Abstract
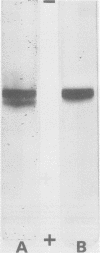
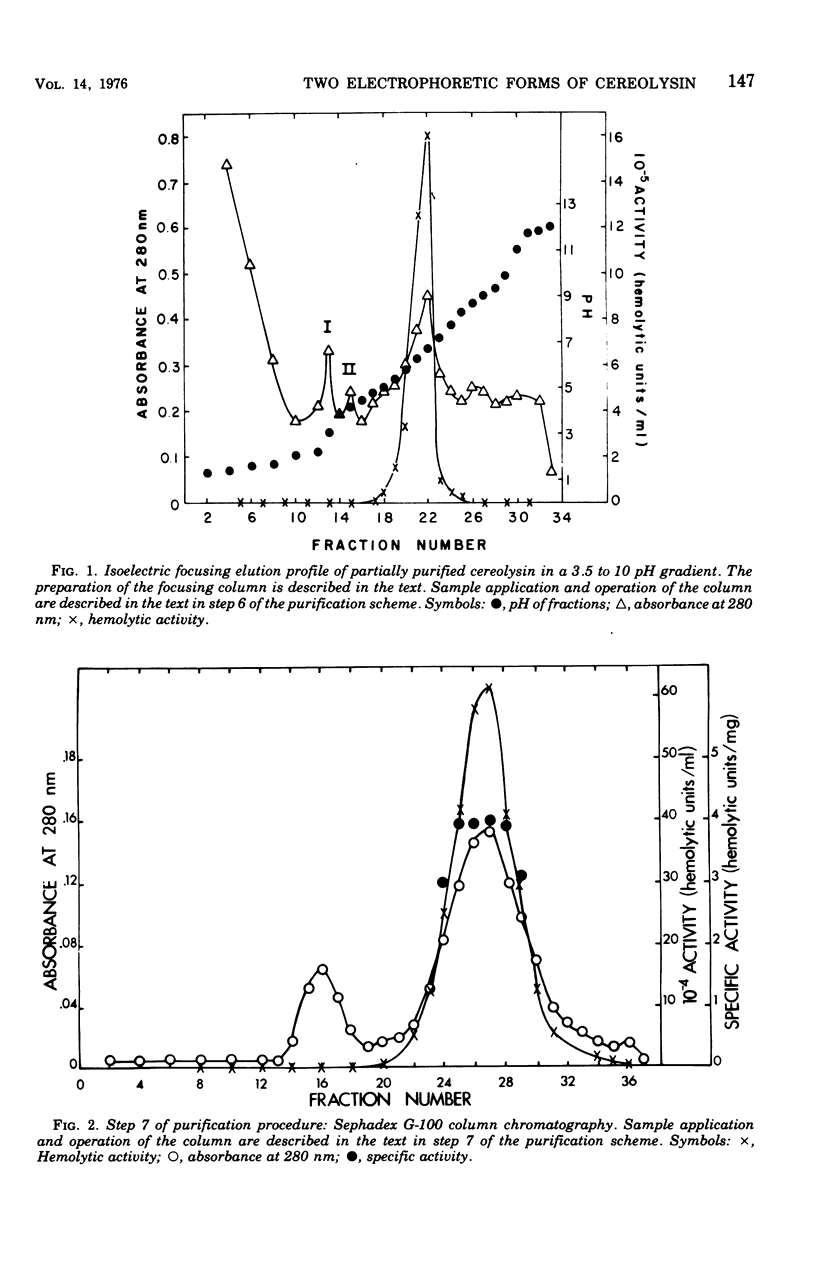
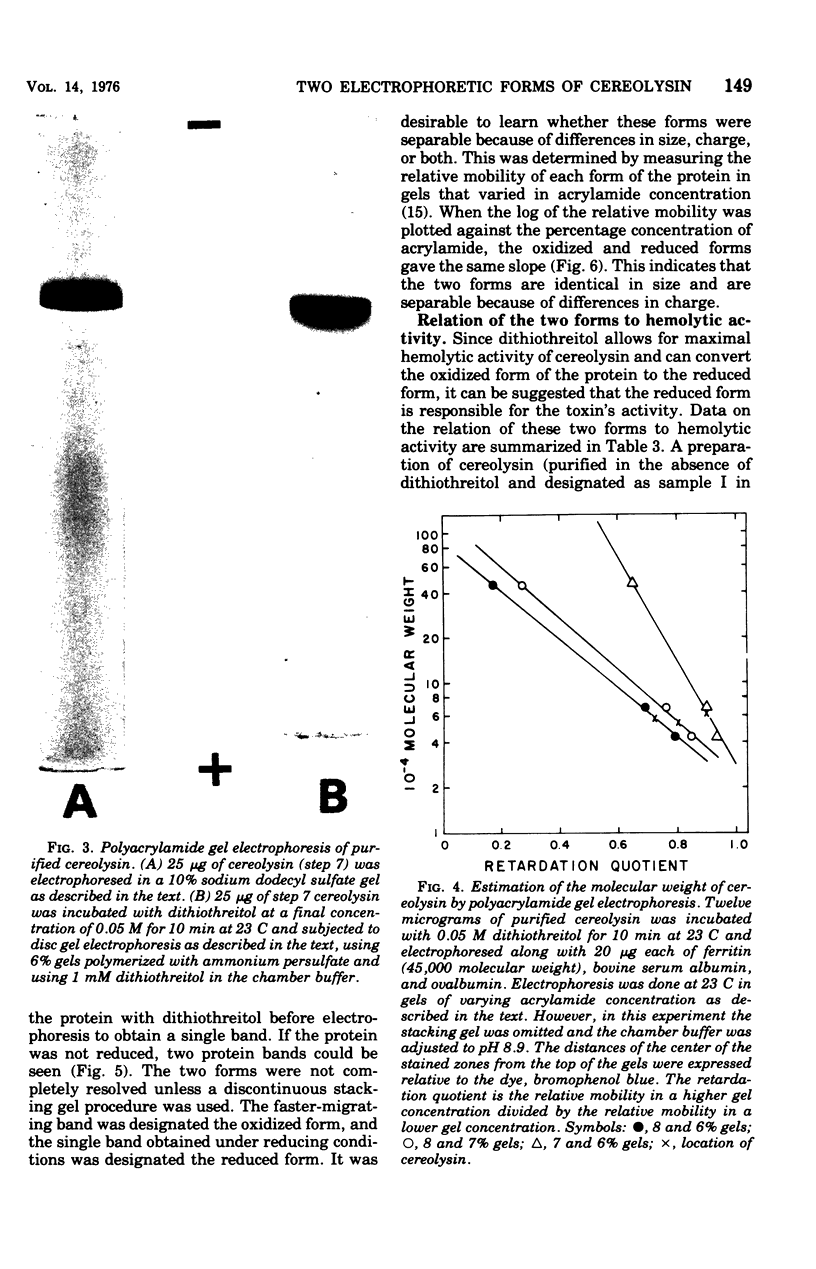
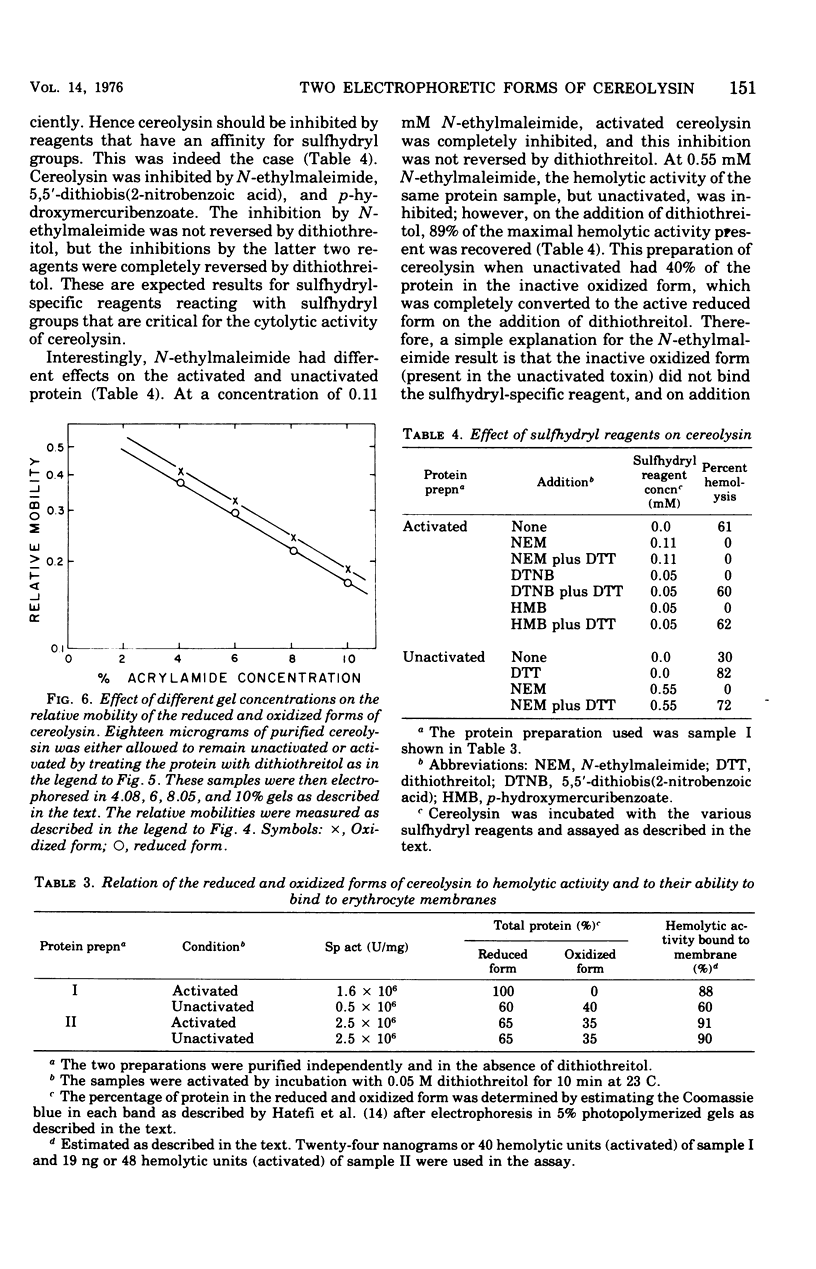
Cereolysin was purified to apparent homogeneity by using ammonium sulfate fractionation, hydrophobic chromatography with AH-Sepharose, isoelectric focusing, and gel filtration. The active form of the toxin had an isoelectric point of 6.6, and the molecular weight of the protein was about 55,500 as judged by sodium dodecyl sulfate-gel electrophoresis, gel filtration, and gel electrophoresis using various concentrations of acrylamide. Cereolysin contained two half-cystine residues and was dependent on reducing agents, such as dithiothreitol, for maximal hemolytic activity and charge homogeneity. By using discontinuous acrylamide electrophoresis, two forms of the toxin could be observed: oxidized and reduced. If the toxin was purified in the absence of dithiothreitol, partial spontaneous oxidation resulted in the formation of an oxidized form of the toxin. Relative to the reduced form, the oxidized form moved slightly closer to the anode in gel electrophoresis at pH 9.0. If the toxin was purified in the presence of 5 mM dithiothreitol or if the spontaneously oxidized toxin was preincubated with dithiothreitol, only the reduced form of the protein was observed. When the logarithims of their relative mobilities were plotted against the concentration of acrylamide in the gels, the slopes for the reduced and oxidized forms were identical. This indicates that the two forms are identical in size and are separable because of different charges. The reduced protein could be inhibited by N-ethylmaleimide, 5,5'-dithiobis(2-nitrobenzoic acid), and p-hydroxymercuribenzoate. Inhibition by the latter two sulfhydryl reagents could be completely reversed by dithiothreitol. The reversibly oxidized form of the toxin did not appear to be inhibited by N-ethylmaleimide and apparently was either unable to bind to or had a decreased affinity for the erythrocyte membrane.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alouf J. E., Raynaud M. Purification and some properties of streptolysin O. Biochimie. 1973;55(10):1187–1193. doi: 10.1016/s0300-9084(74)80322-6. [DOI] [PubMed] [Google Scholar]
- Andrews P. Estimation of the molecular weights of proteins by Sephadex gel-filtration. Biochem J. 1964 May;91(2):222–233. doi: 10.1042/bj0910222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BERNHEIMER A. W., SCHWARTZ L. L. Isolation and composition of staphylococcal alpha toxin. J Gen Microbiol. 1963 Mar;30:455–468. doi: 10.1099/00221287-30-3-455. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Grushoff P., Avigad L. S. Isoelectric analysis of cytolytic bacterial proteins. J Bacteriol. 1968 Jun;95(6):2439–2441. doi: 10.1128/jb.95.6.2439-2441.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernheimer A. W., Grushoff P. Cereolysin: production, purification and partial characterization. J Gen Microbiol. 1967 Jan;46(1):143–150. doi: 10.1099/00221287-46-1-143. [DOI] [PubMed] [Google Scholar]
- Bernheimer A. W., Grushoff P. Extracellular hemolysins of aerobic sporogenic bacilli. J Bacteriol. 1967 May;93(5):1541–1543. doi: 10.1128/jb.93.5.1541-1543.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelhoch H. Spectroscopic determination of tryptophan and tyrosine in proteins. Biochemistry. 1967 Jul;6(7):1948–1954. doi: 10.1021/bi00859a010. [DOI] [PubMed] [Google Scholar]
- HOWARD J. G., WALLACE K. R., WRIGHT G. P. The inhibitory effects of cholesterol and related sterols on haemolysis by streptolysin O. Br J Exp Pathol. 1953 Apr;34(2):174–180. [PMC free article] [PubMed] [Google Scholar]
- Hatefi Y., Davis K. A., Baltscheffsky H., Baltscheffsky M., Johansson B. C. Isolation and properties of succinate dehydrogenase from Rhodospirillum rubrum. Arch Biochem Biophys. 1972 Oct;152(2):613–618. doi: 10.1016/0003-9861(72)90257-3. [DOI] [PubMed] [Google Scholar]
- Hedrick J. L., Smith A. J. Size and charge isomer separation and estimation of molecular weights of proteins by disc gel electrophoresis. Arch Biochem Biophys. 1968 Jul;126(1):155–164. doi: 10.1016/0003-9861(68)90569-9. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mitsui K., Mitsui N., Hase J. Clostridium perfringens exotoxins. II. Purification and some properties of theta-toxin. Jpn J Exp Med. 1973 Oct;43(5):377–391. [PubMed] [Google Scholar]
- Oberley T. D., Duncan J. L. Characteristics of streptolysin O action. Infect Immun. 1971 Dec;4(6):683–687. doi: 10.1128/iai.4.6.683-687.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pendleton I. R., Bernheimer A. W., Grushoff P. Purification and partial characterization of hemolysins from Bacillus thuringiensis. J Invertebr Pathol. 1973 Mar;21(2):131–135. doi: 10.1016/0022-2011(73)90192-4. [DOI] [PubMed] [Google Scholar]
- Pendleton I. R., Kim K. S., Bernheimer A. W. Detection of cholesterol in cell membranes by use of bacterial toxins. J Bacteriol. 1972 May;110(2):722–730. doi: 10.1128/jb.110.2.722-730.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SANGER F., THOMPSON E. O. Halogenation of tyrosine during acid hydrolysis. Biochim Biophys Acta. 1963 May 14;71:468–471. doi: 10.1016/0006-3002(63)91108-9. [DOI] [PubMed] [Google Scholar]
- Shany S., Bernheimer A. W., Grushoff P. S., Kim K. S. Evidence for membrane cholesterol as the common binding site for cereolysin, streptolysin O and saponin. Mol Cell Biochem. 1974 May 30;3(3):179–186. doi: 10.1007/BF01686643. [DOI] [PubMed] [Google Scholar]
- Smyth C. J., Fehrenbach F. J. Isoelectric analysis of haemolysins and enzymes from streptococci of groups A, C and G. Acta Pathol Microbiol Scand B Microbiol Immunol. 1974 Dec;82(6):860–870. doi: 10.1111/j.1699-0463.1974.tb02384.x. [DOI] [PubMed] [Google Scholar]
- Smyth C. J. The identification and purification of multiple forms of theta-haemolysin (theta-toxin) of Clostridium perfringens type A. J Gen Microbiol. 1975 Apr;87(2):219–238. doi: 10.1099/00221287-87-2-219. [DOI] [PubMed] [Google Scholar]
- Van Epps D. E., Andersen B. R. Streptolysin O II. Relationship of Sulfyhdryl Groups to Activity. Infect Immun. 1971 May;3(5):648–652. doi: 10.1128/iai.3.5.648-652.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zwaan J. Estimation of molecular weights of proteins by polyacrylamide gel electrophoresis. Anal Biochem. 1967 Nov;21(2):155–168. doi: 10.1016/0003-2697(67)90177-7. [DOI] [PubMed] [Google Scholar]