Abstract
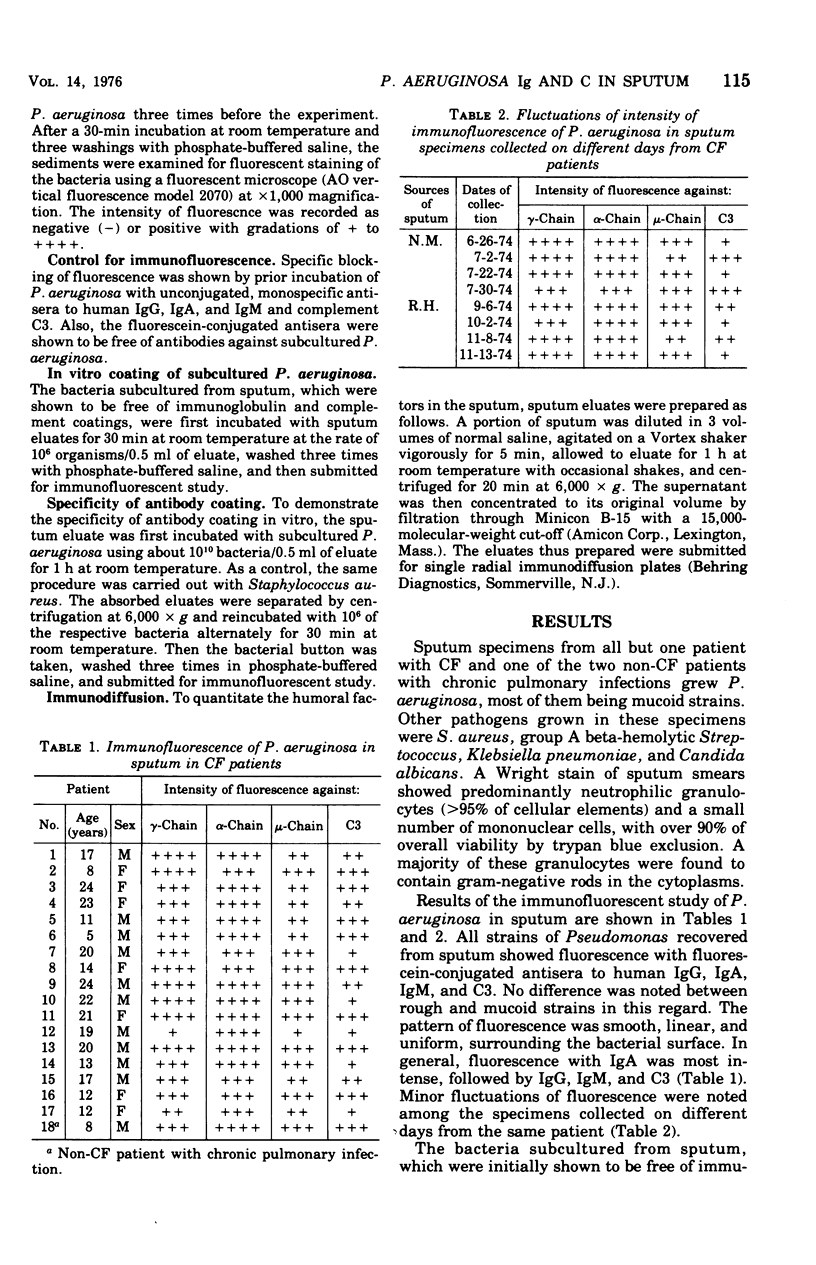
The interactions of Pseudomonas aeruginosa with humoral factors in the sputum of patients with cystic fibrosis were investigated by using an indirect immunofluorescent technique. Fluorescein-conjugated, monovalent antiserum specific to heavy chains of human immunoglobulin A (IgA), IgG, or IgM and to complement C3 were used. All strains of P. aeruginosa recovered from the sputum specimens of patients with cystic fibrosis were found to be coated with antibodies of IgA, IgG, and IgM classes and with C3. The specificity of the antibody coating was determined. The fluorescence was most intense with IgA and was followed in intensity by IgG, IgM, and C3. No difference was noted between rough and mucoid strains of P. aeruginosa. When the subcultured P. aeruginosa was incubated with the sputum eluates, a similar pattern of fluorescence was demonstrated, indicating that these humoral factors are present in the sputum and that the coating process can take place in the lower respiratory tract of the patients. By single radial immunodiffusion, significant quantities of the humoral factors in the sputum eluates were detected. These findings suggest that P. aeruginosa is opsonized in sputum of patients with cystic fibrosis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Biggar W. D., Holmes B., Good R. A. Opsonic defect in patients with cystic fibrosis of the pancreas. Proc Natl Acad Sci U S A. 1971 Aug;68(8):1716–1719. doi: 10.1073/pnas.68.8.1716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxerbaum B., Kagumba A., Matthews L. W. Selective inhibition of phagocytic activity of rabbit alveolar macrophages by cystic fibrosis serum. Am Rev Respir Dis. 1973 Oct;108(4):777–783. doi: 10.1164/arrd.1973.108.4.777. [DOI] [PubMed] [Google Scholar]
- GREEN G. M., KASS E. H. FACTORS INFLUENCING THE CLEARANCE OF BACTERIA BY THE LUNG. J Clin Invest. 1964 Apr;43:769–776. doi: 10.1172/JCI104961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green G. M., Carolin D. The depressant effect of cigarette smoke on the in vitro antibacterial activity of alveolar macrophages. N Engl J Med. 1967 Feb 23;276(8):421–427. doi: 10.1056/NEJM196702232760801. [DOI] [PubMed] [Google Scholar]
- Gugler E., Pallavicini J. C., Swedlow H., Zipkin I., Agnese P. A. Immunological studies of submaxillary saliva from patients with cystic fibrosis and from normal children. J Pediatr. 1968 Oct;73(4):548–559. doi: 10.1016/s0022-3476(68)80270-7. [DOI] [PubMed] [Google Scholar]
- Götze O., Müller-Eberhard H. J. The C3-activator system: an alternate pathway of complement activation. J Exp Med. 1971 Sep 1;134(3 Pt 2):90s–108s. [PubMed] [Google Scholar]
- HUANG N. N., VAN LOON E. L., SHENG K. T. The flora of the respiratory tract of patients with cystic fibrosis of the pancreas. J Pediatr. 1961 Oct;59:512–521. doi: 10.1016/s0022-3476(61)80234-5. [DOI] [PubMed] [Google Scholar]
- IACOCCA V. F., SIBINGA M., BARBERO G. J. RESPIRATORY TRACT BACTERIOLOGY IN CYSTIC FIBROSIS. Am J Dis Child. 1963 Sep;106:315–324. doi: 10.1001/archpedi.1963.02080050317012. [DOI] [PubMed] [Google Scholar]
- Ishizaka T., Ishizaka K., Borsos T., Rapp H. C'-1 fixation by human isoagglutinins: fixation of C'-1 by gamma-G and gamma-M but not by gamma-A antibody. J Immunol. 1966 Nov;97(5):716–726. [PubMed] [Google Scholar]
- Johnston R. B., Jr, Klemperer M. R., Alper C. A., Rosen F. S. The enhancement of bacterial phagocytosis by serum. The role of complement components and two cofactors. J Exp Med. 1969 Jun 1;129(6):1275–1290. doi: 10.1084/jem.129.6.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcer V., Mastella G., Mengoli V. Immunoglobuline nel siero, nell'escreato e nella saliva di pazienti affetti da fibrosi cistica (mucoviscidosi) con mportanti complicanze respiratorie. Riv Clin Pediatr. 1970 Sep-Oct;83(3):135–143. [PubMed] [Google Scholar]
- OREN R., FARNHAM A. E., SAITO K., MILOFSKY E., KARNOVSKY M. L. Metabolic patterns in three types of phagocytizing cells. J Cell Biol. 1963 Jun;17:487–501. doi: 10.1083/jcb.17.3.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith M. R., Shin H. S., Wood W. B., Jr Natural immunity to bacterial infections: the relation of complement to heat-labile opsonins. Proc Natl Acad Sci U S A. 1969 Aug;63(4):1151–1156. doi: 10.1073/pnas.63.4.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- South M. A., Warwick W. J., Wolheim F. A., Good R. A. The IgA system. 3. IgA levels in the serum and saliva of pediatric patients--evidence for a local immunological system. J Pediatr. 1967 Nov;71(5):645–653. doi: 10.1016/s0022-3476(67)80199-9. [DOI] [PubMed] [Google Scholar]
- Thomas V., Shelokov A., Forland M. Antibody-coated bacteria in the urine and the site of urinary-tract infection. N Engl J Med. 1974 Mar 14;290(11):588–590. doi: 10.1056/NEJM197403142901102. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Dossett J. H., Quie P. G. Comparative studies of immunoglobulin opsonins in osteomyelitis and other established infections. Immunology. 1969 Aug;17(2):249–265. [PMC free article] [PubMed] [Google Scholar]
- Winkelstein J. A. Opsonins: their function, identity, and clinical significance. J Pediatr. 1973 May;82(5):747–753. doi: 10.1016/s0022-3476(73)80062-9. [DOI] [PubMed] [Google Scholar]
- Young L. S., Armstrong D. Human immunity to Pseudomonas aeruginosa. I. In-vitro interaction of bacteria, polymorphonuclear leukocytes, and serum factors. J Infect Dis. 1972 Sep;126(3):257–276. doi: 10.1093/infdis/126.3.257. [DOI] [PubMed] [Google Scholar]



