Abstract
The repeat low-dose virus challenge model is commonly used in nonhuman primate studies of HIV transmission and biomedical preventions. For some viruses or challenge routes, it is uncertain whether the repeated exposure design might induce virus-directed innate or adaptive immunity that could affect infection or viremic outcomes. Retrospective cohorts of male Indian rhesus (n=40) and female pigtail (n=46) macaques enrolled in repeat low-dose rectal or vaginal SHIVSF162p3 challenge studies, respectively, were studied to compare the relationship between the number of previous exposures and peak plasma SHIV RNA levels or viral load area under the curve (AUC), surrogate markers of viral control. Repeated mucosal exposures of 10 or 50 TCID50 of virus for rectal and vaginal exposures, respectively, were performed. Virus levels were measured by quantitative reverse-transcriptase real-time PCR. The cumulative number of SHIVSF162p3 exposures did not correlate with observed peak virus levels or with AUC in rectally challenged rhesus macaques [peak: rho (ρ)=0.04, p=0.8; AUC: ρ=0.33, p=0.06] or vaginally challenged pigtail macaques (peak: ρ=−0.09, p=0.7; AUC: ρ=0.11, p=0.6). Infections in these models occur independently of exposure history and provide assurance that neither inoculation route nor number of exposures required for infection correlates with postinfection viremia. These data also indicate that both the vaginal and rectal repeated low-dose virus exposure models using SHIVSF162p3 provide a reliable system for nonhuman primate studies.
The macaque repeat low-dose virus challenge model is commonly used in nonhuman primate modeling of HIV acquisition and HIV biomedical prevention testing. Because of the presence of HIV-exposed, yet uninfected cases in humans,1 many of whom have HIV-specific immune cells,2 it could be questioned whether repeated low-level virus exposures in macaques may also induce virus-specific immunity, possibly affecting infection susceptibility or viremic outcomes. If repeated exposures caused mucosal immune cell activation, we could then hypothesize that the resulting enhanced susceptibility would facilitate more rapid transmission rates and higher, faster rises in plasma virus levels. Abdulhaqq et al. reported evidence for this, showing that repeated vaginal exposures to replication-deficient SIVsmB7 resulted in activated plasmacytoid dendritic cell and CD4+ T cell infiltrate, which then led to faster rates of CD4+ T cell decline and virus replication after macaques were later infected with SIVmac251.3
Alternatively, repeated exposures could also induce potent antiviral immunity. Animals requiring multiple exposures for infection should then also have a greater degree of adaptive immunity, which should then result in delayed infections, with lower levels of systemic viremia due to increased adaptive viral immune control. Then again, macaques could have preexisting genetically based differences in infection resistance (e.g., TRIM-5α alleles4). Late infections due to genetic resistance should also result in lower viral loads because of the animal's natural ability to restrict viral replication before and after infection. These plausible infection scenarios, which may not be mutually exclusive, could be important considerations when using macaque S(H)IV infection models.
Several studies have examined whether repeated mucosal exposures, either vaginally or rectally, induce virus-directed immunity in macaques.5–10 Letvin et al. found little evidence of systemic or local cell-mediated immune responses or local humoral immune responses in uninfected rhesus macaques after repeated rectal virus exposures.6 We previously studied systemic T cell-produced cytokines and proliferation markers in SHIV-infected and -uninfected rhesus macaques after a series of low-dose rectal challenges.5 Despite transient immune activation, T cell activation or the presence of T cell-produced cytokines was not associated with infection, making it unlikely there was an immune response effect on virus acquisition.5 In a vaginal SHIVSF162p3 challenge repeated exposure study performed in pigtail macaques, Promadej-Lanier et al. did detect significant increases in mucosal cytokine levels and virus-specific T cell responses after exposures began.9 However, in the small number of placebo animals, this induction was not protective.9
In another pigtail macaque intravaginal study, Ma et al. also reported increases in virus-specific T cell responses, but these responses were detected just prior to the onset of systemic infection.7 It is possible that immune responses after vaginal or rectal exposures differ, which could differentially affect subsequent infections, as discussed by Abdulhaqq et al.3 In this present retrospective study of both rectal and vaginal repeated exposures, we analyze peak virus levels and viral load area under the curve (AUC) relative to the number of previous challenges before confirmed infection. These data provide an additional perspective, from a large cohort of both rhesus and pigtail macaques and from two different mucosal exposure routes, on the utility of the repeat low-dose virus challenge model using SHIVSF162p3 in nonhuman primates. The findings support the use of this model as a means to closely mimic human mucosal HIV transmission because there was no relationship between viremic control and increasing numbers of exposures to low-level virus inocula during rectal or vaginal challenges.
Adult male Indian rhesus macaques (Macaca mulatta) and female pigtail macaques (Macaca nemestrina) were purchased and housed at CDC in accordance with standards established in the Guide for the Care and Use of Laboratory Animals (published by the National Academy of Science, National Academy Press, Washington, D.C.). All procedures were approved by CDC's Institutional Animal Care and Use Committee. Rectal and vaginal virus exposures were administered with SHIVSF162p311 obtained from the NIH AIDS Research and Reference Reagent Program, expanded on either rhesus or pigtail peripheral blood mononuclear cells, as appropriate. Stocks were titrated in vivo and used at 10 or 50 TCID50 for rectal and vaginal exposures, respectively. Macaque infections and real-time polymerase chain reaction (PCR) measurements of viral loads were previously described.5,12–19 Real-time PCR viral load measurements across all studies were consistent in the use of the same primer/probe sequences targeting the virus core's gag region. Virus exposures and blood collection occurred once or twice per week, depending on respective study designs. Correlations between number of previous virus exposures before confirmed infection and log-transformed peak virus level were estimated using Pearson's correlation coefficient (rho, ρ). Predicted values from linear regression are displayed as lines on data scatterplot graphs (GraphPad Prism, La Jolla, CA; Fig. 1A–F).
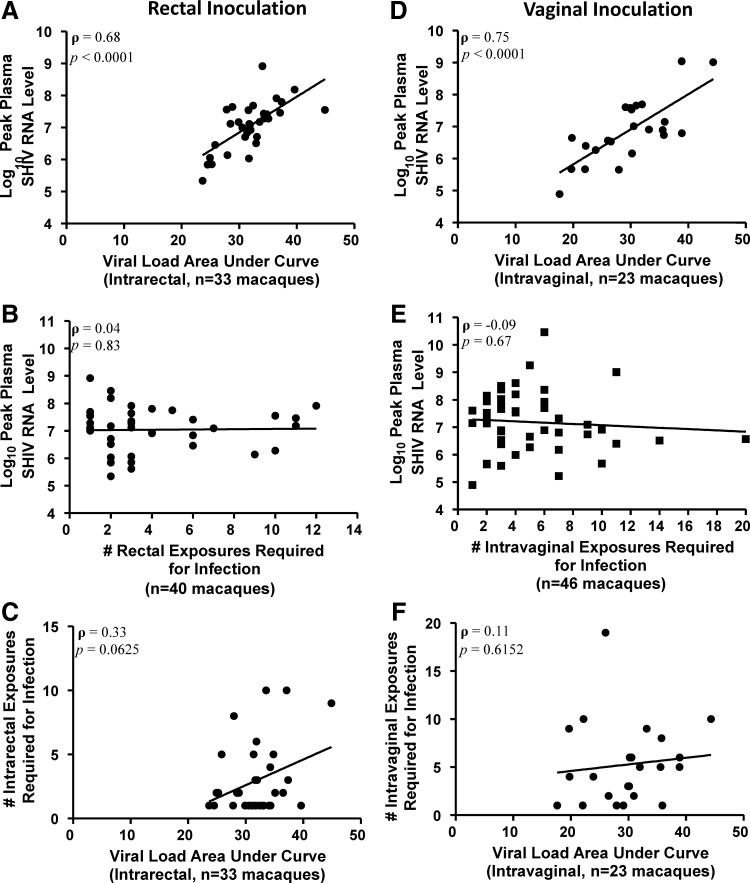
FIG. 1.
ρ (rho) indicates Pearson's correlation coefficient. (A) Scatter plot correlating peak viremia and viral load area under the curve (AUC) from a subset of available samples (n=33) of the rectally challenged rhesus macaque cohort (rho=0.68, p<0.0001). (B) Scatter plot showing lack of correlation between the number of low-dose rectal exposures (x-axis) and peak plasma SHIVSF162p3 RNA levels from the resulting infection (y-axis)(rho=0.04, p=0.83). (C) Scatter plot showing lack of correlation between exposure number and AUC in rectally challenged rhesus macaques (rho=0.33, p=0.06). (D) Scatter plot correlating peak viremia and viral load AUC from a subset of available samples (n=23) of the vaginally challenged pigtail macaque cohort (rho=0.75, p<0.0001). (E) Scatter plot showing no correlation between the number of low-dose vaginal exposures (x-axis) and peak plasma SHIVSF162p3 RNA levels from the resulting infection (y-axis) (rho=−0.09, p=0.67). (F) Scatter plot showing lack of correlation between exposure number and AUC in vaginally challenged pigtail macaques (rho=0.11, p=0.62).
To provide justification for the use of peak viral load as an early measure of immune control and surrogate marker of viral kinetics, we confirmed the correlation between peak viremia and viral load AUC for each cohort (Fig. 1A and D). AUC was calculated from selected longitudinal samples (1 and 2 weeks prepeak, peak viremia, and 4 weeks postpeak). The trapezoidal rule for numerical integration was used to approximate the area under the viral load curve. In addition to linear correlation, nonlinear relationships were evaluated by comparing peak viral load or AUC for each cumulative number of exposures relative to that for only one exposure, i.e., regression models with dummy-coded variables (data not shown).
We analyzed 40 rhesus macaques infected via rectal inoculation with SHIVSF162p3 (Fig. 1A–C). Analyzing a subset of data for animals with longitudinal viral load measurements (n=33), AUC strongly correlated with peak viremia [Pearson's correlation coefficient, rho (ρ)=0.68, p<0.0001; Fig. 1A], supporting the use of peak viremia as a surrogate for viremic control in the analyses. In these 40 rectally infected animals, peak plasma SHIV RNA levels ranged from 5.34 log10 to 8.92 log10 copies/ml. The mean peak virus level was 7.05±0.79 (SD) log10. In this collection of data from multiple studies, there was a median of three challenges prior to infection, with a range of 1 to 12. Peak plasma SHIV RNA levels were not correlated with number of exposures (Pearson's correlation coefficient=0.04; Fig. 1B), nor was a nonlinear relationship observed between virus challenges and peak viremia (data not shown). Similarly, viral load AUC was not correlated with exposure number (Pearson's correlation coefficient=0.33, Fig. 1C). These data suggest the number of rectal virus challenges does not affect the host's immune control of viremia, as measured by peak plasma SHIV RNA levels.
We also analyzed 46 pigtail macaques infected via vaginal inoculation with SHIVSF162p3 (Fig. 1D–F). For this cohort, complete data sets for viral load curve AUC analyses were available for n=23 pigtail macaques (Fig. 1D), and from this subset, AUC was strongly correlated with peak viremia (Pearson's correlation=0.75, p<0.0001; Fig. 1D), again supporting the use of peak viremia as a surrogate for viremic control in the analyses. Peak plasma SHIV RNA levels in the 46 vaginally infected macaques were similar to those of rectally infected macaques, ranging from 4.89 log10 to 10.46 log10 copies/ml and with a mean of 7.18±1.10 log10. The median number of challenges was 4, ranging between 1 and 20, and, as observed for the rectal rhesus cohort, peak viremia was not correlated with cumulative number of virus challenges (Pearson's correlation coefficient=−0.09; Fig. 1E), nor was a nonlinear relationship observed between these variables (data not shown). Furthermore, viral load AUC was not correlated with exposure number (Pearson's correlation coefficient=0.11; Fig. 1F). Similar to observations from rectally infected macaques, these data from vaginally infected macaques also suggest that increased numbers of repeated vaginal virus exposures do not influence viremic immune control postinfection.
These data provide further support for the use the SHIVSF162p3 repeated low-dose exposure model in nonhuman primate studies by providing assurance that an increasing number of exposures does not affect systemic control of the virus postinfection, examining both the linear and nonlinear relationships between virus levels/kinetics and cumulative numbers of exposures. These findings also corroborate Regoes's stochastic mathematical modeling of infection data from repeated exposure challenge experiments in macaques with SIV.20 In the event immune cells were activated or primed, it does not appear to lead to differences in infection susceptibility or control of viral replication.
Given the immunological dynamic milieu and tissue complexity of the female genital tract and previous findings by Promadej-Lanier et al.9 correlating postexposure Nef- and Rev-specific T cell responses with lower plasma viral loads, we hypothesized that repeated exposures via the vaginal route might correlate with systemic virus levels postinfection. However, in this study's larger cohort analysis, this association was not observed. We previously reported that repeated rectal exposures with SHIVSF162p3 were insufficient to induce long-lasting cellular immune responses.5 Also, Butler et al. examined the role of host restriction factor TRIM-5α/CypA in rectal SHIVSF162p3 infections and reported that the presence/absence of these alleles had no bearing on infection or viremic outcomes in rhesus macaques.13 Our current data add to these findings to indicate the number of exposures required for infection using this virus does not affect peak plasma virus levels, further characterizing the rectal repeat low-dose model.
Because we observed no correlation between peak viremia and number of exposures, a likely explanation is that infection kinetics is independent of exposure history. A limitation to this study is the use of peak viremia as a marker of viral immune control, which does not account for time-to-peak or other virus infection kinetics that could also inform questions of immune control. However, we also incorporated the use of viral load AUC (Fig. 1C–F) to better address the question of viral kinetics and support the use of peak viral load in the analyses. Viral set point data could also support analyses of viral kinetics, but adequate sampling time points were not available, and SHIVSF162P3 is rapidly controlled often to undetectable levels within approximately 12 weeks. We acknowledge that CD4+ T cell decline would also be an appropriate marker of immune control, but again, this was not assessed during the original macaque prevention studies. Although CD4+ T cell numbers are significantly reduced immediately following detection of plasma virus, with SHIVSF162p3 infection, this loss is transient and the cell levels soon rebound to preinfection levels and stabilize.21–23 Thus, we opted to focus analyses on peak viremia, an acute marker of infection and immune control, while acknowledging that the absolute peak virus level may not have been captured with the once or twice weekly sampling design used in the studies.
These studies did not assess differing virus doses. It is possible that challenging with a higher TCID50 may result in a greater degree of immune response priming or higher peak plasma viral loads, or that repeated exposures with lower concentrations of virus might have contributed to increased resistance to infection. Moreover, the use of a different challenge virus might also result in differing outcomes. Nonetheless, for research using the repeat low-dose model using SHIVSF162p3, these data ensure that both the vaginal and rectal models provide a reliable model system with which to perform SHIV/HIV transmission and biomedical prevention testing studies without concerns that early viral kinetics are affected by inoculation route or frequency of challenges required for infection.
Acknowledgments
The authors gratefully acknowledge the intellectual contributions of Dr. Sal Butera, as well as the previously published macaque infection work and contributions of the Preclinical Evaluation Team's animal procedure staff, James Mitchell, Elizabeth Sweeney, Shanon Ellis, Frank Deyounks, Lecreesia Jenkins, Kristen Kelley, David Garber, and others, without whom these studies would not have been possible. The authors also acknowledge the excellent laboratory support work of Debra Adams, Patricia Guenthner, Priya Srinivasan, Sunita Sharma, Mian-er Cong, and Rolieria West. A portion of the animal studies was funded by an intraagency agreement (Y1-A1-0681-02) between CDC and NIH. SHIVSF162P3 was obtained through the NIH AIDS Reagent Program, NIAID, NIH from Drs. Janet Harouse, Cecilia Cheng-Mayer, Ranajit Pal, and DAIDS/NIAID. The findings and conclusions in this article are those of the authors and do not necessarily represent the views of the CDC.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Fowke KR, Nagelkerke NJ, Kimani J, et al. : Resistance to HIV-1 infection among persistently seronegative prostitutes in Nairobi, Kenya. Lancet 1996;348(9038):1347–1351 [DOI] [PubMed] [Google Scholar]
- 2.Kaul R, Rowland-Jones SL, Kimani J, et al. : New insights into HIV-1 specific cytotoxic T-lymphocyte responses in exposed, persistently seronegative Kenyan sex workers. Immunol Lett 2001;79(1–2):3–13 [DOI] [PubMed] [Google Scholar]
- 3.Abdulhaqq SA, Martinez MI, Kang G, et al. : Serial cervicovaginal exposures with replication-deficient SIVsm induce higher dendritic cell (pDC) and CD4+ T-cell infiltrates not associated with prevention but a more severe SIVmac251 infection of rhesus macaques. J Acquir Immune Defic Syndr 2014;65(4):405–413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kirmaier A, Wu F, Newman RM, et al. : TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol 2010;8(8):e1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kersh EN, Luo W, Adams DR, et al. : Repeated rectal SHIVSF162P3 exposures do not consistently induce sustained T cell responses prior to systemic infection in the repeat-low dose preclinical macaque model. AIDS Res Hum Retroviruses 2009;25(9):905–917 [DOI] [PubMed] [Google Scholar]
- 6.Letvin NL, Rao SS, Dang V, et al. : No evidence for consistent virus-specific immunity in simian immunodeficiency virus-exposed, uninfected rhesus monkeys. J Virol 2007;81(22):12368–12374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ma ZM, Abel K, Rourke T, et al. : A period of transient viremia and occult infection precedes persistent viremia and antiviral immune responses during multiple low-dose intravaginal simian immunodeficiency virus inoculations. J Virol 2004;78(24):14048–14052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McDermott AB, Mitchen J, Piaskowski S, et al. : Repeated low-dose mucosal simian immunodeficiency virus SIVmac239 challenge results in the same viral and immunological kinetics as high-dose challenge: A model for the evaluation of vaccine efficacy in nonhuman primates. J Virol 2004;78(6):3140–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Promadej-Lanier N, Srinivasan P, Curtis K, et al. : Systemic and mucosal immunological responses during repeated mucosal SHIV(162P3) challenges prior to and following infection in pigtailed macaques. Virology 2008;375(2):492–503 [DOI] [PubMed] [Google Scholar]
- 10.Trivedi P, Horejsh D, Hinds SB, et al. : Intrarectal transmission of simian immunodeficiency virus in rhesus macaques: Selective amplification and host responses to transient or persistent viremia. J Virol 1996;70(10):6876–6883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harouse JM, Gettie A, Tan RC, et al. : Distinct pathogenic sequela in rhesus macaques infected with CCR5 or CXCR4 utilizing SHIVs. Science 1999;284(5415):816–819 [DOI] [PubMed] [Google Scholar]
- 12.Aidoo M, Otten RA, Rodriguez V, et al. : Absence of SHIV infection in gut and lymph node tissues in rhesus monkeys after repeated rectal challenges following HIV-1 DNA/MVA immunizations. Vaccine 2007;25(35):6474–6481 [DOI] [PubMed] [Google Scholar]
- 13.Butler K, Morgan JS, Hanson DL, et al. : Susceptibility to repeated, low-dose, rectal SHIVSF162P3 challenge is independent of TRIM5 genotype in rhesus macaques. AIDS Res Hum Retroviruses 2013;29(7):1091–1094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dobard C, Sharma S, Parikh UM, et al. : Postexposure protection of macaques from vaginal SHIV infection by topical integrase inhibitors. Sci Transl Med 2014;6(227):227ra235. [DOI] [PubMed] [Google Scholar]
- 15.Kersh EN, Luo W, Adams DR, et al. : Short communication: No evidence of occult SHIV infection as demonstrated by CD8+ cell depletion after chemoprophylaxis-induced protection from mucosal infection in rhesus macaques. AIDS Res Hum Retroviruses 2008;24(4):543–546 [DOI] [PubMed] [Google Scholar]
- 16.Otten RA, Adams DR, Kim CN, et al. : Multiple vaginal exposures to low doses of R5 simian-human immunodeficiency virus: Strategy to study HIV preclinical interventions in nonhuman primates. J Infect Dis 2005;191(2):164–173 [DOI] [PubMed] [Google Scholar]
- 17.Parikh UM, Dobard C, Sharma S, et al. : Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol 2009;83(20):10358–10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Radzio J, Aung W, Holder A, et al. : Prevention of vaginal SHIV transmission in macaques by a coitally-dependent Truvada regimen. PLoS One 2012;7(12):e50632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smith JM, Rastogi R, Teller RS, et al. : Intravaginal ring eluting tenofovir disoproxil fumarate completely protects macaques from multiple vaginal simian-HIV challenges. Proc Natl Acad Sci USA 2013;110(40):16145–16150 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Regoes RR: The role of exposure history on HIV acquisition: Insights from repeated low-dose challenge studies. PLoS Comput Biol 2012;8(11):e1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Harouse JM, Gettie A, Eshetu T, et al. : Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3). J Virol 2001;75(4):1990–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kersh EN, Luo W, Zheng Q, et al. : Reduced inflammation and CD4 loss in acute SHIV infection during oral pre-exposure prophylaxis. J Infect Dis 2012;206(5):770–779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pahar B, Wang X, Dufour J, et al. : Virus-specific T cell responses in macaques acutely infected with SHIV(sf162p3). Virology 2007;363(1):36–47 [DOI] [PMC free article] [PubMed] [Google Scholar]