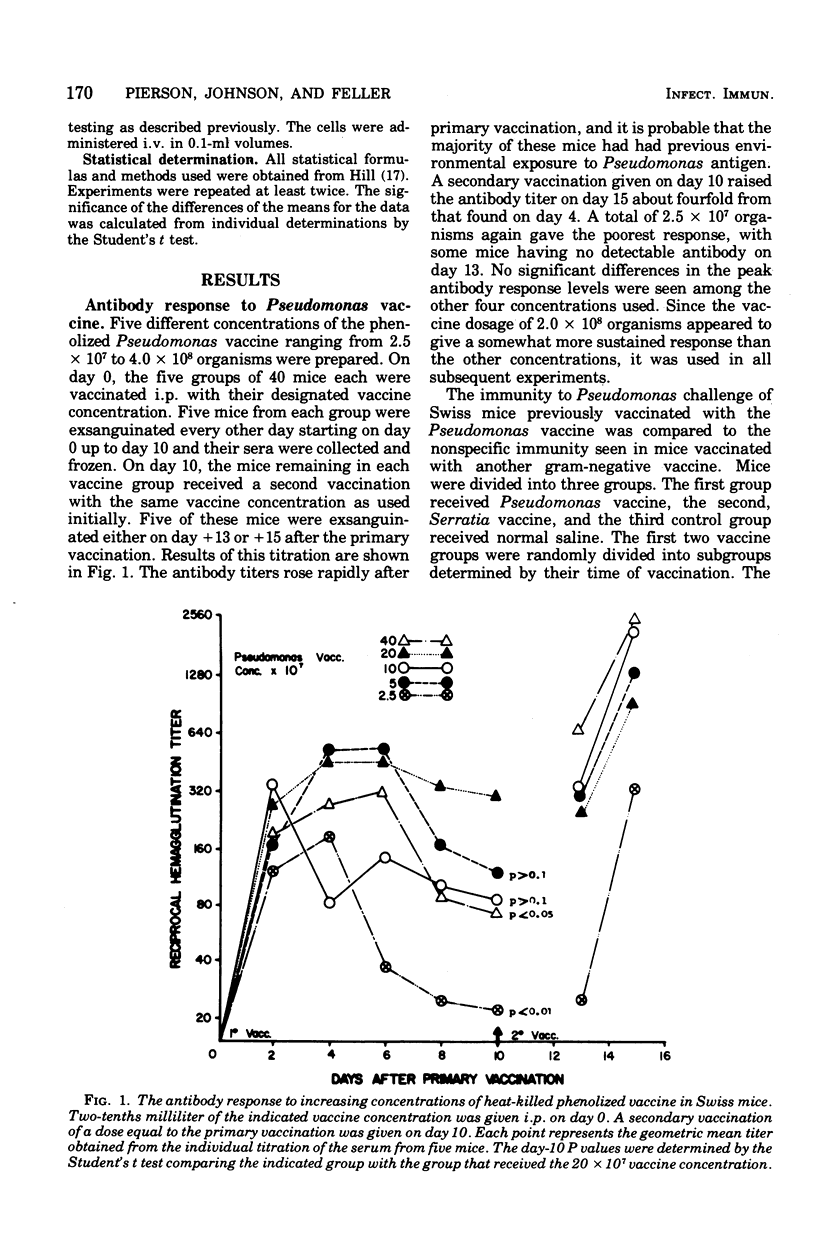
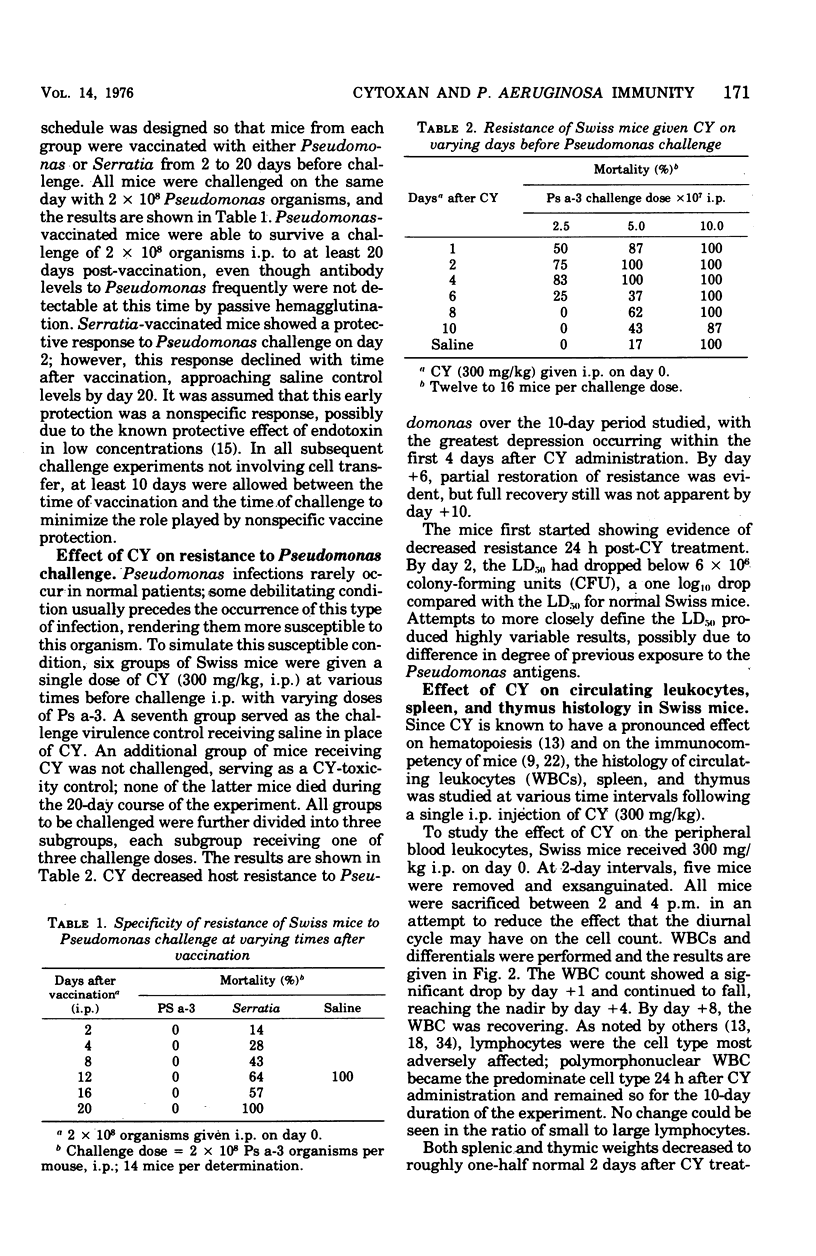
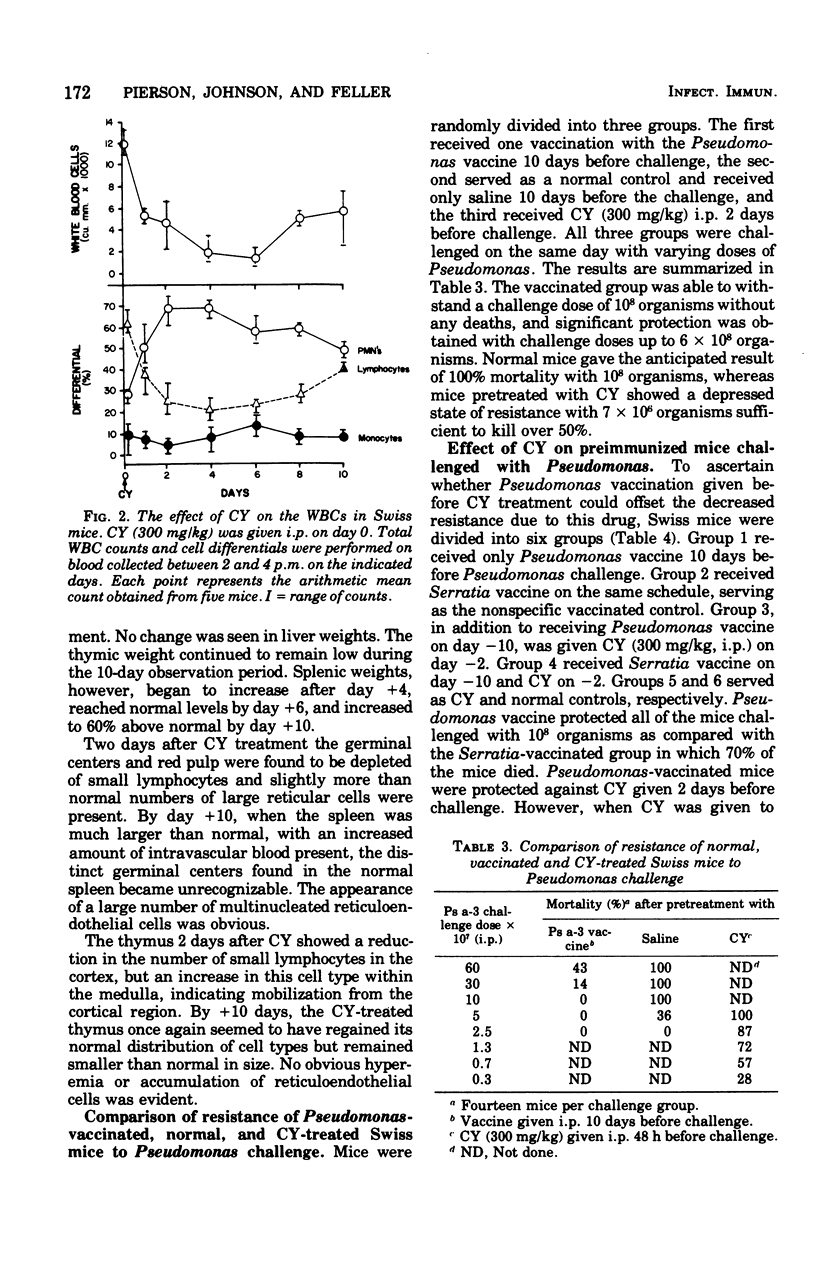
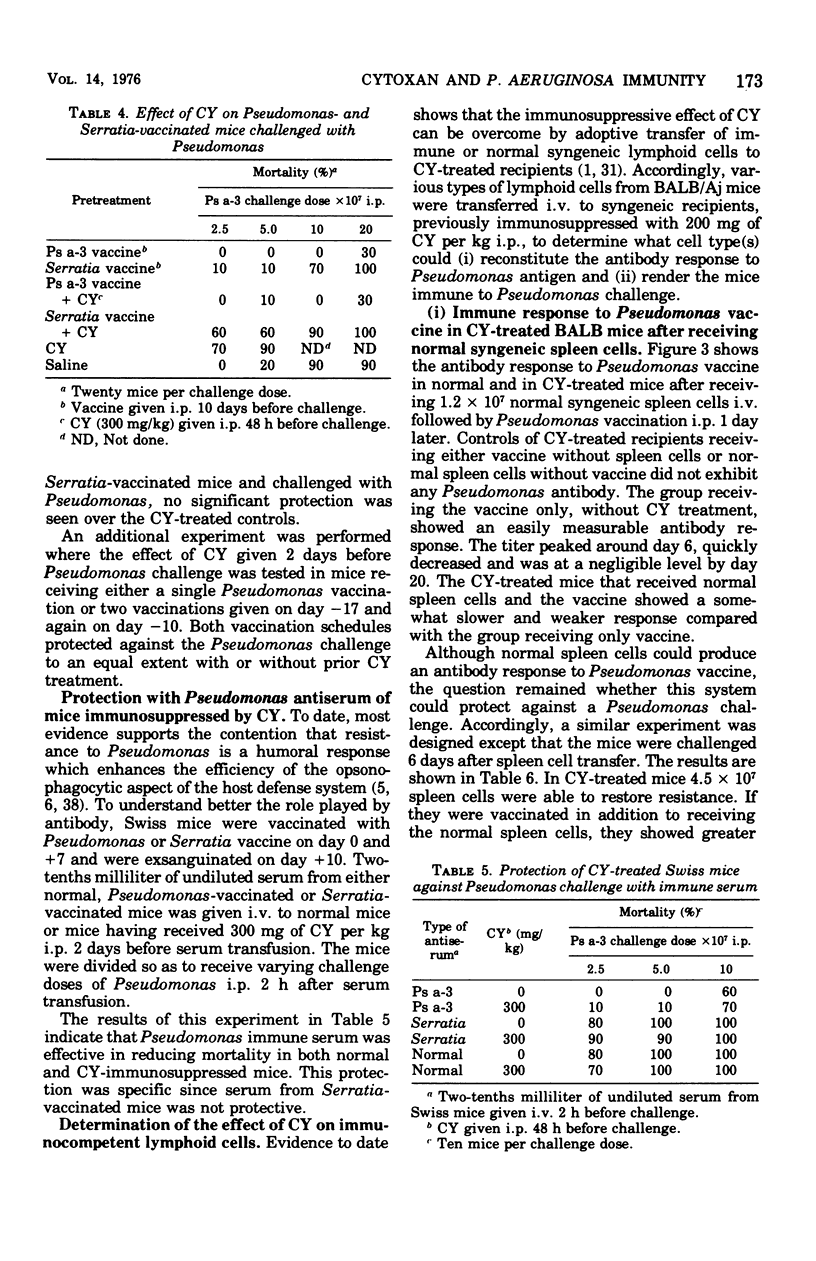
Abstract
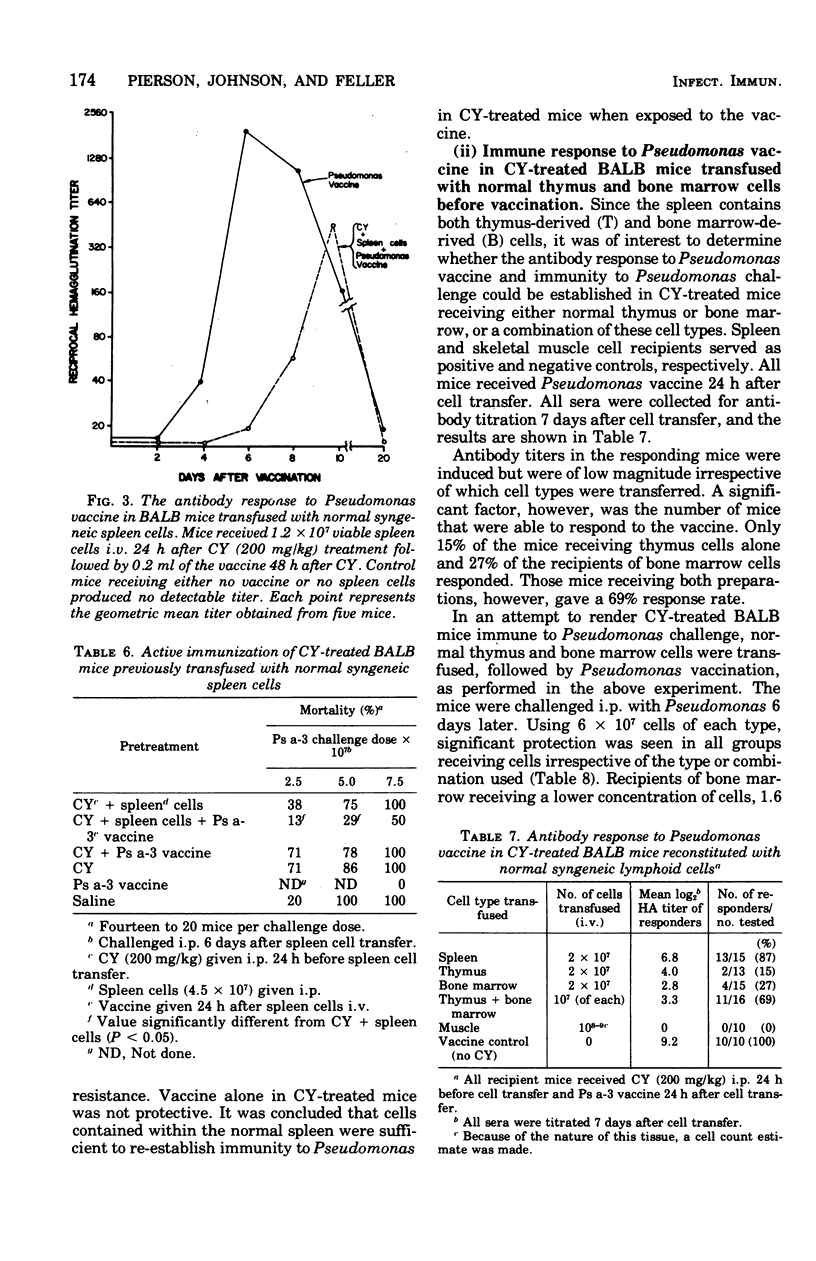
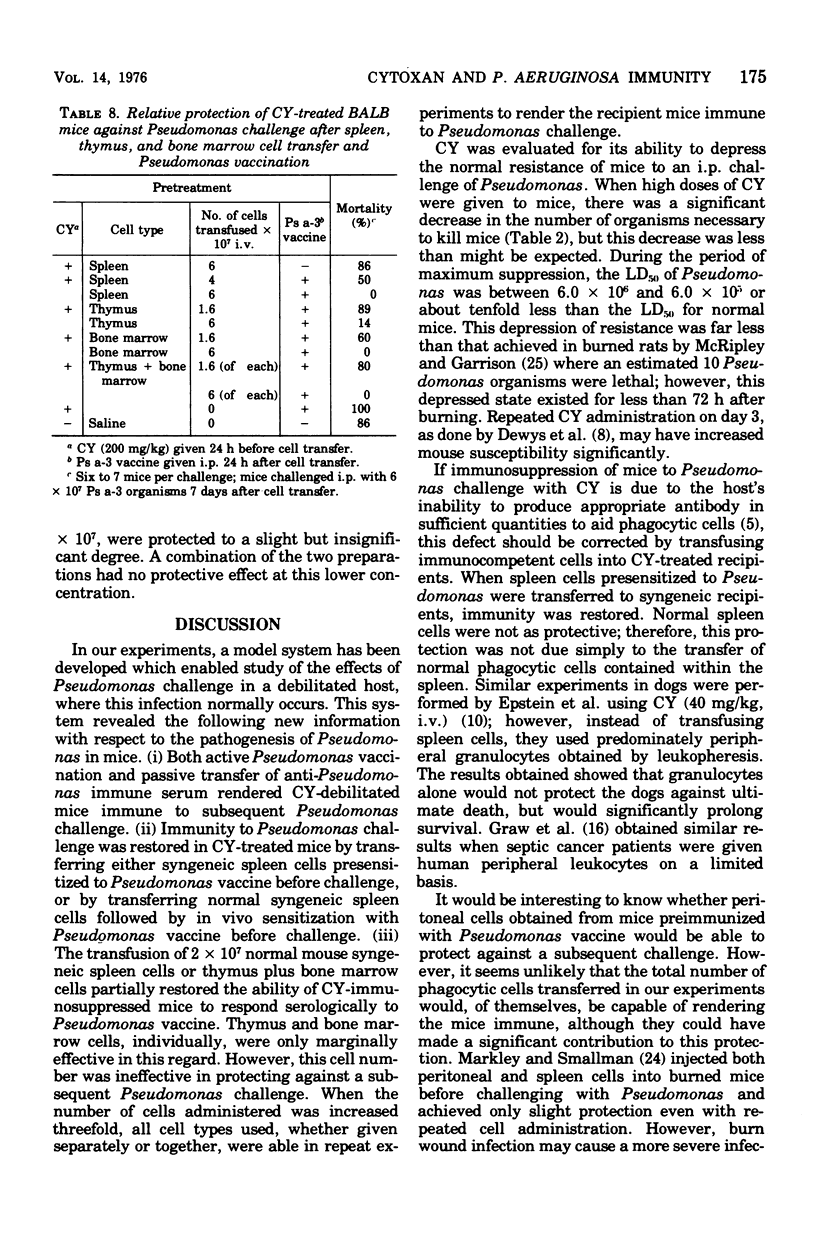
Natural resistance in mice to Pseudomonas aeruginosa was decreased 10-fold with a single dose of 300 mg of cyclophosphamide (CY) per kg intraperitoneally. Mice were resistant to infection when immunized actively with Pseudomonas vaccine or passively with Pseudomonas immune serum before receiving CY. Syngeneic spleen, thymus and/or bone marrow cells were transfused into CY-treated recipient mice. Protective anti-Pseudomonas antibody was elicited in the recipient mice when they were vaccinated 1 day after receiving normal spleen cells and challenged 8 days after vaccination. When 1.6 X 10(7) normal thymus and bone marrow cells were infused before vaccination, 69% of the recipients of both cell preparations responded serologically compared with 15 and 27% of those receiving either thymus or bone marrow cells, respectively. CY-treated thymus or bone marrow cell recipients were resistant to Pseudomonas infection when 6 X 10(7) of either cell population was transfused.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aisenberg A. C., Davis C. The thymus and recovery from cyclophosphamide-induced tolerance to sheep erythrocytes. J Exp Med. 1968 Jul 1;128(1):35–46. doi: 10.1084/jem.128.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alms T. H., Bass J. A. Immunization against Pseudomonas aeruginosa. II. Purification and characterization of the protective factor from the alcohol-precipitated fraction. J Infect Dis. 1967 Jun;117(3):257–264. doi: 10.1093/infdis/117.3.257. [DOI] [PubMed] [Google Scholar]
- Bennett J. V. Nosocomial infections due to Pseudomonas. J Infect Dis. 1974 Nov;130 (Suppl)(0):S4–S7. doi: 10.1093/infdis/130.supplement.s4. [DOI] [PubMed] [Google Scholar]
- Bjornson A. B., Michael J. G. Biological Activities of Rabbit Immunoglobulin M and Immunoglobulin G Antibodies to Pseudomonas aeruginosa. Infect Immun. 1970 Oct;2(4):453–461. doi: 10.1128/iai.2.4.453-461.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowder J. G., Fisher M. W., White A. Type-specific immunity in pseudomonas diseases. J Lab Clin Med. 1972 Jan;79(1):47–54. [PubMed] [Google Scholar]
- Dale D. C., Reynolds H. Y., Pennington J. E., Elin R. J., Pitts T. W., Graw R. G., Jr Granulocyte transfusion therapy of experimental Pseudomonas pneumonia. J Clin Invest. 1974 Sep;54(3):664–671. doi: 10.1172/JCI107804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeWys W. D., Goldin A., Man, El N. Hematopoietic recovery after large doses of cyclophosphamide: correlation of proliferative state with sensitivity. Cancer Res. 1970 Jun;30(6):1692–1697. [PubMed] [Google Scholar]
- Dumont F. Destruction and regeneration of lymphocyte populations in the mouse spleen after cyclophosphamide treatment. Int Arch Allergy Appl Immunol. 1974;47(1):110–123. doi: 10.1159/000231206. [DOI] [PubMed] [Google Scholar]
- Epstein R. B., Waxman F. J., Bennett B. T., Andersen B. R. Pseudomonas septicemia in neutropenic dogs. I. Treatment with granulocyte transfusions. Transfusion. 1974 Jan-Feb;14(1):51–57. doi: 10.1111/j.1537-2995.1974.tb04484.x. [DOI] [PubMed] [Google Scholar]
- FELLER I., VIAL A. B., CALLAHAN W., WALDYKE J. USE OF VACCINE AND HYPERIMMUNE SERUM FOR PROTECTION AGAINST PSEUDOMONAS SEPTICEMIA. J Trauma. 1964 Jul;4:451–456. doi: 10.1097/00005373-196407000-00002. [DOI] [PubMed] [Google Scholar]
- Feller I., Pierson C. Pseudomonas vaccine and hyperimmune plasma for burned patients. Arch Surg. 1968 Aug;97(2):225–229. doi: 10.1001/archsurg.1968.01340020089010. [DOI] [PubMed] [Google Scholar]
- Finland M. Excursions into epidemiology: selected studies during the past four decades at Boston City Hospital. J Infect Dis. 1973 Jul;128(1):76–124. doi: 10.1093/infdis/128.1.76. [DOI] [PubMed] [Google Scholar]
- Fisher M. W., Devlin H. B., Gnabasik F. J. New immunotype schema for Pseudomonas aeruginosa based on protective antigens. J Bacteriol. 1969 May;98(2):835–836. doi: 10.1128/jb.98.2.835-836.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graw R. G., Jr, Herzig G., Perry S., Henderson E. S. Normal granulocyte transfusion therapy: treatment of septicemia due to gram-negative bacteria. N Engl J Med. 1972 Aug 24;287(8):367–371. doi: 10.1056/NEJM197208242870801. [DOI] [PubMed] [Google Scholar]
- Hoffsten P. E., Dixon F. J. Effect of irradiation and cyclophosphamide on anti-KLH antibody formation in mice. J Immunol. 1974 Feb;112(2):564–572. [PubMed] [Google Scholar]
- Lagrange P. H., Mackaness G. B., Miller T. E. Potentiation of T-cell-mediated immunity by selective suppression of antibody formation with cyclophosphamide. J Exp Med. 1974 Jun 1;139(6):1529–1539. doi: 10.1084/jem.139.6.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linna T. J., Frommel D., Good R. A. Effects of early cyclophosphamide treatment on the development of lymphoid organs and immunological functions in the chickens. Int Arch Allergy Appl Immunol. 1972;42(1):20–39. doi: 10.1159/000230590. [DOI] [PubMed] [Google Scholar]
- MCRIPLEY R. J., GARRISON D. W. INCREASED SUSCEPTIBILITY OF BURNED RATS TO PSEUDOMONAS AERUGINOSA. Proc Soc Exp Biol Med. 1964 Feb;115:336–338. doi: 10.3181/00379727-115-28906. [DOI] [PubMed] [Google Scholar]
- Many A., Schwartz R. S. Drug-induced immunologic tolerance: site of action of cyclophosphamide. Proc Soc Exp Biol Med. 1970 Mar;133(3):754–757. doi: 10.3181/00379727-133-34558. [DOI] [PubMed] [Google Scholar]
- Markley K., Smallman E. Protection by vaccination against Pseudomonas infection after thermal injury. J Bacteriol. 1968 Oct;96(4):867–874. doi: 10.1128/jb.96.4.867-874.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markley K., Smallman E. Protection of burned mice challenged with pseudomonas by peritoneal exudate cells. J Reticuloendothel Soc. 1969 Oct-Dec;6(5):448–456. [PubMed] [Google Scholar]
- Munster A. M., Leary A. G., Spicer S. S., Fisher M. W. Effect of lymphocytotherapy on the course of experimental Pseudomonas sepsis. Ann Surg. 1974 Apr;179(4):482–488. doi: 10.1097/00000658-197404000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Möller G., Michael G. Frequency of antigen-sensitive cells to thymus-independent antigens. Cell Immunol. 1971 Aug;2(4):309–316. doi: 10.1016/0008-8749(71)90065-7. [DOI] [PubMed] [Google Scholar]
- Pruitt B. A., Jr Infections caused by Pseudomonas species in patients with burns and in other surgical patients. J Infect Dis. 1974 Nov;130 (Suppl)(0):S8–13. doi: 10.1093/infdis/130.supplement.s8. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y., Thompson R. E. Pulmonary host defenses. I. Analysis of protein and lipids in bronchial secretions and antibody responses after vaccination with pseudomonas aeruginosa. J Immunol. 1973 Aug;111(2):358–368. [PubMed] [Google Scholar]
- Sandberg J. S., Owens A. H., Jr, Santos G. W. Clinical and pathologic characteristics of graft-versus-host disease produced in cyclophosphamide-treated adult mice. J Natl Cancer Inst. 1971 Jan;46(1):151–160. [PubMed] [Google Scholar]
- Saslaw S., Carlisle H. N., Moheimani M. Effect of vincristine sulfate on Pseudomonas infections in monkeys. Infect Immun. 1972 Aug;6(2):149–155. doi: 10.1128/iai.6.2.149-155.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorvari T., Toivanen A., Toivanen P. Transplantation of bursal stem cells into cyclophosphamide-treated chicks. Redevelopment of bursal follicles. Transplantation. 1974 Jun;17(6):584–592. doi: 10.1097/00007890-197406000-00007. [DOI] [PubMed] [Google Scholar]
- Stockman G. D., Heim L. R., South M. A., Trentin J. J. Differential effects of cyclophosphamide on the B and T cell compartments of adult mice. J Immunol. 1973 Jan;110(1):277–282. [PubMed] [Google Scholar]
- Tapper M. L., Armstrong D. Bacteremia due to Pseudomonas aeruginosa complicating neoplastic disease: a progress report. J Infect Dis. 1974 Nov;130 (Suppl)(0):S14–S23. doi: 10.1093/infdis/130.supplement.s14. [DOI] [PubMed] [Google Scholar]
- WALKER H. L., MASON A. D., Jr, RAULSTON G. L. SURFACE INFECTION WITH PSEUDOMONAS AERUGINOSA. Ann Surg. 1964 Aug;160:297–305. doi: 10.1097/00000658-196408000-00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt P. J., Okubadejo O. A. Changes in incidence and aetiology of bacteraemia arising in hospital practice. Br Med J. 1967 Jan 28;1(5534):210–211. doi: 10.1136/bmj.1.5534.210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesley J., Fisher A., Fisher M. W. Immunization against Pseudomonas in infection after thermal injury. J Infect Dis. 1974 Nov;130 (Suppl)(0):S152–S158. doi: 10.1093/infdis/130.supplement.s152. [DOI] [PubMed] [Google Scholar]
- Young L. S. Human immunity to Pseudomonas aeruginosa. II. Relationship between heat-stable opsonins and type-specific lipopolysaccharides. J Infect Dis. 1972 Sep;126(3):277–287. doi: 10.1093/infdis/126.3.277. [DOI] [PubMed] [Google Scholar]
- Young L. S., Meyer R. D., Armstrong D. Pseudomonas aeruginosa vaccine in cancer patients. Ann Intern Med. 1973 Oct;79(4):518–527. doi: 10.7326/0003-4819-79-4-518. [DOI] [PubMed] [Google Scholar]



