Abstract
Characterization of the immune correlates of protection against HIV infection is crucial for the development of preventive strategies. This study examined HIV-1 envelope (Env) glycoproteins, specifically immunoglobulin G (IgG), in systemic and mucosal compartments of female Beninese commercial sex workers (CSWs). Samples of 23 HIV-1-positive and 20 highly exposed HIV-1-seronegative (HESN) CSWs were studied. HIV-1 Env-specific IgG detection in sera and cervicovaginal lavages (CVLs) from the study population was done by cell-based ELISA. The HIV neutralizing activity was evaluated with a neutralization assay. The HIV-1-specific antibody-dependent cellular cytotoxicity (ADCC) response of the cohort was measured with a FACS-based assay evaluating the ADCC-mediated elimination of gp120-coated target cells. No anti-HIV-1 Env-specific IgG neutralizing or ADCC activities were detected in samples from HESN CSWs. Samples from HIV-1-infected CSWs presented ADCC activity in both sera and CVLs. Anti-Env IgG from sera and CVLs from HIV-1-infected CSWs preferentially recognized Env in its CD4-bound conformation. HIV-1-infected CSWs have ADCC-mediating IgG that preferentially recognizes Env in its CD4-bound conformation at the mucosal site.
Globally, more than 90% of HIV-1 transmission occurs across the mucosal surfaces via heterosexual or homosexual contacts.1,2 A better understanding of the mechanisms of transmission and HIV-specific immune responses at the initial site of infection is therefore pivotal to the design of preventive strategies. The antibody-dependent cell-mediated cytotoxicity (ADCC) response is an antibody-driven immune mechanism that recruits effector cells and activates them against infected cells expressing surface antigens, resulting in their elimination, and may play a protective role at mucosal surfaces. Analysis of correlates of protection from the RV144 vaccine trial suggested that increased ADCC activity was linked to decreased HIV-1 acquisition.3 Unfortunately, mucosal samples were not collected during the RV144 trial to assess mucosal envelope (Env)-specific Ig levels and antibody functions.
In other studies, ADCC-mediating antibodies were observed in cervicovaginal lavages (CVLs) of HIV-infected women5 and ADCC activity was negatively correlated with genital viral loads.6 The study of highly exposed HIV-seronegative (HESN) individuals, such as HIV-1-uninfected commercial sex workers (CSWs), who may represent a model of natural immunity to HIV-1, may yield important clues to the development of preventive approaches. High levels of antiinflammatory and neutralizing proteins were found in the genital mucosa of HESN CSWs.7 To date, a number of reports in the literature8–11 have failed to detect anti-HIV-1 Env-specific immunoglobulin G (IgG) in HESN. However, in these studies the detection of anti-HIV-1 Env-specific IgG was performed by looking at gp120 Env in its monomeric context10–12 and/or looking at the two Env subunits gp120 and gp41 separately.11 To our knowledge, none looked at Env trimer in its entirety.
The importance of trimeric Env is pivotal because it is the only virus-specific antigen present at the surface of viral particles and as such, it is highly exposed and thus elicits neutralizing and non-neutralizing antibodies. Env assembles into spike-like projections consisting of three gp120-gp41 heterodimers that form the Env trimer, upon which the virus depends for attachment and fusion with target cells.13 In the current study, we screened sera and CVLs from HIV-1-infected and -uninfected CSWs for the presence of anti-Env IgG as well as for their potential to neutralize HIV-1 viral particles and/or mediate ADCC. This study was approved by the Ministère de la Santé du Bénin and by the CHUM human research ethics boards. Written consent was obtained from every participant.
The study population consisted of untreated HIV-1-positive female CSWs with less than 3 years of prostitution (n=23) and HIV-1-negative female CSWs with more than 7 years of prostitution (n=20) enrolled through a sex worker clinic in Cotonou, Benin. Women who were less than 18 years of age, pregnant, menstruating during the visit, or taking oral contraceptives were excluded from the study. Demographic information and sexual behavioral information are shown in Table 1. Participants also underwent a genital examination by a physician. None of these women had vaginosis, candidiasis, or other sexually transmitted infections at the time of the visit. CVL samples were obtained from all study participants by a physician using a 10-ml syringe filled with sterile phosphate-buffered solution and aimed directly into the cervical os. The specimen collection and processing were performed using methods described previously.14
Table 1.
Sociodemographic and Behavioral Characteristics of Beninese Sex Workers
| HIV-1-uninfected CSWs N=20 | HIV-1-infected CSWs N=23 | p-valuea | |
|---|---|---|---|
| Years of sex work | ≥7 | ≤3 | <0.0001 |
| Age (mean, SD) | 40.5 (10.1) | 37.9 (8.8) | NS |
| Clients in the last 7 days (mean, SD) | 15.1 (14.3) | 17.3 (21.0) | NS |
| Vaginal douching | 100% (20/20) | 100% (23/23) | NS |
| Condom always used in the last 7 days | 93.3% (14/15) | 95.0% (19/20) | NS |
p-value; unpaired t-test for comparison between the HIV-1-infected CSWs and HIV-1-uninfected. The level of HIV exposure was determined based on the prevalence of HIV infection among male clients, the number of clients per week, unprotected sex encounters, and years of sex work. HIV-1-uninfected and HIV-1-infected CSWs had 45 and 15 high-risk HIV exposures, respectively.
CSW, commercial sex worker; HIV-1, human immunodefiency virus type 1; N, number of participants; NS, nonsignificant.
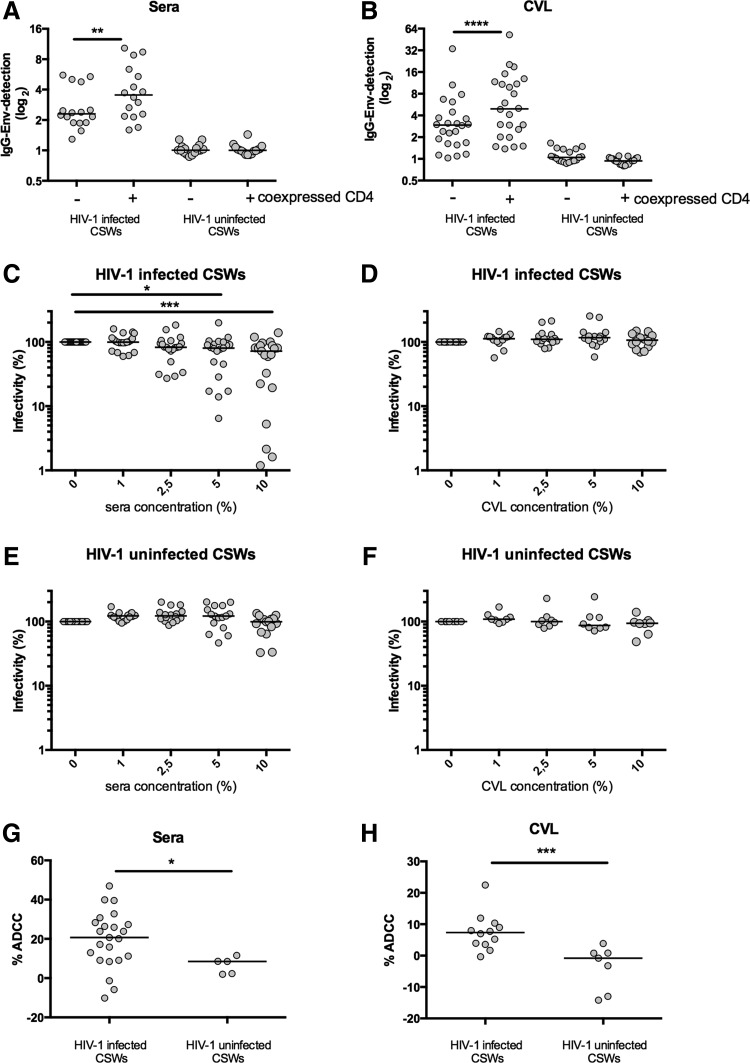
To assess whether protection from HIV-1 in the HESN CSWs is associated with HIV-1 Env-specific IgGs, we first screened sera and CVLs of the study participants through a cell-based ELISA assay that expresses fully cleaved mature HIV-1 Env glycoprotein trimers at the surface of cells.15–17 The detection of trimeric HIV-1 Env at the surface of Hos cells was performed by cell-based ELISA as previously described.15–17 Briefly, 2×104 Hos cells were plated in 96-well plates and transfected the next day using a standard polyethylenimine (PEI) transfection technique with 0.15 μg of the pSVIII plasmid expressing a cytoplasmic tail-deleted HIV-1YU2 envelope glycoprotein and 0.01 μg of a Tat-expressing plasmid per well, as reported.15–17 For coexpression of CD4, 0.007 μg of pCDNA3.1 CD4 expressing plasmid15,16 was added to the transfection mix. After 48 h, the detection of trimeric HIV-1 Env was performed in triplicate for each sample. The murine leukemia virus (MLV) Env was used as a negative control. Sera and CVL samples from 20 HIV-1-negative non-CSWs from the general population (no risk of HIV exposure) were also tested as controls. No anti-HIV-1 Env-specific IgGs were detected in sera and CVLs from HIV-1-negative CSWs (Fig. 1A and B) and non-CSWs from the general population (data not shown).
FIG. 1.
Anti-immunoglobulin G (IgG) envelope HIV-1-specific antibody detection, neutralization, and antibody-dependent cellular cytotoxicity (ADCC) by sera and cervicovaginal lavages (CVLs) from the study population. In both sera and CVLs of HIV-1-positive commercial sex workers (CSWs), we observed that coexpression of cellular CD4 increased the detection of IgGs. Sera (A) and CVLs (B) from the cohort described in Table 1 were screened by a cell-based ELISA assay for the presence of IgG directed against Env in the absence or presence of coexpressed cellular CD4 receptor. The detection of IgG for each patient was calculated taking the relative luminescence unit (RLU) value for each condition subtracted by the RLU for murine leukemia virus (MLV) of each individual. The paired t-test (**p<0.01, ***p<0.001, ****p<0.0001) was used to assess the significance between groups. Sera from HIV-1-positive CSWs but not from HIV-1-negative CSWs neutralized HIV-1. Increasing serum and CVL dilutions from HIV-1-positive CSWs (C, D) or HIV-1-negative CSWs (E, F) were incubated for 1 h at 37°C with HIV-1 viral particles bearing the primary clade B (HIV-1YU2) envelope before infection. Sera sample from every individual was also screened with MLV to test the specificity of the reduction of infectivity (not shown). The Wilcoxon matched-pairs test (*p<0.05, **p<0.01, ***p<0.001) was used to assess the significance between the different conditions. Sera and CVLs of some HIV-1-positive CSWs were able to mediate elimination of gp120-coated target cells by ADCC. HIV-1-positive CSWs and HIV-1-negative CSWs were screened by serum-mediated (G) and CVL-mediated (H) gp120-coated target CEM-NK cells. ADCC killing was measured using peripheral blood mononuclear cells (PBMCs) from healthy donors as effector cells. The Mann–Whitney test was used to assess statistical significance (*p<0.05, **p<0.01, ***p<0.001).
The latter result is in sharp contrast to a previous study from Carrillo et al. that found IgGs that recognized cell-surface-expressed Env and CD4-induced epitopes in the plasma of HESN.18 However, the signal that they obtained with HESN samples was very close to background levels. Moreover, instead of using a heterologous Env to control for specificity, they used a secondary antibody alone, which cannot control for nonspecific binding of plasma from HESN. All HIV-1-positive CSWs had IgGs that were able to recognize the trimeric Env in their sera and CVLs. Importantly, anti-HIV-1 Env-specific IgGs found in HIV-1-positive CSWs better recognized Env when it was coexpressed with CD4 (Fig. 1A and B) and thus sampled the CD4-bound conformation.15,16
We then performed an HIV-1-specific neutralization screening assay with envelope-pseudotyped viral particles in order to determine whether protection might be associated with the production of highly potent neutralizing factors, other than IgGs, in both sera and CVLs (Fig. 1C–F). The neutralization assay was performed as previously reported.19 Briefly, HIV-1 viral particles were produced by calcium phosphate transfection into 293T human embryonic cells lines with an env-deleted pNL4-3 molecular clone expressing the luciferase reporter gene (pNL4-3-LucΔEnv) and pseudotyped by cotransfecting HIV-1 Env expressors from clades A and B. Cf2Th-CD4/CCR5 cells were placed in 96-well plates (2×104 cells per well) and infected the next day. Previous to infection, infectious viral particles were preincubated with serial dilutions of patient's serum or CVLs before infecting cells.19,20 Luciferase activity was measured 48 h later with a luminometer. Comparing the luciferase activity across the samples, serial dilutions assessed the neutralizing activity of each sample. Neutralizing assays were done in quadruplicate for each sample. The MLV envelope was used as a negative control.
Our assay failed to detect neutralizing factors in sera or CVLs from HIV-1-negative CSWs. The lower infectivity detected at higher dilutions of the sera of HIV-1-negative CSWs could be due to nonspecific cytotoxicity (Fig. 1E). Sera, but not CVL, samples of HIV-1-positive CSWs were able to specifically reduce the infectivity of HIV-1 viral particles (Fig. 1C and D). Because it has been reported that neutralizing antibodies are generally strain specific, being able to neutralize only the patient's own (autologous) virus,21 we repeated these neutralization assays with viral particles bearing a clade A Env (predominant subtype in Benin)22 and again we did not detect any significant neutralization in the samples of HIV-1-uninfected CSWs (not shown).
We then investigated whether sera and CVL samples of CSWs could eliminate gp120-coated target cells through ADCC. Recombinant HIV-1YU2 gp120 proteins were produced and purified as previously described.17,19 CEM.NKr target cells were coated with 50 ng of recombinant HIV-1 gp120 (per 7.5×105 cells) for 30 min at 37°C before being stained for 20 min with a viability marker (AquaVivid, Invitrogen) and a cellular marker (eFluor 670, eBiosciences). Cryopreserved peripheral blood mononuclear cells (PBMCs) from healthy donors were labeled with a cellular marker (eFluor450, eBiosciences) and used as effector cells in this assay. Cell labeling was performed according to the manufacturer's directions.
Target and effector cells were placed together and incubated together for 4–6 h with serum diluted 1:1,000 or CVL 1:100, cells were fixed with 2% phosphate-buffered saline (PBS)-formaldehyde solution containing a constant amount of flow cytometry particles (5×104/ml) (AccuCount Blank Particles, 5.3 μm; Spherotech). A constant number of particles (1×103) was counted during cytometry acquisition in order to normalize the number of viable targets cells that survived and to calculate the percentage of ADCC-mediated killing. Each sample was acquired with an LSRII, and data analysis was performed using FlowJovX.0.6.23 Interestingly, ADCC activity was found in both sera and CVLs of HIV-1-positive CSWs and the levels were significantly higher than those observed in samples from HIV-1-negative women where no specific anti-Env was detected (Fig. 1G and H). It is possible that antibodies that do not recognize the CD4-bound conformation are present in the samples of HIV-1-positive CSWs and might contribute to ADCC; however, those recognizing the CD4-bound conformation account for the majority of the ADCC response.15
HIV-1-negative CSWs had levels of ADCC similar to those observed in HIV-1-negative non-CSWs from the general population (data not shown). Finally, we observed no correlation in the ability of sera from HIV-1-positive CSWs to neutralize the virus and to mediate an effective gp120-coated ADCC response (data not shown).
In the present study we used highly sensitive and HIV-1-specific assays and found that protection from HIV-1 in HESN CSWs from Benin was not associated with HIV-1-specific neutralizing factors or the ability of the host to produce ADCC-mediated Abs against gp120 epitopes in both the systemic and genital mucosal compartments. As mentioned previously, the relevance of the presence of antibodies recognizing the CD4-bound conformation comes from the RV144 trial3 where antibodies isolated from vaccinees were shown to mediate ADCC4,15 and to preferentially recognize the CD4-bound conformation.15
Although, the CD4 receptor on infected cells might be downregulated upon exposure to HIV, recent studies by our group suggest that the degree of CD4 downregulation initiated by the transmitter/founder viruses might be compatible with a certain degree of CD4i exposure (unpublished data). In addition, there were no anti-HIV-1 Env-specific IgGs in either the serum or CVLs from HESN CSWs, as detected by our cell-based ELISA assay using trimeric Env. Therefore, we found no evidence that HIV-1 resistance in the HESN CSWs from Benin was associated with HIV-1-specific neutralizing and ADCC functions. This protection may be rather linked with the host's capacity to preserve systemic integrity by constraining immune activity and controlling inflammatory conditions at the mucosal point of entry, as recently suggested.7 HIV-1-positive CSWs have the ability to develop anti-HIV-1 Env-specific IgGs that detect trimeric Env in both blood and mucosal fluids. Moreover, these HIV-1 Env-specific IgGs might be responsible for both the HIV-1-specific neutralizing and ADCC activities observed in the sera and CVLs of some of these women. More importantly, our results show for the first time that HIV-1 Env-specific IgGs in genital mucosal compartments preferentially target Env in its CD4 bound conformation and that these IgGs might be involved in ADCC responses at the mucosal site.
Altogether, these findings suggest that future mucosal vaccines should aim to induce the production of CD4i anti-Env antibodies in the genital mucosa to mediate a more effective ADCC response to eliminate HIV-infected cells at the portal site of entry. Future work should aim to investigate the IgA response in the whole study population because IgA binding to the same epitopes may compete with and prevent the interaction of IgG with trimers.
Acknowledgments
This work was supported by Grant HOP-79213 from the Canadian Institutes of Health Research (CIHR) to M.R. and by CIHR catalyst 126630, CRCHUM continuum, and the Réseau SIDA from the Fonds de la Recherche en Santé du Québec (FRSQ) grants to M.R. and A.F. A.F. is a recipient of a Canada Research Chair on Retroviral Entry.
M.R. and A.F. are the lead investigators of this study and with LA.B., DE.K., and J.P. designed the experiments and LA.B., M.V., and J.R. performed the experiments. M.R., A.F., and LA.B wrote the article. AC.L., F.G., and M.A. were responsible for the participants' recruitment and provided clinical and laboratory data. All authors edited and approved the final version of the manuscript.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Kresina TF. and Mathieson B: Human immunodeficiency virus type 1 infection, mucosal immunity, and pathogenesis and extramural research programs at the National Institutes of Health. J Infect Dis 1999;179(Suppl 3):S392–396 [DOI] [PubMed] [Google Scholar]
- 2.Overbaugh J, Kreiss J, Poss M, et al. : Studies of human immunodeficiency virus type 1 mucosal viral shedding and transmission in Kenya. J Infect Dis 1999;179(Suppl 3):S401–404 [DOI] [PubMed] [Google Scholar]
- 3.Haynes BF, Gilbert PB, McElrath MJ, et al. : Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012;366(14):1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bonsignori M, Pollara J, Moody MA, et al. : Antibody-dependent cellular cytotoxicity-mediating antibodies from an HIV-1 vaccine efficacy trial target multiple epitopes and preferentially use the VH1 gene family. J Virol 2012;86(21):11521–11532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battle-Miller K, Eby CA, Landay AL, et al. : Antibody-dependent cell-mediated cytotoxicity in cervical lavage fluids of human immunodeficiency virus type 1-infected women. J Infect Dis 2002;185(4):439–447 [DOI] [PubMed] [Google Scholar]
- 6.Nag P, Kim J, Sapiega V, et al. : Women with cervicovaginal antibody-dependent cell-mediated cytotoxicity have lower genital HIV-1 RNA loads. J Infect Dis 2004;190(11):1970–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Poudrier J, Thibodeau V, and Roger M: Natural immunity to HIV: A delicate balance between strength and control. Clin Dev Immunol 2012;2012:875821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buchacz K, Parekh BS, Padian NS, et al. : HIV-specific IgG in cervicovaginal secretions of exposed HIV-uninfected female sexual partners of HIV-infected men. AIDS Res Hum Retroviruses 2001;17(18):1689–1693 [DOI] [PubMed] [Google Scholar]
- 9.Dorrell L, Hessell AJ, Wang M, et al. : Absence of specific mucosal antibody responses in HIV-exposed uninfected sex workers from the Gambia. AIDS 2000;14(9):1117–1122 [DOI] [PubMed] [Google Scholar]
- 10.Kaul R, Trabattoni D, Bwayo JJ, et al. : HIV-1-specific mucosal IgA in a cohort of HIV-1-resistant Kenyan sex workers. AIDS 1999;13(1):23–29 [DOI] [PubMed] [Google Scholar]
- 11.Mestecky J, Wright PF, Lopalco L, et al. : Scarcity or absence of humoral immune responses in the plasma and cervicovaginal lavage fluids of heavily HIV-1-exposed but persistently seronegative women. AIDS Res Hum Retroviruses 2011;27(5):469–486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mazzoli S, Trabattoni D, Lo Caputo S, et al. : HIV-specific mucosal and cellular immunity in HIV-seronegative partners of HIV-seropositive individuals. Nat Med 1997;3(11):1250–1257 [DOI] [PubMed] [Google Scholar]
- 13.Wyatt R. and Sodroski J: The HIV-1 envelope glycoproteins: Fusogens, antigens, and immunogens. Science 1998;280(5371):1884–1888 [DOI] [PubMed] [Google Scholar]
- 14.Lajoie J, Poudrier J, Massinga-Loembe M, et al. : Differences in immunoregulatory cytokine expression patterns in the systemic and genital tract compartments of HIV-1-infected commercial sex workers in Benin. Mucosal Immunol 2008;1(4):309–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Veillette M, Desormeaux A, Medjahed H, et al. : Interaction with cellular CD4 exposes HIV-1 envelope epitopes targeted by antibody-dependent cell-mediated cytotoxicity. J Virol 2014;88(5):2633–2644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Veillette M, Coutu M, Richard J, et al. : Conformational evaluation of HIV-1 trimeric envelope glycoproteins using a cell-based ELISA assay. J Visualize Exp 2014;91:e51995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Desormeaux A, Coutu M, Medjahed H, et al. : The highly conserved layer-3 component of the HIV-1 gp120 inner domain is critical for CD4-required conformational transitions. J Virol 2013;87(5):2549–2562 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Carrillo J, Restrepo C, Rallon NI, et al. : HIV exposed seronegative individuals show antibodies specifically recognizing native HIV envelope glycoprotein. AIDS 2013;27(9):1375–1385 [DOI] [PubMed] [Google Scholar]
- 19.Finzi A, Xiang SH, Pacheco B, et al. : Topological layers in the HIV-1 gp120 inner domain regulate gp41 interaction and CD4-triggered conformational transitions. Mol Cell 2010;37(5):656–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.LaBonte JA, Patel T, Hofmann W, and Sodroski J: Importance of membrane fusion mediated by human immunodeficiency virus envelope glycoproteins for lysis of primary CD4-positive T cells. J Virol 2000;74(22):10690–10698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wei X, Decker JM, Wang S, et al. : Antibody neutralization and escape by HIV-1. Nature 2003;422(6929):307–312 [DOI] [PubMed] [Google Scholar]
- 22.Chamberland A, Diabate S, Sylla M, et al. : Transmission of HIV-1 drug resistance in Benin could jeopardise future treatment options. Sex Transm Infect 2012;88(3):179–183 [DOI] [PubMed] [Google Scholar]
- 23.Richard J, Veillette M, Batraville LA, et al. : Flow cytometry-based assay to study HIV-1 gp120 specific antibody-dependent cellular cytotoxicity responses. J Virol Methods 2014;208C:107–114 [DOI] [PubMed] [Google Scholar]