar]
- 101.Nichols JE, Cortiella JC. Engineering of a Complex Organ Progress Toward Development of a Tissue-engineered Lung. Proc Am Thorac Soc. 2008;5:723–30. doi: 10.1513/pats.200802-022AW. [DOI] [PubMed] [Google Scholar]
- 102.Panoskaltsis-Mortari A, Weiss DJ. Breathing new life into lung transplantation therapy. Mol Ther. 2010;18:1581–83. doi: 10.1038/mt.2010.177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Price AP, et al. Development of a decellularized lung bioreactor system for bioengineering the lung: the matrix reloaded. Tissue Eng Part A. 2010;16:2581–91. doi: 10.1089/ten.tea.2009.0659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Cortiella J, et al. Influence of acellular natural lung matrix on murine embryonic stem cell differentiation and tissue formation. Tissue Eng Part A. 2010 doi: 10.1089/ten.tea.2009.0730. [DOI] [PubMed] [Google Scholar]
- 105.Petersen TH, et al. Tissue-engineered lungs for in vivo implantation. Science. 2010;329:538–41. doi: 10.1126/science.1189345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ott HC, Clippinger B, Conrad C, Schuetz C, Pomerantseva I, Ikonomou L, Kotton D, Vacanti JP. Regeneration and orthotopic transplantation of a bioartificial lung. Nat Med. 2010;16:927–33. doi: 10.1038/nm.2193. [DOI] [PubMed] [Google Scholar]
- 107.Bonenfant N, Sokocevic D, Wagner DE, Borg ZD, Lathrop M, Lam YW, Deng B, DeSarno M, Ashikaga T, Loi R, Weiss DJ. The effects of storage and sterilization on de-cellularized and re-cellularized whole lung. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2013.01.031. [in press] 10 1016/j.biomaterials. 2013.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bonvillain RW, Danchuk S, Sullivan DE, Betancourt AM, Semon JA, Eagle ME, et al. A nonhuman primate model of lung regeneration: detergent-mediated cecellularization and initial in vitro recellularization with mesenchymal stem cells. Tissue Eng Part A. 2012;18:2437–52. doi: 10.1089/ten.tea.2011.0594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Daly AB, Wallis JM, Borg ZD, Bonvillain RW, Deng B, Ballif BA, Jaworski DM, Allen GB, Weiss DJ. Initial binding and re-cellularization of de-cellularized mouse lung scaffolds with bone marrow-derived mesenchymal stromal cells. Tiss Eng Part A. 2012;18(1-2):1–16. doi: 10.1089/ten.tea.2011.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Jensen T, Roszell B, Zang F, Girard E, Matson A, Thrall R, Jaworski DM, Hatton C, Weiss DJ, Finck CM. A Rapid Lung De-Cellularization Protocol Supports Embryonic Stem Cell Differentiation In Vitro and Following Implantation. Tiss Eng Part C: Methods. 2012;18(8):632–46. doi: 10.1089/ten.tec.2011.0584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sokocevic D, Bonenfant N, Wagner DE, Borg ZD, Lathrop M, Lam YW, Deng B, DeSarno M, Ashikaga T, Loi R, Hoffman AM, Weiss DJ. The effect of age and emphysematous and fibrotic injury on the re-cellularization of de-cellularized lungs. Biomaterials. 2013 doi: 10.1016/j.biomaterials.2013.01.028. [in press] 10.1016/j.biomaterials.2013.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Wallis JM, Borg ZD, Daly AB, Deng B, Ballif BA, Allen GB, Jaworski DM, Weiss DJ. Comparative assessment of detergent-based protocols for mouse lung de cellularization and re-cellularization. Tiss Eng Part C: Methods. 2012;18(6):420–32. doi: 10.1089/ten.tec.2011.0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Nichols JE, Niles JA, Cortiella J. Production and utilization of acellular lung scaffolds in tissue engineering. J Cell Biochem. 2012;113(7):2185–92. doi: 10.1002/jcb.24112. [DOI] [PubMed] [Google Scholar]
- 114.Huh D, et al. Reconstituting organ-level lung functions on a chip. Science. 2010;328(5986):1662–8. doi: 10.1126/science.1188302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Huh D, et al. A human disease model of drug toxicity-induced pulmonary edema in a lung-on-a-chip microdevice. Sci Transl Med. 2012;4(159):147. doi: 10.1126/scitranslmed.3004249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kotton DN. Next Generation Regeneration: the Hope and Hype of Lung Stem Cell Research. Am J Respir Crit Care Med. 2012;12:1255–1260. doi: 10.1164/rccm.201202-0228PP. [DOI] [PubMed] [Google Scholar]
- 117.Weiss DJ, Rojas M. MSCs in Chronic Lung Diseases: COPD and Lung Fibrosis. In: Berlin GmbH, Gross G, Häupl G,T., editors. Stem Cell-Dependent Therapies. DeGruyter Publisher; 2013. [Google Scholar]



