Abstract
Significance: Heart disease is the primary cause of death in the industrialized world. Cardiac failure is dictated by an uncompensated reduction in the number of viable and fully functional cardiomyocytes. While current pharmacological therapies alleviate the symptoms associated with cardiac deterioration, heart transplantation remains the only therapy for advanced heart failure. Therefore, there is a pressing need for novel therapeutic modalities. Cell-based therapies involving cardiac stem cells (CSCs) constitute a promising emerging approach for the replenishment of the lost tissue and the restoration of cardiac contractility. Recent Advances: CSCs reside in the adult heart and govern myocardial homeostasis and repair after injury by producing new cardiomyocytes and vascular structures. In the last decade, different classes of immature cells expressing distinct stem cell markers have been identified and characterized in terms of their growth properties, differentiation potential, and regenerative ability. Phase I clinical trials, employing autologous CSCs in patients with ischemic cardiomyopathy, are being completed with encouraging results. Critical Issues: Accumulating evidence concerning the role of CSCs in heart regeneration imposes a reconsideration of the mechanisms of cardiac aging and the etiology of heart failure. Deciphering the molecular pathways that prevent activation of CSCs in their environment and understanding the processes that affect CSC survival and regenerative function with cardiac pathologies, commonly accompanied by alterations in redox conditions, are of great clinical importance. Future Directions: Further investigations of CSC biology may be translated into highly effective and novel therapeutic strategies aiming at the enhancement of the endogenous healing capacity of the diseased heart. Antioxid. Redox Signal. 21, 2002–2017.
Introduction
Experimental and clinical evidence of the regenerative potential of the adult human heart has accumulated over the years (11, 40, 65, 68, 79, 87, 123, 137, 159). The discovery of resident cardiac stem cells (CSCs) in the myocardium (9, 10, 56, 95, 99, 105, 107, 114, 135, 157, 159) elicited extensive investigations by multiple groups in an attempt to understand CSC biology, the mechanisms of cardiac repair, and the therapeutic implications of this process.
Stem cells are distinguished from more mature cells by their ability to differentiate into multiple specialized cell types and concurrently generate an identical stem cell following asymmetric division. This process may occur after long periods of inactivity, since stem cells replicate rarely. Stem cells are postulated to serve as an internal tissue repair system for the replenishment of damaged parenchymal cells.
Adult tissue-resident CSCs with the capacity to differentiate into cardiomyocytes, and vascular endothelial cells and smooth muscle cells have been identified in early 2000 (9, 10, 137, 157). When activated, CSCs proliferate and form lineage-committed progenitors, which will progressively acquire a fully mature phenotype, forming new contractile muscle and blood vessels for tissue oxygenation (1, 52, 79, 80). Preclinical studies have established that transplanted or resident CSCs have the potential to reduce the infarct size and improve cardiac function in a variety of animal models of myocardial injury (1, 40, 71, 90, 153). Thus, there is growing interest in the implementation of CSCs for the management of the diseased heart. Several laboratories have reported the identification of CSCs expressing distinct stem cell antigens. In this review, we will discuss the current understanding of the mechanisms controlling the activity of tissue-resident CSCs, the impact of changes in redox biology on CSC biology, and the possibility of using CSCs for the treatment of human heart failure.
Adult Resident CSC Populations
C-kit-positive CSCs
Beltrami et al. were the first to identify a unique population of cells in the myocardium that have the phenotypic appearance of primitive cells and express the stem cell marker, c-kit (10). Overall, these cells occur at a frequency of 1:30,000 myocardial cells. The expression of VEGF-receptor 2 (KDR) in the pool of c-kit-positive CSCs distinguishes myocyte progenitor cells (KDR negative) and vasculogenic progenitor cells (KDR positive), both of which can differentiate into cardiomyocytes, endothelial cells, and smooth muscle cells; however, myocyte progenitor cells have a greater propensity to produce cardiomyocytes, whereas vascular progenitors preferentially acquire vascular smooth muscle cell and endothelial cell fates (Fig. 1) (28). In the heart, a small fraction of c-kit-positive CSCs expresses the transcription factors Nkx2.5 and GATA-4, indicating their commitment to the myogenic lineage. Isolated c-kit-positive CSCs form in vitro endothelial cells, fibroblasts, smooth muscle cells, and cardiomyocytes, although spontaneously beating myocytes have not been demonstrated as yet (9, 10, 28). Upon transplantation into the damaged heart, c-kit-positive CSCs generate a large pool of functionally competent cardiomyocytes, resistance arterioles, and capillary profiles repairing, in part, the infarcted myocardium, reducing the infarct size, and attenuating ventricular remodeling (10, 27–29, 44, 46, 59, 120, 158).
FIG. 1.
Differentiation of vascular progenitor cells (VPCs) and myocyte progenitor cells (MPCs). (A) Human VPCs differentiated predominantly into endothelial cells (ECs) (von Willebrand factor [vWf ], yellow) and smooth muscle cells (SMCs) (α-smooth muscle actin [α-SMA], green), while MPCs formed mostly cardiomyocytes (α-sarcomeric actin [α-SA], red). (B) Percentage of VPCs and MPCs differentiating into ECs, SMCs, and myocytes. The EC phenotype was recognized based on the expression of the transcription factor Ets1 and the surface markers CD31 and vWf; the SMC phenotype was defined based on the presence of the transcription factor GATA-6, the membrane antigen TGF-β1 receptor (TGF-β1R), and the contractile protein α-SMA. The myocyte phenotype was identified based on the expression of the transcription factors Nkx2.5 and MEF2c, and the sarcomeric proteins α-cardiac actinin (α-CA) and α-SA. *p<0.05 versus ECs and SMCs.
Cardiac side population cells
Pfister and Liao selectively isolated cardiac side population (SP) cells, which are characterized by the efflux of Hoechst 33342 DNA-binding fluorescent dye due to the presence of ATP-binding cassette transporters, Abcg2 and MDR1 (114). SP cells account for 1.2% to 2% of adult myocardial cells. Abcg2 expression enhances proliferation and survival of SP cells, although inhibiting their differentiation (115). SP cells generally express the stem cell antigen-1 (Sca-1) and PECAM-1, also termed CD31. The minority of cardiac SP cells that are CD31-negative have been shown to be endowed with cardiomyogenic potential (114). Following culture, the expression of the cardiogenic transcription factors GATA-4 and MEF2c, and the sarcomeric proteins α-actinin and troponin I, is increased in SP cells lacking CD31. In coculture with adult cardiomyocytes, cardiac SP cells differentiate, develop organized sarcomeres, and efficiently contract (95, 109, 114, 153).
Sca-1-positive CSCs
Oh et al. demonstrated that Sca-1-positive cells with a phenotype distinct from hematopoietic lineages reside in the adult heart and are colocalized with small capillary vessels (105). In resting state, Sca-1-positive cells are uncommitted and do not express markers of cardiac or endothelial lineages; however, these cells are capable of adapting cardiomyogenic fate during embryogenesis and differentiate into myocytes after transplantation (47, 96, 97, 105, 161). In the myocardial interstitium, the population of Sca-1-expressing cells accounts for ∼2% of myocardial cells. Sca-1-positive cells express also CD29 (β1-integrin) and CD44 (hyaluronic acid receptor), but are negative for CD31, CD45 (pan-leukocyte marker), and c-kit. Of interest, cardiac SP cells are highly enriched for Sca-1 (102, 114), suggesting a relationship between Sca-1-positive cells and SP cells in the heart (32).
Cardiosphere-derived cells
Smith et al. introduced a methodology for the isolation and expansion of cells able to acquire the cardiomyocyte phenotype; they are derived from nonadherent multicellular clusters termed cardiospheres, which are obtained from endomyocardial biopsies (135). The cardiosphere-derived cells (CDCs) represent a heterogeneous cell population consisting of undifferentiated and committed cells, expressing c-kit and Sca-1; they are also positive for CD105 (endogelein), CD90 (Thy-1), and CD29 (β1-integrin), typical epitopes of mesenchymal stromal cells (MSCs). CDCs can differentiate into beating cardiomyocytes when cocultured with mature myocytes; vascular smooth muscle cells and endothelial cells can also derive from differentiation of CDCs (99, 146). Other putative stem cell classes have been described in the heart, including bone marrow-derived hematopoietic cells (87, 108), MSCs (57, 147), and epicardial cells (16). The cardiomyogenic potential and clinical significance of these cells are discussed in (34, 58, 71, 98, 123, 124, 142, 162).
The Role of CSCs in Heart Homeostasis and Injury
The maintenance of cardiac function requires the continuous replacement of lost cardiomyocytes (1, 80). The magnitude of myocyte renewal in the adult heart and the decline in the endogenous regenerative capacity of the aging myocardium are currently the subject of intense debate (11, 65, 68, 140). Additionally, there is no agreement concerning the origin of newly formed myocytes: dedifferentiation and proliferation of pre-existing myocytes (126) have been proposed, and transdifferentiation of cells derived from the epicardium (134) or from extracardiac origins (67, 118) has been described. In this review, we will focus on the critical role of resident CSCs in the control of the development, postnatal growth, and aging of the heart. Accumulating evidence indicates that a direct lineage relationship exists between CSCs and cardiomyogenesis. Similarly, the generation of the various classes of the coronary circulation via activation and lineage specification of CSCs has repeatedly been shown.
Embryonic and fetal hearts contain a defined population of uncommitted c-kit-positive CSCs, which regulate the growth and maturation of the prenatal organ and generate myocytes upon transplantation (38). In addition, CDCs from a neonatal heart include a high number of undifferentiated cells that express c-kit or Sca-1, and have strong regenerative ability in a model of myocardial infarction (132). Mathematical modeling predicts that the size and growth properties of the c-kit-positive population in the course of embryogenesis account for the number of myocytes present in the ventricle of a fully formed neonatal heart (38).
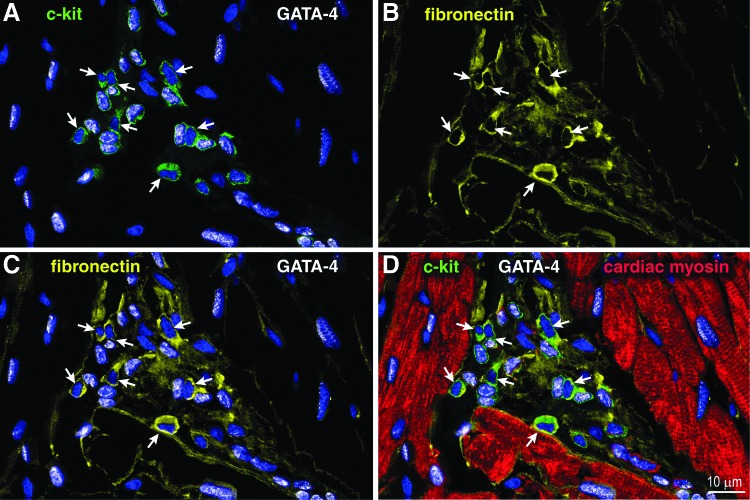
CSCs and early lineage-committed cells are nested in specialized microdomains of the myocardial interstitium defined as stem cell niches (156) (Fig. 2). Myocyte progenitors within the niches express c-kit and the myocyte-specific transcription factors GATA-4, Nkx2.5, or MEF2c. The more differentiated myocyte precursors retain the stem cell antigen and contain the contractile proteins sarcomeric α-actinin and troponin. With further maturation, amplifying myocytes are formed; they lack c-kit and have the ability to divide and concurrently differentiate until the adult cell phenotype is acquired.
FIG. 2.
Cellular and extracellular components of cardiac niches. Apical niche containing 23 c-kit-positive (A, D: green) cardiac stem cells (CSCs). The distribution of fibronectin (B–D: yellow) within the niche is shown. Fibronectin is present throughout the niche and accumulates in close proximity of 7 c-kit-positive GATA-4-negative CSCs (arrows). GATA-4 is illustrated in white in (A, C), and (D). Sixteen c-kit-positive cells express GATA-4 in their nuclei.
The activation of endogenous CSCs, accompanied by proliferation and differentiation, occurs in humans in response to injury, including myocardial infarction and chronic aortic stenosis (10, 157, 159). Myocardial regeneration mediated by growth and commitment of multipotent CSCs has been shown in human heart failure of ischemic and nonischemic origin (64, 158). Experimentally, genetic tagging of c-kit-positive CSCs has established that, following delivery into the damaged myocardium, a single human CSC is capable of creating multilineage progeny consisting of myocytes, vascular smooth muscle cells, and endothelial cells (51). In the settings of xenotransplantation when human CSCs are administered to an infarcted rodent heart, the donor cells generate a band of new human myocardium that replaces the acutely infarcted portion of the left ventricular wall. As a result of this intervention, myocardial recovery is dramatically improved (10, 27, 29, 44, 53, 62, 84, 96, 101, 120). The human origin of the regenerated tissue has been documented by the presence of human-specific Alu sequences in the DNA of the newly formed myocytes (Fig. 3). Independent studies by numerous groups have confirmed that with acute ischemia, the intramyocardial administration or intracoronary delivery of CSCs leads to tissue regeneration due to successful homing of the donor cells to the site of damage, their differentiation into cardiac lineages, and functional coupling with pre-existing myocytes and coronary vessels [for recent review, see Refs. (1, 30, 75, 79, 80, 124)].
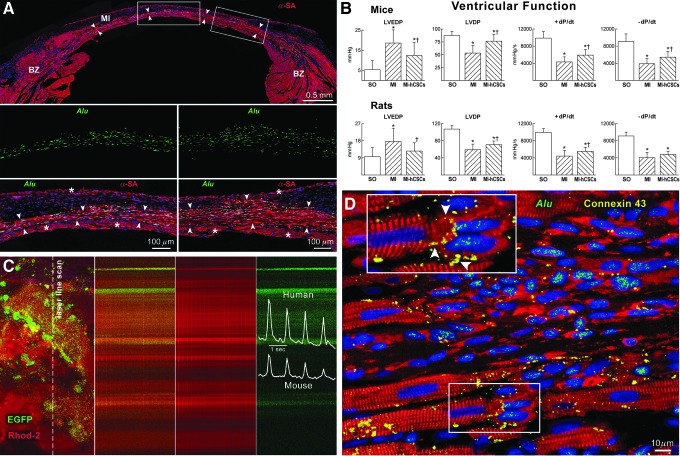
FIG. 3.
Human CSCs (hCSCs) regenerate infarcted myocardium. Myocardial infarction (MI) was produced in anesthetized female immunodeficient Scid mice and Fischer 344 rats treated with a standard immunosuppressive regimen. Shortly after coronary occlusion, two injections of ∼40,000 hCSCs were made at the opposite sites of the border zone. Animals were killed 5–21 days after surgery. (A) Mouse heart 21 days after infarction and injection of hCSCs. Human myocardium (arrowheads) is present within the infarct (MI). BZ, border zone. Areas in rectangles are shown at higher magnification below. Human myocytes are α-SA (red) positive and Alu (green) positive. Asterisks indicate spared myocytes. (B) Ventricular hemodynamics was measured in sham-operated (SO), untreated infarcted (MI), and hCSC-treated infarcted animals (MI-hCSCs). Results are mean±SD. * and † p<0.05, versus SO and MI, respectively. (C) Calcium transient in EGFP-positive human myocytes and EGFP-negative mouse myocytes recorded by two-photon microscopy and laser line-scan imaging (calcium indicator Rhod-2, red). (D) Myocardial regeneration at 3 weeks. Connexin 43 (yellow; arrowheads in the inset) is present between human myocytes (α-SA, red; Alu, green) and spared rat myocytes (α-SA, red; Alu negative).
In analogy with other tissues, physiological aging and diseases negatively impact on the stem cell compartment of the heart (2, 5, 33, 63, 68, 80, 104, 129, 131, 138, 148). The population of CSCs in the failing myocardium displays characteristics of cellular senescence (18, 21, 31, 116), an impaired capacity to migrate to the areas of need (45, 46), and reduced differentiation ability (P.G., unpublished observations). The myocyte progeny derived from old CSCs rapidly acquires a senescent phenotype characterized by depressed contractile ability and a shorter lifespan (21, 46, 63, 68, 148, 159). The decline in CSC function with age may condition the deterioration in the structural integrity of the myocardium, leading to heart failure. Indeed, in rodent models of cardiac aging or chemotherapy-induced cardiomyopathy, transplantation of the CSCs from young hearts results in enhanced cardiomyogenesis and ameliorates the performance of the senescent hearts (31, 46).
Molecular Mechanisms That Regulate CSC Growth and Activation
Despite their high potential for myocardial regeneration, the activity of CSCs remains inadequate in the setting of extensive damage (1). Importantly, a subset of CSCs with preserved growth reserve persists in very old and sick hearts, and can be isolated for further propagation and clinical applications (13, 28, 63, 68). Significant effort has been applied to the understanding of the factors that limit the endogenous regenerative capacity of CSCs and the pathways that potentiate CSC growth and lineage specification.
CSCs reside in myocardial niches, in which parenchymal cells, for example, myocytes, serve to sustain their function. CSCs are structurally connected to the supporting cells via cadherin-mediated adherence junctions and connexin-mediated gap junctions (156) (Fig. 4). This intercellular communication affects multiple aspects of CSC behavior, including proliferation and self-renewal, survival in stressful conditions, migration from the niches, and differentiation. For example, activation of the Notch receptor by the Jagged1 ligand, which is expressed on cardiomyocytes, stimulates CSC division and drives myocyte lineage commitment. This signaling mechanism sustains the formation of a pool of transiently amplifying immature myocytes (14). If the Notch pathway is inhibited, myocardial repair is perturbed (Fig. 5). Pharmacological ablation of the Notch activity in the first week of postnatal life causes a dilated cardiomyopathy, underscoring the importance of Notch signaling for heart growth and myocyte formation (155).
FIG. 4.
Cardiac niches and supporting cells. Within the niches, CSCs are structurally and functionally connected to the supporting cells, cardiomyocytes, and fibroblasts, by gap and adherens junctions. Connexin 43 and 45 and N- and E-cadherin in atrial niches containing c-kit-positive CSCs (green) are represented by yellow dots located between two CSCs (arrowheads), a CSC and a fibroblast (procollagen, white; arrows), and a CSC and a myocyte (α-SA, red; double arrowheads). Areas in the rectangles are shown at higher magnification in the insets.
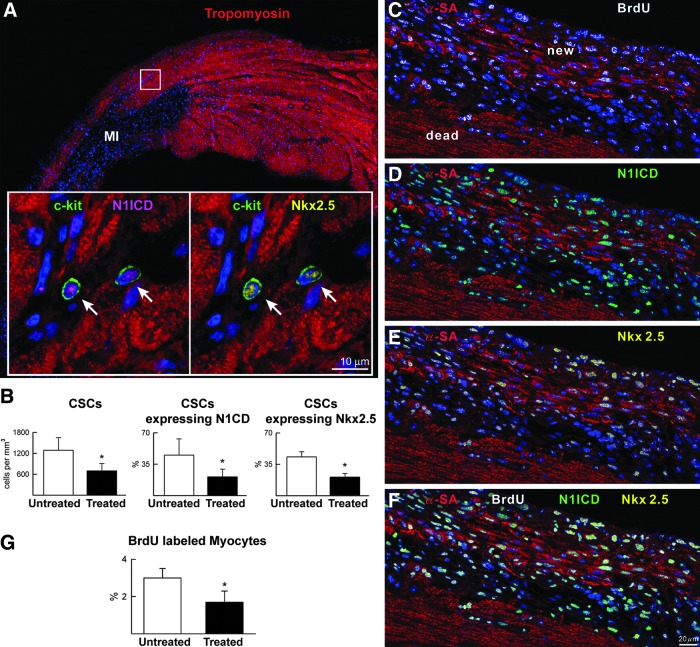
FIG. 5.
Myocardial regeneration and Notch1 function in vivo. Regenerative response of infarcted mice injected with a γ-secretase inhibitor (treated) or vehicle (untreated). (A) Border zone of an untreated infarct in which the area included in rectangles is shown at higher magnification in the insets. CSCs (c-kit, green) express activated Notch1 intracellular domain (N1ICD: magenta, arrows) and Nkx2.5 (yellow). Cardiomyocytes: tropomyosin, red. (B) Number of CSCs and percentage of CSCs positive for N1ICD and Nkx2.5 in the border zone of untreated and treated infarcts. *p<0.05 versus untreated. (C–F) Area of regeneration (new) located within the infarct (dead). New myocytes are BrdU positive (C), N1ICD positive (D), and Nkx2.5 positive (E). (F) Merge of (C–E). (G) γ-secretase inhibition decreases the fraction of BrdU-labeled myocytes. *p<0.05 versus untreated.
An additional example of the direct role of myocytes in the control of CSC fate has been described in a recent study, which suggests that microRNAs, specifically miR-499, are actively transported via gap junctions from mature myocytes into CSCs, promoting their commitment and differentiation (53).
The potential contribution of MSCs to cardiac repair has also been postulated [reviewed in Refs. (15, 162)]. Although it has not been established whether cardiac-resident MSCs act as supporting cells within myocardial niches, transplanted bone marrow-derived MSCs successfully engraft in the heart after infarction, connect with the resident progenitors, and stimulate proliferation and myogenic commitment of endogenous c-kit-positive CSCs (49).
Cell-to-cell contacts are favorable for CSCs; cardiac SP cells in three-dimensional cell aggregates are protected from cell death induced by oxidative stress and anoxia/reoxygenation. This approach improves stem cell survival after transplantation in the hostile environment of the infarcted myocardium (6).
The components of the extracellular matrix (ECM) regulate multiple cellular processes by direct binding to the cells via specific receptors, by providing mechanical stimuli, or by modulating the signaling pathways triggered by several growth factors (8). In the myocardial niches, undifferentiated CSCs express the ECM receptor α4-integrin (CD49d, VLA-4) (156), which mediates retention and mobilization of stem cells in the hematopoietic system (96, 111). Recently, it was demonstrated that after myocardial infarction, an accumulation of the ECM component fibronectin is necessary for CSC expansion and tissue regeneration (74). Moreover, CSCs produce the ECM proteins in an autocrine fashion and the profile of the secreted ECM changes in the course of myogenic differentiation (7, 156). The ex vivo expansion of CDCs enhances the expression of the ECM and adhesion molecules, which have a beneficial effect on the function of CDCs after delivery to the injured heart (83).
One of the major regulators of CSC growth is Wnt signaling. The canonical Wnt pathway, through a downstream effector IGFBP3, limits the renewal of adult cardiac SP cells, blocks endogenous regeneration, and impairs cardiac performance (107). In contrast, Wnt/β-catenin is upregulated in c-kit-positive CSCs that are induced to proliferate in vivo by stem cell factor (SCF) secreted by cardiomyocytes (166). The SCF activates c-kit receptor. Gene transfer of SCF into myocytes promotes the expansion of the c-kit CSC compartment in the mouse heart after infarction and increases the recruitment of these cells to the injured area (166).
In addition to SCF, insulin-like growth factors (IGF) -1 and -2 have been shown to critically affect CSC behavior. IGF-1 signaling activates nuclear phospho-Akt and telomerase activity, diminishes oxidative stress, and delays CSC aging and death (148). Moreover, the expression of the IGF-1 receptor distinguishes a pool of phenotypically young CSCs, characterized by longer telomeres, a higher telomerase activity, enhanced proliferation, and higher resistance to apoptosis (27, 89). In an autocrine and a paracrine fashion, IGF-2 potentiates the myogenic differentiation of CSCs (27). Conversely, CSCs that present the IGF-2 receptor, rather than the IGF-1 receptor, and coexpress the angiotensin II receptor type 1, exhibit impaired growth and a high rate of cell death. The activation of IGF signaling in CSCs by pretreatment with the growth factor before transplantation or by in vivo administration using self-assembling peptide nanofibers facilitates cardiomyogenesis and vasculogenesis, ameliorating cardiac recovery after infarction (27, 110). Notably, IGF-1 can induce the expression of a downstream target of Akt kinase, named Pim-1 [for review Ref. (43)], which is an important regulator of CSC proliferation, differentiation, and survival in the infarcted myocardium (25, 26, 101, 151).
Abcg2 and MDR1 are known to contribute to multidrug resistance and play a role in protecting the CSCs from toxins. Cardiac SP cells from Abcg2 knockout mice show augmented cell death and reduced proliferation ability, whereas Abcg2 overexpression enhances CSC growth, but limits their myogenic differentiation (113). Additionally, Abcg2 controls the fate of CSCs, favoring symmetric cell division and stem cell renewal (127). Of interest, oscillations in Ca2+ levels in c-kit-positive CSCs similarly affect the cell cycle progression and dictate the pattern of stem cell proliferation. The spontaneous rises in intracellular Ca2+, which are generated by IP3 receptor activation, condition the asymmetrical stem cell division potentiating myocyte lineage specification (38, 39).
The mobilization of CSCs to the site of injury is of paramount importance for their regenerative potential. Multiple factors affect CSC trafficking in the myocardium. For instance, the cytokine SDF-1 (CXCL12) is upregulated in the ischemic tissue (3, 17). SDF-1 activates the CXCR4 receptor, and this axis stimulates the migration of cardiac SP cells, Sca-1-positive cells, and CDCs (86, 146). Hepatocyte growth factor/scatter factor (HGF/SF) operates by binding to a high-affinity receptor c-Met. HGF has been shown to accumulate in the myocardium after infarction, and the expression of c-Met is controlled by hypoxia (17, 152). C-kit-positive CSCs contain functional c-Met and are mobilized to the infarcted region by the intramyocardial administration of HGF (89, 158), facilitating cardiac regeneration (46, 120). In addition to cytokines, the interaction of CSCs with cardiomyocytes controls cell translocation. The cell-guidance receptor EphA2 is preferentially expressed in c-kit-positive CSCs, whereas one of its ligands, ephrin A1, is presented in a complimentary fashion on cardiomyocytes (44). Intramyocardial administration of ephrin A1 promotes the mobilization of resident CSCs (44) and ameliorates heart recovery after myocardial infarction (36). EphA2 activation by ephrin A1 increases the chemotaxis of CSCs to HGF, improving stem cell recruitment and cardiac repair after infarction (44).
Redox Control of CSC Function
Reactive oxygen and nitrogen species (ROS) such as superoxide anion (O2•−) and hydrogen peroxide (H2O2), are highly chemically reactive by-products of aerobic metabolism. The major sources of ROS in cells are mitochondria and NADPH oxidase (4). Balanced ROS production and sequestration are necessary for proper cell responses to environmental stimuli, including activation of the signaling by growth factor receptors, regulation of cell adhesion, and differentiation [recently reviewed in Refs. (41, 73, 122)]. On the other hand, it is generally accepted that an excessive ROS level is harmful to cells, since it produces DNA damage, lipid and carbohydrate peroxidation, and protein oxidation and inactivation, leading to cellular senescence or death (42, 73, 128, 136). In pathological conditions, for example, atherosclerosis, heart failure, hypertension, diabetes, and aging, ROS create an inflammatory and oxidative microenvironment (167). Mitochondrial ROS emission is a major contributor to the overall oxidizing environment in cardiovascular diseases and heart failure. It is now emerging that the monoamine oxidase activity augments mitochondrial ROS generation and exacerbates heart dysfunction in the state of chronic stress (69–71).
In CSCs, oxidative stress has been shown to induce DNA lesions, upregulation of p53 and p16INK4a, telomeric shortening, and death of stem and progenitor cells (Fig. 6). For example, diabetic hearts are characterized by a marked reduction in CSC abundance. High glucose promotes ROS accumulation, which causes CSC dysfunction in a p66shc-dependent manner (119), as well as inhibition of the pentose phosphate and prosurvival signaling pathways, leading to cell apoptosis (72). Similarly, doxorubicin-induced cardiotoxicity is related to oxidative stress and is accompanied by accelerated apoptosis and premature senescence of CSCs (31, 45, 54, 116). This impairs the regenerative capacity of the heart following antineoplastic treatments.
FIG. 6.
Heart failure and CSC senescence. Shown are control myocardium (A), acute infarcts (C, E), and chronic infarcts (B, D, and F). c-kit-positive CSCs (green; arrows, A–F) express p16INK4a (yellow, A and B) and p53 (magenta, C and D). The detection of telomeres in c-kit-positive CSCs is shown by small white fluorescence dots (E and F).
Of interest, myocardial aging is associated with an increase in CSC numbers (68); however, CSCs in old hearts display signs of cellular senescence and an inferior growth and differentiation ability (18, 21, 46, 63, 66, 148). This is in accordance with numerous studies demonstrating that stem cell exhaustion due to extensive replication conditions organ aging [for review, see Refs. (2, 60, 130, 131)]. Surprisingly, a residual compartment of CSCs with preserved growth reserve is detectable in the senescent heart (18, 46, 65); yet, this stem cell population is idle and does not adequately contribute to myocardial repair. One of the most intriguing aspects of ROS function is the control of stem cell quiescence. Recent findings in the aging heart document an accumulation of quiescent CSCs, which persist in a hypoxic state due to alterations in their microenvironment and changes in metabolism (121). Although these CSCs possess longer telomeres and a significant proliferative ability, the low level of cellular ROS precludes re-entry into the cell cycle and new myocyte formation. Similarly, quiescent hematopoietic stem cells with the highest potential to reconstitute the circulating blood cells are found in the hypoxic microenvironment of the bone marrow (106, 112). The low endogenous amount of ROS is correlated with hematopoietic stem cell quiescence and the preservation of the undifferentiated phenotype (61, 73, 103). The increase in cellular ROS, specifically, H2O2, has been shown to trigger hematopoietic stem cell proliferation, differentiation, and migration (20, 81, 94, 117). For instance, after ischemic injury, accelerated ROS production is required to modulate the activity of HIF-1α, a key regulator of the intracellular response to hypoxia that induces hematopoietic stem cell expansion in the bone marrow and their mobilization into the circulation (17, 154). In agreement, experimental hyperoxia produces changes in the redox state of CSCs residing in the old myocardium, promoting their activation and ameliorating the aging heart phenotype (121).
In this regard, if the rise in cellular ROS is not well adjusted, as in the case of the genetic ablation of the ATM or FoxO proteins, the self-renewal capacity and differentiation pattern of hematopoietic stem cells are severely compromised (55, 100, 149, 164, 165). In a similar manner, the lack of expression of a transcription factor Prdm16 causes oxidative stress, interfering with the maintenance and function of the neural stem cell compartment (23). Importantly, as discussed above, uncontrolled generation of ROS by mitochondria and a depressed antioxidant defense are among the major contributors to the overall oxidizing environment in cardiac pathologies and heart failure (19, 139, 150).
In contrast to these findings, an increased oxidative status promotes activity of neuroepithelial stem cells in the central nervous system (76). Likewise, oxidation favors self-renewal and division of cells that generate neurons and hippocampal cells (88).
Changes in cellular redox state, due to a decrease in ROS generation by mitochondria, are implicated in cardiomyogenic differentiation during development (50). Conversely, models of ex vivo differentiation suggest that mitochondrial oxidative metabolism and high ROS formation facilitate the acquisition of the cardiac phenotype by stem cells (24, 35, 77, 82). Recently, it has been shown that HIF-1α links redox pathways and cellular metabolism (133, 144, 145). Thus, physiologic induction of ROS in CSCs may be regulated by cell-intrinsic mechanisms, including cell energetics, the metabolic state, and the microenvironment (73, 141, 160). In cardiac and embryonic stem cells, the concept of oxidative optimum for the control of genetic stability has been proposed (85), implying that ROS production is tightly regulated to promote cell proliferation, differentiation, and survival; the adequate ROS level for these processes may depend on the cellular context.
It is important to indicate that nitrogen oxide (NO) plays a central role in cardiovascular physiology, and the interplay between NO and ROS, that is, the nitroso/redox balance, is a key regulator of cardiovascular homeostasis [for comprehensive review, see Refs. (48, 125)]. Future research may help to identify the impact of NO and ROS on CSC behavior and the potential therapeutic targets for the restoration of stem cell function in the pathologic heart.
Current CSC Therapies for Heart Diseases
The recognition that CSCs govern cardiac homeostasis and are endowed with regenerative capacity led to the development of stem cell-based therapies for myocardial repair. Numerous cell types have been introduced clinically in an attempt to restore cardiac structure and ameliorate heart function in patients. The results of the clinical trials with stem cells from extracardiac origins, such as circulating CD34-positive cells and bone marrow-derived MSCs, are extensively discussed elsewhere (58, 123, 124, 142, 162).
The therapeutic efficacy of resident c-kit-positive CSCs was recently assessed in the phase I trial Cardiac Stem Cells in Patients with Ischemic Cardiomyopathy (SCIPIO) (12). In this trial, c-kit-positive cells were obtained from the right atrial appendage of patients for autologous transplantation. The isolated CSCs were successfully expanded ex vivo and lacked the markers of lineage commitment, which reflects the features of primitive stem cells. The infusion of CSCs in the SCIPIO trial produced no adverse effects and exerted a lasting beneficial impact on the cardiac performance and quality of life in the patient population (22). Another therapeutic approach, which was employed in the Cardiosphere-Derived Autologous Stem Cells to Reverse Ventricular Dysfunction (CADECEUS) proof-of-concept clinical trial (92), involved the injection of CDCs that were expanded from endomyocardial biopsies. Although this trial failed to detect an improvement in cardiac function, a reduction in the size of scar tissue and an increase in the viable myocardium were observed in treated patients. Additionally, in a small-scale preliminary trial Autologous Human Cardiac-Derived Stem Cell To Treat Ischemic Cardiomyopathy (ALCADIA), the administration of undifferentiated cardiac cells together with a controlled-release formulation of basic fibroblast growth factor was evaluated in patients with ischemic cardiomyopathy (143). The findings from these clinical trials confirm the feasibility and safety of autologous CSC transplantation, and open an avenue for further efforts to harness the regenerative capacity of stem cells for cardiovascular therapies.
Innovation
There are significant clinical and experimental evidences demonstrating that the heart can heal itself, although to a limited extent. The repair process requires the activity of cardiac stem cells (CSCs) residing in the adult myocardium. Different classes of CSCs have been described and recently employed in clinical trials for the treatment of heart diseases, producing encouraging results. Advances in the understanding of CSC biology indicate that cell-autonomous and environmental changes in the redox status also control CSC growth, survival, differentiation and, ultimately, regenerative potential. Thus, establishing approaches to modulate the redox signaling in the heart have important therapeutic implications.
Future Directions
Although considerable strides have been made in the course of the past decade to gain insight on the biology of CSCs, there are important aspects of CSC function that remain to be investigated. The molecular mechanisms leading to a decline in CSC regenerative potential with age constitute a fundamental question pertaining to the etiology of the aging myopathy and heart failure. Conceivably, an increasingly pro-oxidizing environment of the old myocardium impedes the CSC activity. Furthermore, in view of the potential implementation of CSCs for autologous transplantation in the elderly, the age-associated decrease in stem cell potency may significantly limit their therapeutic efficacy. Similarly, it is unclear why the endogenous regenerative reserve is inadequate to repair an extensive damage, which leads to myocardial scarring and progressive deterioration in the structural integrity of the heart. Thus, therapeutic strategies able to enhance the function of resident CSCs may prove to be required to interfere with the aging myopathy and heart failure in the old patient population.
It is equally important to study the factors that affect the efficacy of CSC transplantation. Approaches are to be developed to optimize cell preparation and routes of delivery, which will ensure a better survival of donor CSCs, adequate engraftment, and successful differentiation into mechanically efficient myocytes and integrated coronary vessels. Tissue engineering and combination therapies consisting of cotransplantation of multiple cell types, or CSC preconditioning with various factors are discussed in the literature and examined in preclinical settings (58, 71, 78, 93, 98, 163). To this end, treatments with antioxidants to prevent CSC senescence and death (37, 91), or establishing the tools that modulate the redox status of CSCs and thereby their activation and differentiation have significant therapeutic potential.
Abbreviations Used
- α-CA
α-cardiac actinin
- α-SA
α-sarcomeric actin
- α-SMA
α-smooth muscle actin
- CDCs
cardiosphere-derived cells
- CSCs
cardiac stem cells
- ECs
endothelial cells
- ECM
extracellular matrix
- H2O2
hydrogen peroxide
- hCSCs
human cardiac stem cells
- HGF/SF
hepatocyte growth factor/scatter factor
- IGF
insulin-like growth factor
- MI
myocardial infarction
- MPCs
myocyte progenitor cells
- MSCs
mesenchymal stroma cells
- NICD
Notch1 intracellular domain
- NO
nitric oxide
- ROS
reactive oxygen species
- Sca-1
stem cell antigen-1
- SCF
stem cell factor
- SMCs
smooth muscle cells
- SP
side population
- SO
sham-operated
- TGF-β1R
TGF-β1 receptor
- VPCs
vascular progenitor cells
- vWf
von Willebrand factor
Acknowledgment
The research in the authors' laboratories was supported by National Institutes of Health.
References
- 1.Anversa P, Kajstura J, Rota M, and Leri A. Regenerating new heart with stem cells. J Clin Invest 123: 62–70, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 2.Anversa P, Rota M, Urbanek K, Hosoda T, Sonnenblick EH, Leri A, Kajstura J, and Bolli R. Myocardial aging—a stem cell problem. Basic Res Cardiol 100: 482–493, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Askari AT, Unzek S, Popovic ZB, Goldman CK, Forudi F, Kiedrowski M, Rovner A, Ellis SG, Thomas JD, DiCorleto PE, Topol EJ, and Penn MS. Effect of stromal-cell-derived factor 1 on stem-cell homing and tissue regeneration in ischaemic cardiomyopathy. Lancet 362: 697–703, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Bae YS, Oh H, Rhee SG, and Yoo YD. Regulation of reactive oxygen species generation in cell signaling. Mol Cells 32: 491–509, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ballard VL. Stem cells for heart failure in the aging heart. Heart Fail Rev 15: 447–456, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Bauer M, Kang L, Qiu Y, Wu J, Peng M, Chen HH, Camci-Unal G, Bayomy AF, Sosnovik DE, Khademhosseini A, and Liao R. Adult cardiac progenitor cell aggregates exhibit survival benefit both in vitro and in vivo. PLoS One 7: e50491, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bax NA, van Marion MH, Shah B, Goumans MJ, Bouten CV, and van der Schaft DW. Matrix production and remodeling capacity of cardiomyocyte progenitor cells during in vitro differentiation. J Mol Cell Cardiol 53: 497–508, 2012 [DOI] [PubMed] [Google Scholar]
- 8.Bayomy AF, Bauer M, Qiu Y, and Liao R. Regeneration in heart disease—is ECM the key? Life Sci 91: 823–827, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bearzi C, Rota M, Hosoda T, Tillmanns J, Nascimbene A, De Angelis A, Yasuzawa-Amano S, Trofimova I, Siggins RW, Lecapitaine N, Cascapera S, Beltrami AP, D'Alessandro DA, Zias E, Quaini F, Urbanek K, Michler RE, Bolli R, Kajstura J, Leri A, and Anversa P. Human cardiac stem cells. Proc Natl Acad Sci U S A 104: 14068–14073, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Beltrami AP, Barlucchi L, Torella D, Baker M, Limana F, Chimenti S, Kasahara H, Rota M, Musso E, Urbanek K, Leri A, Kajstura J, Nadal-Ginard B, and Anversa P. Adult cardiac stem cells are multipotent and support myocardial regeneration. Cell 114: 763–776, 2003 [DOI] [PubMed] [Google Scholar]
- 11.Bergmann O, Bhardwaj RD, Bernard S, Zdunek S, Barnabe-Heider F, Walsh S, Zupicich J, Alkass K, Buchholz BA, Druid H, Jovinge S, and Frisen J. Evidence for cardiomyocyte renewal in humans. Science 324: 98–102, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bolli R, Chugh AR, D'Amario D, Loughran JH, Stoddard MF, Ikram S, Beache GM, Wagner SG, Leri A, Hosoda T, Sanada F, Elmore JB, Goichberg P, Cappetta D, Solankhi NK, Fahsah I, Rokosh DG, Slaughter MS, Kajstura J, and Anversa P. Cardiac stem cells in patients with ischaemic cardiomyopathy (SCIPIO): initial results of a randomised phase 1 trial. Lancet 378: 1847–1857, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 13.Bolli R, Tang XL, Sanganalmath SK, Rimoldi O, Mosna F, Abdel-Latif A, Jneid H, Rota M, Leri A, and Kajstura J. Intracoronary delivery of autologous cardiac stem cells improves cardiac function in a porcine model of chronic ischemic cardiomyopathy. Circulation 128: 122–131, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boni A, Urbanek K, Nascimbene A, Hosoda T, Zheng H, Delucchi F, Amano K, Gonzalez A, Vitale S, Ojaimi C, Rizzi R, Bolli R, Yutzey KE, Rota M, Kajstura J, Anversa P, and Leri A. Notch1 regulates the fate of cardiac progenitor cells. Proc Natl Acad Sci U S A 105: 15529–15534, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 15.Boyle AJ, McNiece IK, and Hare JM. Mesenchymal stem cell therapy for cardiac repair. Methods Mol Biol 660: 65–84, 2010 [DOI] [PubMed] [Google Scholar]
- 16.Cai CL, Martin JC, Sun Y, Cui L, Wang L, Ouyang K, Yang L, Bu L, Liang X, Zhang X, Stallcup WB, Denton CP, McCulloch A, Chen J, and Evans SM. A myocardial lineage derives from Tbx18 epicardial cells. Nature 454: 104–108, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ceradini DJ, Kulkarni AR, Callaghan MJ, Tepper OM, Bastidas N, Kleinman ME, Capla JM, Galiano RD, Levine JP, and Gurtner GC. Progenitor cell trafficking is regulated by hypoxic gradients through HIF-1 induction of SDF-1. Nat Med 10: 858–864, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Cesselli D, Beltrami AP, D'Aurizio F, Marcon P, Bergamin N, Toffoletto B, Pandolfi M, Puppato E, Marino L, Signore S, Livi U, Verardo R, Piazza S, Marchionni L, Fiorini C, Schneider C, Hosoda T, Rota M, Kajstura J, Anversa P, Beltrami CA, and Leri A. Effects of age and heart failure on human cardiac stem cell function. Am J Pathol 179: 349–366, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen K. and Keaney JF, Jr. Evolving concepts of oxidative stress and reactive oxygen species in cardiovascular disease. Curr Atheroscler Rep 14: 476–483, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chen ML, Logan TD, Hochberg ML, Shelat SG, Yu X, Wilding GE, Tan W, Kujoth GC, Prolla TA, Selak MA, Kundu M, Carroll M, and Thompson JE. Erythroid dysplasia, megaloblastic anemia, and impaired lymphopoiesis arising from mitochondrial dysfunction. Blood 114: 4045–4053, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chimenti C, Kajstura J, Torella D, Urbanek K, Heleniak H, Colussi C, Di Meglio F, Nadal-Ginard B, Frustaci A, Leri A, Maseri A, and Anversa P. Senescence and death of primitive cells and myocytes lead to premature cardiac aging and heart failure. Circ Res 93: 604–613, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Chugh AR, Beache GM, Loughran JH, Mewton N, Elmore JB, Kajstura J, Pappas P, Tatooles A, Stoddard MF, Lima JA, Slaughter MS, Anversa P, and Bolli R. Administration of cardiac stem cells in patients with ischemic cardiomyopathy: the SCIPIO trial: surgical aspects and interim analysis of myocardial function and viability by magnetic resonance. Circulation 126: S54–S64, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chuikov S, Levi BP, Smith ML, and Morrison SJ. Prdm16 promotes stem cell maintenance in multiple tissues, partly by regulating oxidative stress. Nat Cell Biol 12: 999–1006, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chung S, Dzeja PP, Faustino RS, Perez-Terzic C, Behfar A, and Terzic A. Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat Clin Pract Cardiovasc Med 4Suppl 1: S60–S67, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cottage CT, Bailey B, Fischer KM, Avitable D, Collins B, Tuck S, Quijada P, Gude N, Alvarez R, Muraski J, and Sussman MA. Cardiac progenitor cell cycling stimulated by pim-1 kinase. Circ Res 106: 891–901, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cottage CT, Neidig L, Sundararaman B, Din S, Joyo AY, Bailey B, Gude N, Hariharan N, and Sussman MA. Increased mitotic rate coincident with transient telomere lengthening resulting from Pim-1 overexpression in cardiac progenitor cells. Stem Cells 30: 2512–2522, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.D'Amario D, Cabral-Da-Silva MC, Zheng H, Fiorini C, Goichberg P, Steadman E, Ferreira-Martins J, Sanada F, Piccoli M, Cappetta D, D'Alessandro DA, Michler RE, Hosoda T, Anastasia L, Rota M, Leri A, Anversa P, and Kajstura J. Insulin-like growth factor-1 receptor identifies a pool of human cardiac stem cells with superior therapeutic potential for myocardial regeneration. Circ Res 108: 1467–1481, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 28.D'Amario D, Fiorini C, Campbell PM, Goichberg P, Sanada F, Zheng H, Hosoda T, Rota M, Connell JM, Gallegos RP, Welt FG, Givertz MM, Mitchell RN, Leri A, Kajstura J, Pfeffer MA, and Anversa P. Functionally competent cardiac stem cells can be isolated from endomyocardial biopsies of patients with advanced cardiomyopathies. Circ Res 108: 857–861, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dawn B, Stein AB, Urbanek K, Rota M, Whang B, Rastaldo R, Torella D, Tang XL, Rezazadeh A, Kajstura J, Leri A, Hunt G, Varma J, Prabhu SD, Anversa P, and Bolli R. Cardiac stem cells delivered intravascularly traverse the vessel barrier, regenerate infarcted myocardium, and improve cardiac function. Proc Natl Acad Sci U S A 102: 3766–3771, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Almeida PE, van Rappard JR, and Wu JC. In vivo bioluminescence for tracking cell fate and function. Am J Physiol Heart Circ Physiol 301: H663–H671, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.De Angelis A, Piegari E, Cappetta D, Marino L, Filippelli A, Berrino L, Ferreira-Martins J, Zheng H, Hosoda T, Rota M, Urbanek K, Kajstura J, Leri A, Rossi F, and Anversa P. Anthracycline cardiomyopathy is mediated by depletion of the cardiac stem cell pool and is rescued by restoration of progenitor cell function. Circulation 121: 276–292, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dey D, Han L, Bauer M, Sanada F, Oikonomopoulos A, Hosoda T, Unno K, De Almeida P, Leri A, and Wu JC. Dissecting the molecular relationship among various cardiogenic progenitor cells. Circ Res 112: 1253–1262, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dimmeler S. and Leri A. Aging and disease as modifiers of efficacy of cell therapy. Circ Res 102: 1319–1330, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dimmeler S. and Zeiher AM. Cell therapy of acute myocardial infarction: open questions. Cardiology 113: 155–160, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Ding Y, Choi KJ, Kim JH, Han X, Piao Y, Jeong JH, Choe W, Kang I, Ha J, Forman HJ, Lee J, Yoon KS, and Kim SS. Endogenous hydrogen peroxide regulates glutathione redox via nuclear factor erythroid 2-related factor 2 downstream of phosphatidylinositol 3-kinase during muscle differentiation. Am J Pathol 172: 1529–1541, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dries JL, Kent SD, and Virag JA. Intramyocardial administration of chimeric ephrinA1-Fc promotes tissue salvage following myocardial infarction in mice. J Physiol 589: 1725–1740, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Drowley L, Okada M, Beckman S, Vella J, Keller B, Tobita K, and Huard J. Cellular antioxidant levels influence muscle stem cell therapy. Mol Ther 18: 1865–1873, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ferreira-Martins J, Ogorek B, Cappetta D, Matsuda A, Signore S, D'Amario D, Kostyla J, Steadman E, Ide-Iwata N, Sanada F, Iaffaldano G, Ottolenghi S, Hosoda T, Leri A, Kajstura J, Anversa P, and Rota M. Cardiomyogenesis in the developing heart is regulated by c-kit-positive cardiac stem cells. Circ Res 110: 701–715, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 39.Ferreira-Martins J, Rondon-Clavo C, Tugal D, Korn JA, Rizzi R, Padin-Iruegas ME, Ottolenghi S, De Angelis A, Urbanek K, Ide-Iwata N, D'Amario D, Hosoda T, Leri A, Kajstura J, Anversa P, and Rota M. Spontaneous calcium oscillations regulate human cardiac progenitor cell growth. Circ Res 105: 764–774, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fine GC, Liao R, and Sohn RL. Cell therapy for cardiac repair. Panminerva Med 50: 129–137, 2008 [PubMed] [Google Scholar]
- 41.Finkel T. Signal transduction by reactive oxygen species. J Cell Biol 194: 7–15, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Finkel T. and Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247, 2000 [DOI] [PubMed] [Google Scholar]
- 43.Fischer KM, Cottage CT, Konstandin MH, Volkers M, Khan M, and Sussman MA. Pim-1 kinase inhibits pathological injury by promoting cardioprotective signaling. J Mol Cell Cardiol 51: 554–558, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goichberg P, Bai Y, D'Amario D, Ferreira-Martins J, Fiorini C, Zheng H, Signore S, del Monte F, Ottolenghi S, D'Alessandro DA, Michler RE, Hosoda T, Anversa P, Kajstura J, Rota M, and Leri A. The ephrin A1-EphA2 system promotes cardiac stem cell migration after infarction. Circ Res 108: 1071–1083, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 45.Goichberg P, Kannappan R, Cimini M, Bai Y, Sanada F, Sorrentino A, Signore S, Kajstura J, Rota M, Anversa P, and Leri A. Age-associated defects in EphA2 signaling impair the migration of human cardiac progenitor cells. Circulation 128: 2211–2223, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Gonzalez A, Rota M, Nurzynska D, Misao Y, Tillmanns J, Ojaimi C, Padin-Iruegas ME, Muller P, Esposito G, Bearzi C, Vitale S, Dawn B, Sanganalmath SK, Baker M, Hintze TH, Bolli R, Urbanek K, Hosoda T, Anversa P, Kajstura J, and Leri A. Activation of cardiac progenitor cells reverses the failing heart senescent phenotype and prolongs lifespan. Circ Res 102: 597–606, 2008 [DOI] [PubMed] [Google Scholar]
- 47.Goumans MJ, de Boer TP, Smits AM, van Laake LW, van Vliet P, Metz CH, Korfage TH, Kats KP, Hochstenbach R, Pasterkamp G, Verhaar MC, van der Heyden MA, de Kleijn D, Mummery CL, van Veen TA, Sluijter JP, and Doevendans PA. TGF-beta1 induces efficient differentiation of human cardiomyocyte progenitor cells into functional cardiomyocytes in vitro. Stem Cell Res 1: 138–149, 2007 [DOI] [PubMed] [Google Scholar]
- 48.Hare JM, Beigi F, and Tziomalos K. Nitric oxide and cardiobiology-methods for intact hearts and isolated myocytes. Methods Enzymol 441: 369–392, 2008 [DOI] [PubMed] [Google Scholar]
- 49.Hatzistergos KE, Quevedo H, Oskouei BN, Hu Q, Feigenbaum GS, Margitich IS, Mazhari R, Boyle AJ, Zambrano JP, Rodriguez JE, Dulce R, Pattany PM, Valdes D, Revilla C, Heldman AW, McNiece I, and Hare JM. Bone marrow mesenchymal stem cells stimulate cardiac stem cell proliferation and differentiation. Circ Res 107: 913–922, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hom JR, Quintanilla RA, Hoffman DL, de Mesy Bentley KL, Molkentin JD, Sheu SS, and Porter GA, Jr. The permeability transition pore controls cardiac mitochondrial maturation and myocyte differentiation. Dev Cell 21: 469–478, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hosoda T, D'Amario D, Cabral-Da-Silva MC, Zheng H, Padin-Iruegas ME, Ogorek B, Ferreira-Martins J, Yasuzawa-Amano S, Amano K, Ide-Iwata N, Cheng W, Rota M, Urbanek K, Kajstura J, Anversa P, and Leri A. Clonality of mouse and human cardiomyogenesis in vivo. Proc Natl Acad Sci U S A 106: 17169–17174, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hosoda T, Rota M, Kajstura J, Leri A, and Anversa P. Role of stem cells in cardiovascular biology. J Thromb Haemost 9Suppl 1: 151–161, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hosoda T, Zheng H, Cabral-da-Silva M, Sanada F, Ide-Iwata N, Ogorek B, Ferreira-Martins J, Arranto C, D'Amario D, del Monte F, Urbanek K, D'Alessandro DA, Michler RE, Anversa P, Rota M, Kajstura J, and Leri A. Human cardiac stem cell differentiation is regulated by a mircrine mechanism. Circulation 123: 1287–1296, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 54.Huang C, Zhang X, Ramil JM, Rikka S, Kim L, Lee Y, Gude NA, Thistlethwaite PA, Sussman MA, Gottlieb RA, and Gustafsson AB. Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation 121: 675–683, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ito K, Hirao A, Arai F, Matsuoka S, Takubo K, Hamaguchi I, Nomiyama K, Hosokawa K, Sakurada K, Nakagata N, Ikeda Y, Mak TW, and Suda T. Regulation of oxidative stress by ATM is required for self-renewal of haematopoietic stem cells. Nature 431: 997–1002, 2004 [DOI] [PubMed] [Google Scholar]
- 56.Itzhaki-Alfia A, Leor J, Raanani E, Sternik L, Spiegelstein D, Netser S, Holbova R, Pevsner-Fischer M, Lavee J, and Barbash IM. Patient characteristics and cell source determine the number of isolated human cardiac progenitor cells. Circulation 120: 2559–2566, 2009 [DOI] [PubMed] [Google Scholar]
- 57.Jain M, Pfister O, Hajjar RJ, and Liao R. Mesenchymal stem cells in the infarcted heart. Coron Artery Dis 16: 93–97, 2005 [DOI] [PubMed] [Google Scholar]
- 58.Jakob P. and Landmesser U. Current status of cell-based therapy for heart failure. Curr Heart Fail Rep 10: 165–176, 2013 [DOI] [PubMed] [Google Scholar]
- 59.Johnston PV, Sasano T, Mills K, Evers R, Lee ST, Smith RR, Lardo AC, Lai S, Steenbergen C, Gerstenblith G, Lange R, and Marban E. Engraftment, differentiation, and functional benefits of autologous cardiosphere-derived cells in porcine ischemic cardiomyopathy. Circulation 120: 1075–1083, 7 p following 1083, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Jones DL. and Rando TA. Emerging models and paradigms for stem cell ageing. Nat Cell Biol 13: 506–512, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Juntilla MM, Patil VD, Calamito M, Joshi RP, Birnbaum MJ, and Koretzky GA. AKT1 and AKT2 maintain hematopoietic stem cell function by regulating reactive oxygen species. Blood 115: 4030–4038, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kajstura J, Bai Y, Cappetta D, Kim J, Arranto C, Sanada F, D'Amario D, Matsuda A, Bardelli S, Ferreira-Martins J, Hosoda T, Leri A, Rota M, Loscalzo J, and Anversa P. Tracking chromatid segregation to identify human cardiac stem cells that regenerate extensively the infarcted myocardium. Circ Res 111: 894–906, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Kajstura J, Gurusamy N, Ogorek B, Goichberg P, Clavo-Rondon C, Hosoda T, D'Amario D, Bardelli S, Beltrami AP, Cesselli D, Bussani R, del Monte F, Quaini F, Rota M, Beltrami CA, Buchholz BA, Leri A, and Anversa P. Myocyte turnover in the aging human heart. Circ Res 107: 1374–1386, 2010 [DOI] [PubMed] [Google Scholar]
- 64.Kajstura J, Leri A, Finato N, Di Loreto C, Beltrami CA, and Anversa P. Myocyte proliferation in end-stage cardiac failure in humans. Proc Natl Acad Sci U S A 95: 8801–8805, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kajstura J, Rota M, Cappetta D, Ogorek B, Arranto C, Bai Y, Ferreira-Martins J, Signore S, Sanada F, Matsuda A, Kostyla J, Caballero MV, Fiorini C, D'Alessandro DA, Michler RE, del Monte F, Hosoda T, Perrella MA, Leri A, Buchholz BA, Loscalzo J, and Anversa P. Cardiomyogenesis in the aging and failing human heart. Circulation 126: 1869–1881, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 66.Kajstura J, Rota M, Urbanek K, Hosoda T, Bearzi C, Anversa P, Bolli R, and Leri A. The telomere-telomerase axis and the heart. Antioxid Redox Signal 8: 2125–2141, 2006 [DOI] [PubMed] [Google Scholar]
- 67.Kajstura J, Rota M, Whang B, Cascapera S, Hosoda T, Bearzi C, Nurzynska D, Kasahara H, Zias E, Bonafe M, Nadal-Ginard B, Torella D, Nascimbene A, Quaini F, Urbanek K, Leri A, and Anversa P. Bone marrow cells differentiate in cardiac cell lineages after infarction independently of cell fusion. Circ Res 96: 127–137, 2005 [DOI] [PubMed] [Google Scholar]
- 68.Kajstura J, Urbanek K, Perl S, Hosoda T, Zheng H, Ogorek B, Ferreira-Martins J, Goichberg P, Rondon-Clavo C, Sanada F, D'Amario D, Rota M, Del Monte F, Orlic D, Tisdale J, Leri A, and Anversa P. Cardiomyogenesis in the adult human heart. Circ Res 107: 305–315, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 69.Kaludercic N, Carpi A, Nagayama T, Sivakumaran V, Zhu G, Lai EW, Bedja D, De Mario A, Chen K, Gabrielson KL, Lindsey ML, Pacak K, Takimoto E, Shih JC, Kass DA, Di Lisa F, and Paolocci N. Monoamine oxidase B prompts mitochondrial and cardiac dysfunction in pressure overloaded hearts. Antioxid Redox Signal 20: 267–280, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kaludercic N, Takimoto E, Nagayama T, Feng N, Lai EW, Bedja D, Chen K, Gabrielson KL, Blakely RD, Shih JC, Pacak K, Kass DA, Di Lisa F, and Paolocci N. Monoamine oxidase A-mediated enhanced catabolism of norepinephrine contributes to adverse remodeling and pump failure in hearts with pressure overload. Circ Res 106: 193–202, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Karantalis V, Balkan W, Schulman IH, Hatzistergos KE, and Hare JM. Cell-based therapy for prevention and reversal of myocardial remodeling. Am J Physiol Heart Circ Physiol 303: H256–H270, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katare R, Oikawa A, Cesselli D, Beltrami AP, Avolio E, Muthukrishnan D, Munasinghe PE, Angelini G, Emanueli C, and Madeddu P. Boosting the pentose phosphate pathway restores cardiac progenitor cell availability in diabetes. Cardiovasc Res 97: 55–65, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kobayashi CI. and Suda T. Regulation of reactive oxygen species in stem cells and cancer stem cells. J Cell Physiol 227: 421–430, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Konstandin MH, Toko H, Gastelum GM, Quijada PJ, De La Torre A, Quintana M, Collins B, Din S, Avitabile D, Volkers MJ, Gude NA, Fassler R, and Sussman MA. Fibronectin is essential for reparative cardiac progenitor cell response following myocardial infarction. Circ Res 113: 115–125, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kreke M, Smith RR, Marban L, and Marban E. Cardiospheres and cardiosphere-derived cells as therapeutic agents following myocardial infarction. Expert Rev Cardiovasc Ther 10: 1185–1194, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Le Belle JE, Orozco NM, Paucar AA, Saxe JP, Mottahedeh J, Pyle AD, Wu H, and Kornblum HI. Proliferative neural stem cells have high endogenous ROS levels that regulate self-renewal and neurogenesis in a PI3K/Akt-dependant manner. Cell Stem Cell 8: 59–71, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lee S, Tak E, Lee J, Rashid MA, Murphy MP, Ha J, and Kim SS. Mitochondrial H2O2 generated from electron transport chain complex I stimulates muscle differentiation. Cell Res 21: 817–834, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Leri A. and Anversa P. Stem cells and myocardial regeneration: cooperation wins over competition. Circulation 127: 165–168, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Leri A, Kajstura J, and Anversa P. Mechanisms of myocardial regeneration. Trends Cardiovasc Med 21: 52–58, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Leri A, Kajstura J, and Anversa P. Role of cardiac stem cells in cardiac pathophysiology: a paradigm shift in human myocardial biology. Circ Res 109: 941–961, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 81.Lewandowski D, Barroca V, Duconge F, Bayer J, Van Nhieu JT, Pestourie C, Fouchet P, Tavitian B, and Romeo PH. In vivo cellular imaging pinpoints the role of reactive oxygen species in the early steps of adult hematopoietic reconstitution. Blood 115: 443–452, 2010 [DOI] [PubMed] [Google Scholar]
- 82.Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, Steger K, Krause KH, and Jaconi ME. The NADPH oxidase NOX4 drives cardiac differentiation: role in regulating cardiac transcription factors and MAP kinase activation. Mol Biol Cell 17: 3978–3988, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Li TS, Cheng K, Lee ST, Matsushita S, Davis D, Malliaras K, Zhang Y, Matsushita N, Smith RR, and Marban E. Cardiospheres recapitulate a niche-like microenvironment rich in stemness and cell-matrix interactions, rationalizing their enhanced functional potency for myocardial repair. Stem Cells 28: 2088–2098, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marban L, and Marban E. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol 59: 942–953, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li TS. and Marban E. Physiological levels of reactive oxygen species are required to maintain genomic stability in stem cells. Stem Cells 28: 1178–1185, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Liang SX, Tan TY, Gaudry L, and Chong B. Differentiation and migration of Sca1+/CD31- cardiac side population cells in a murine myocardial ischemic model. Int J Cardiol 138: 40–49, 2010 [DOI] [PubMed] [Google Scholar]
- 87.Liao R, Pfister O, Jain M, and Mouquet F. The bone marrow—cardiac axis of myocardial regeneration. Prog Cardiovasc Dis 50: 18–30, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Limoli CL, Rola R, Giedzinski E, Mantha S, Huang TT, and Fike JR. Cell-density-dependent regulation of neural precursor cell function. Proc Natl Acad Sci U S A 101: 16052–16057, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Linke A, Muller P, Nurzynska D, Casarsa C, Torella D, Nascimbene A, Castaldo C, Cascapera S, Bohm M, Quaini F, Urbanek K, Leri A, Hintze TH, Kajstura J, and Anversa P. Stem cells in the dog heart are self-renewing, clonogenic, and multipotent and regenerate infarcted myocardium, improving cardiac function. Proc Natl Acad Sci U S A 102: 8966–8971, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu J, Narsinh KH, Lan F, Wang L, Nguyen PK, Hu S, Lee A, Han L, Gong Y, Huang M, Nag D, Rosenberg J, Chouldechova A, Robbins RC, and Wu JC. Early stem cell engraftment predicts late cardiac functional recovery: preclinical insights from molecular imaging. Circ Cardiovasc Imaging 5: 481–490, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Liu TC, Ismail S, Brennan O, Hastings C, and Duffy GP. Encapsulation of cardiac stem cells in superoxide dismutase-loaded alginate prevents doxorubicin-mediated toxicity. J Tissue Eng Regen Med 7: 302–311, 2013 [DOI] [PubMed] [Google Scholar]
- 92.Makkar RR, Smith RR, Cheng K, Malliaras K, Thomson LE, Berman D, Czer LS, Marban L, Mendizabal A, Johnston PV, Russell SD, Schuleri KH, Lardo AC, Gerstenblith G, and Marban E. Intracoronary cardiosphere-derived cells for heart regeneration after myocardial infarction (CADUCEUS): a prospective, randomised phase 1 trial. Lancet 379: 895–904, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Malliaras K, Li TS, Luthringer D, Terrovitis J, Cheng K, Chakravarty T, Galang G, Zhang Y, Schoenhoff F, Van Eyk J, Marban L, and Marban E. Safety and efficacy of allogeneic cell therapy in infarcted rats transplanted with mismatched cardiosphere-derived cells. Circulation 125: 100–112, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mantel C, Messina-Graham SV, and Broxmeyer HE. Superoxide flashes, reactive oxygen species, and the mitochondrial permeability transition pore: potential implications for hematopoietic stem cell function. Curr Opin Hematol 18: 208–213, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Martin CM, Meeson AP, Robertson SM, Hawke TJ, Richardson JA, Bates S, Goetsch SC, Gallardo TD, and Garry DJ. Persistent expression of the ATP-binding cassette transporter, Abcg2, identifies cardiac SP cells in the developing and adult heart. Dev Biol 265: 262–275, 2004 [DOI] [PubMed] [Google Scholar]
- 96.Matsuura K, Honda A, Nagai T, Fukushima N, Iwanaga K, Tokunaga M, Shimizu T, Okano T, Kasanuki H, Hagiwara N, and Komuro I. Transplantation of cardiac progenitor cells ameliorates cardiac dysfunction after myocardial infarction in mice. J Clin Invest 119: 2204–2217, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Matsuura K, Nagai T, Nishigaki N, Oyama T, Nishi J, Wada H, Sano M, Toko H, Akazawa H, Sato T, Nakaya H, Kasanuki H, and Komuro I. Adult cardiac Sca-1-positive cells differentiate into beating cardiomyocytes. J Biol Chem 279: 11384–11391, 2004 [DOI] [PubMed] [Google Scholar]
- 98.Mazhari R. and Hare JM. Translational findings from cardiovascular stem cell research. Trends Cardiovasc Med 22: 1–6, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Messina E, De Angelis L, Frati G, Morrone S, Chimenti S, Fiordaliso F, Salio M, Battaglia M, Latronico MV, Coletta M, Vivarelli E, Frati L, Cossu G, and Giacomello A. Isolation and expansion of adult cardiac stem cells from human and murine heart. Circ Res 95: 911–921, 2004 [DOI] [PubMed] [Google Scholar]
- 100.Miyamoto K, Araki KY, Naka K, Arai F, Takubo K, Yamazaki S, Matsuoka S, Miyamoto T, Ito K, Ohmura M, Chen C, Hosokawa K, Nakauchi H, Nakayama K, Nakayama KI, Harada M, Motoyama N, Suda T, and Hirao A. Foxo3a is essential for maintenance of the hematopoietic stem cell pool. Cell Stem Cell 1: 101–112, 2007 [DOI] [PubMed] [Google Scholar]
- 101.Mohsin S, Khan M, Toko H, Bailey B, Cottage CT, Wallach K, Nag D, Lee A, Siddiqi S, Lan F, Fischer KM, Gude N, Quijada P, Avitabile D, Truffa S, Collins B, Dembitsky W, Wu JC, and Sussman MA. Human cardiac progenitor cells engineered with Pim-I kinase enhance myocardial repair. J Am Coll Cardiol 60: 1278–1287, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nagai T, Matsuura K, and Komuro I. Cardiac side population cells and sca-1-positive cells. Methods Mol Biol 1036: 63–74, 2013 [DOI] [PubMed] [Google Scholar]
- 103.Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, Park SY, Lu J, Protopopov A, and Silberstein LE. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol 15: 533–543, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.North BJ. and Sinclair DA. The intersection between aging and cardiovascular disease. Circ Res 110: 1097–1108, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oh H, Bradfute SB, Gallardo TD, Nakamura T, Gaussin V, Mishina Y, Pocius J, Michael LH, Behringer RR, Garry DJ, Entman ML, and Schneider MD. Cardiac progenitor cells from adult myocardium: homing, differentiation, and fusion after infarction. Proc Natl Acad Sci U S A 100: 12313–12318, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Oh IH. and Kwon KR. Concise review: multiple niches for hematopoietic stem cell regulations. Stem Cells 28: 1243–1249, 2010 [DOI] [PubMed] [Google Scholar]
- 107.Oikonomopoulos A, Sereti KI, Conyers F, Bauer M, Liao A, Guan J, Crapps D, Han JK, Dong H, Bayomy AF, Fine GC, Westerman K, Biechele TL, Moon RT, Force T, and Liao R. Wnt signaling exerts an antiproliferative effect on adult cardiac progenitor cells through IGFBP3. Circ Res 109: 1363–1374, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Orlic D, Kajstura J, Chimenti S, Jakoniuk I, Anderson SM, Li B, Pickel J, McKay R, Nadal-Ginard B, Bodine DM, Leri A, and Anversa P. Bone marrow cells regenerate infarcted myocardium. Nature 410: 701–705, 2001 [DOI] [PubMed] [Google Scholar]
- 109.Oyama T, Nagai T, Wada H, Naito AT, Matsuura K, Iwanaga K, Takahashi T, Goto M, Mikami Y, Yasuda N, Akazawa H, Uezumi A, Takeda S, and Komuro I. Cardiac side population cells have a potential to migrate and differentiate into cardiomyocytes in vitro and in vivo. J Cell Biol 176: 329–341, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Padin-Iruegas ME, Misao Y, Davis ME, Segers VF, Esposito G, Tokunou T, Urbanek K, Hosoda T, Rota M, Anversa P, Leri A, Lee RT, and Kajstura J. Cardiac progenitor cells and biotinylated insulin-like growth factor-1 nanofibers improve endogenous and exogenous myocardial regeneration after infarction. Circulation 120: 876–887, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Papayannopoulou T. Mechanisms of stem-/progenitor-cell mobilization: the anti-VLA-4 paradigm. Semin Hematol 37: 11–18, 2000 [DOI] [PubMed] [Google Scholar]
- 112.Parmar K, Mauch P, Vergilio JA, Sackstein R, and Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci U S A 104: 5431–5436, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pfister O. and Liao R. Pump to survive: novel cytoprotective strategies for cardiac progenitor cells. Circ Res 102: 998–1001, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Pfister O, Mouquet F, Jain M, Summer R, Helmes M, Fine A, Colucci WS, and Liao R. CD31- but Not CD31+cardiac side population cells exhibit functional cardiomyogenic differentiation. Circ Res 97: 52–61, 2005 [DOI] [PubMed] [Google Scholar]
- 115.Pfister O, Oikonomopoulos A, Sereti KI, Sohn RL, Cullen D, Fine GC, Mouquet F, Westerman K, and Liao R. Role of the ATP-binding cassette transporter Abcg2 in the phenotype and function of cardiac side population cells. Circ Res 103: 825–835, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Piegari E, De Angelis A, Cappetta D, Russo R, Esposito G, Costantino S, Graiani G, Frati C, Prezioso L, Berrino L, Urbanek K, Quaini F, and Rossi F. Doxorubicin induces senescence and impairs function of human cardiac progenitor cells. Basic Res Cardiol 108: 334–351, 2013 [DOI] [PubMed] [Google Scholar]
- 117.Pollard PJ. and Kranc KR. Hypoxia signaling in hematopoietic stem cells: a double-edged sword. Cell Stem Cell 7: 276–278, 2010 [DOI] [PubMed] [Google Scholar]
- 118.Rota M, Kajstura J, Hosoda T, Bearzi C, Vitale S, Esposito G, Iaffaldano G, Padin-Iruegas ME, Gonzalez A, Rizzi R, Small N, Muraski J, Alvarez R, Chen X, Urbanek K, Bolli R, Houser SR, Leri A, Sussman MA, and Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci U S A 104: 17783–17788, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Rota M, LeCapitaine N, Hosoda T, Boni A, De Angelis A, Padin-Iruegas ME, Esposito G, Vitale S, Urbanek K, Casarsa C, Giorgio M, Luscher TF, Pelicci PG, Anversa P, Leri A, and Kajstura J. Diabetes promotes cardiac stem cell aging and heart failure, which are prevented by deletion of the p66shc gene. Circ Res 99: 42–52, 2006 [DOI] [PubMed] [Google Scholar]
- 120.Rota M, Padin-Iruegas ME, Misao Y, De Angelis A, Maestroni S, Ferreira-Martins J, Fiumana E, Rastaldo R, Arcarese ML, Mitchell TS, Boni A, Bolli R, Urbanek K, Hosoda T, Anversa P, Leri A, and Kajstura J. Local activation or implantation of cardiac progenitor cells rescues scarred infarcted myocardium improving cardiac function. Circ Res 103: 107–116, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Sanada F, Kim J, Czarna A, Chan NY, Signore S, Ogorek B, Isobe K, Wybieralska EW, Borghetti G, Pesapane A, Sorrentino A, Mangano EN, Cappetta D, Mangiaracina CM, Ricciardi M, Cimini M, Ifedigbo E, Perrella MA, Goichberg P, Choi AM, Kajstura J, Hosoda T, Rota M, Anversa P, and Leri A. c-kit-positive cardiac stem cells nested in hypoxic niches are activated by stem cell factor reversing the aging myopathy. Circ Res 114: 41–55, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sardina JL, Lopez-Ruano G, Sanchez-Sanchez B, Llanillo M, and Hernandez-Hernandez A. Reactive oxygen species: are they important for haematopoiesis? Crit Rev Oncol Hematol 81: 257–274, 2012 [DOI] [PubMed] [Google Scholar]
- 123.Schoenfeld M, Frishman WH, Leri A, Kajstura J, and Anversa P. The existence of myocardial repair: mechanistic insights and enhancements. Cardiol Rev 21: 111–120, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Schulman IH. and Hare JM. Key developments in stem cell therapy in cardiology. Regen Med 7: 17–24, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Schulman IH. and Hare JM. Regulation of cardiovascular cellular processes by S-nitrosylation. Biochim Biophys Acta 1820: 752–762, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Senyo SE, Steinhauser ML, Pizzimenti CL, Yang VK, Cai L, Wang M, Wu TD, Guerquin-Kern JL, Lechene CP, and Lee RT. Mammalian heart renewal by pre-existing cardiomyocytes. Nature 493: 433–436, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Sereti KI, Oikonomopoulos A, Unno K, Cao X, Qiu Y, and Liao R. ATP-binding cassette G-subfamily transporter 2 regulates cell cycle progression and asymmetric division in mouse cardiac side population progenitor cells. Circ Res 112: 27–34, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Shao D, Oka S, Brady CD, Haendeler J, Eaton P, and Sadoshima J. Redox modification of cell signaling in the cardiovascular system. J Mol Cell Cardiol 52: 550–558, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Sheydina A, Riordon DR, and Boheler KR. Molecular mechanisms of cardiomyocyte aging. Clin Sci (Lond) 121: 315–329, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Signer RA. and Morrison SJ. Mechanisms that regulate stem cell aging and life span. Cell Stem Cell 12: 152–165, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Sikora E, Arendt T, Bennett M, and Narita M. Impact of cellular senescence signature on ageing research. Ageing Res Rev 10: 146–152, 2011 [DOI] [PubMed] [Google Scholar]
- 132.Simpson DL, Mishra R, Sharma S, Goh SK, Deshmukh S, and Kaushal S. A strong regenerative ability of cardiac stem cells derived from neonatal hearts. Circulation 126: S46–S53, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, Schneider JW, Zhang CC, and Sadek HA. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell 7: 380–390, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Smart N, Bollini S, Dube KN, Vieira JM, Zhou B, Davidson S, Yellon D, Riegler J, Price AN, Lythgoe MF, Pu WT, and Riley PR. De novo cardiomyocytes from within the activated adult heart after injury. Nature 474: 640–644, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Smith RR, Barile L, Cho HC, Leppo MK, Hare JM, Messina E, Giacomello A, Abraham MR, and Marban E. Regenerative potential of cardiosphere-derived cells expanded from percutaneous endomyocardial biopsy specimens. Circulation 115: 896–908, 2007 [DOI] [PubMed] [Google Scholar]
- 136.Sohal RS. and Orr WC. The redox stress hypothesis of aging. Free Radic Biol Med 52: 539–555, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Sohn RL, Jain M, and Liao R. Adult stem cells and heart regeneration. Expert Rev Cardiovasc Ther 5: 507–517, 2007 [DOI] [PubMed] [Google Scholar]
- 138.Stamm C, Nasseri B, Drews T, and Hetzer R. Cardiac cell therapy: a realistic concept for elderly patients? Exp Gerontol 43: 679–690, 2008 [DOI] [PubMed] [Google Scholar]
- 139.Stanley BA, Sivakumaran V, Shi S, McDonald I, Lloyd D, Watson WH, Aon MA, and Paolocci N. Thioredoxin reductase-2 is essential for keeping low levels of H(2)O(2) emission from isolated heart mitochondria. J Biol Chem 286: 33669–33677, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Steinhauser ML, Bailey AP, Senyo SE, Guillermier C, Perlstein TS, Gould AP, Lee RT, and Lechene CP. Multi-isotope imaging mass spectrometry quantifies stem cell division and metabolism. Nature 481: 516–519, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Suda T, Takubo K, and Semenza GL. Metabolic regulation of hematopoietic stem cells in the hypoxic niche. Cell Stem Cell 9: 298–310, 2011 [DOI] [PubMed] [Google Scholar]
- 142.Suncion VY, Schulman IH, and Hare JM. Concise review: the role of clinical trials in deciphering mechanisms of action of cardiac cell-based therapy. Stem Cells Transl Med 1: 29–35, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Takehara N, Ogata T, Nakata M, Kami D, Nakamura T, Matoba S, Gojo S, Sawada T, Yaku H, and Matsubara H. The ALCADIA (autologous human cardiac-derived stem cell to treat ischemic cardiomyopathy) trial. Circulation 126: 2783, 2012 [Google Scholar]
- 144.Takubo K, Goda N, Yamada W, Iriuchishima H, Ikeda E, Kubota Y, Shima H, Johnson RS, Hirao A, Suematsu M, and Suda T. Regulation of the HIF-1alpha level is essential for hematopoietic stem cells. Cell Stem Cell 7: 391–402, 2010 [DOI] [PubMed] [Google Scholar]
- 145.Takubo K, Nagamatsu G, Kobayashi CI, Nakamura-Ishizu A, Kobayashi H, Ikeda E, Goda N, Rahimi Y, Johnson RS, Soga T, Hirao A, Suematsu M, and Suda T. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell 12: 49–61, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Tang YL, Zhu W, Cheng M, Chen L, Zhang J, Sun T, Kishore R, Phillips MI, Losordo DW, and Qin G. Hypoxic preconditioning enhances the benefit of cardiac progenitor cell therapy for treatment of myocardial infarction by inducing CXCR4 expression. Circ Res 104: 1209–1216, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Toma C, Pittenger MF, Cahill KS, Byrne BJ, and Kessler PD. Human mesenchymal stem cells differentiate to a cardiomyocyte phenotype in the adult murine heart. Circulation 105: 93–98, 2002 [DOI] [PubMed] [Google Scholar]
- 148.Torella D, Rota M, Nurzynska D, Musso E, Monsen A, Shiraishi I, Zias E, Walsh K, Rosenzweig A, Sussman MA, Urbanek K, Nadal-Ginard B, Kajstura J, Anversa P, and Leri A. Cardiac stem cell and myocyte aging, heart failure, and insulin-like growth factor-1 overexpression. Circ Res 94: 514–524, 2004 [DOI] [PubMed] [Google Scholar]
- 149.Tothova Z, Kollipara R, Huntly BJ, Lee BH, Castrillon DH, Cullen DE, McDowell EP, Lazo-Kallanian S, Williams IR, Sears C, Armstrong SA, Passegue E, DePinho RA, and Gilliland DG. FoxOs are critical mediators of hematopoietic stem cell resistance to physiologic oxidative stress. Cell 128: 325–339, 2007 [DOI] [PubMed] [Google Scholar]
- 150.Tsutsui H, Kinugawa S, and Matsushima S. Oxidative stress and heart failure. Am J Physiol Heart Circ Physiol 301: H2181–H2190, 2011 [DOI] [PubMed] [Google Scholar]
- 151.Tufan H, Zhang XH, Haghshenas N, Sussman MA, Cleemann L, and Morad M. Cardiac progenitor cells engineered with Pim-1 (CPCeP) develop cardiac phenotypic electrophysiological properties as they are co-cultured with neonatal myocytes. J Mol Cell Cardiol 53: 695–706, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Ueda H, Nakamura T, Matsumoto K, Sawa Y, and Matsuda H. A potential cardioprotective role of hepatocyte growth factor in myocardial infarction in rats. Cardiovasc Res 51: 41–50, 2001 [DOI] [PubMed] [Google Scholar]
- 153.Unno K, Jain M, and Liao R. Cardiac side population cells: moving toward the center stage in cardiac regeneration. Circ Res 110: 1355–1363, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Urao N, Inomata H, Razvi M, Kim HW, Wary K, McKinney R, Fukai T, Ushio-and Fukai M. Role of nox2-based NADPH oxidase in bone marrow and progenitor cell function involved in neovascularization induced by hindlimb ischemia. Circ Res 103: 212–220, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Urbanek K, Cabral-da-Silva MC, Ide-Iwata N, Maestroni S, Delucchi F, Zheng H, Ferreira-Martins J, Ogorek B, D'Amario D, Bauer M, Zerbini G, Rota M, Hosoda T, Liao R, Anversa P, Kajstura J, and Leri A. Inhibition of notch1-dependent cardiomyogenesis leads to a dilated myopathy in the neonatal heart. Circ Res 107: 429–441, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Urbanek K, Cesselli D, Rota M, Nascimbene A, De Angelis A, Hosoda T, Bearzi C, Boni A, Bolli R, Kajstura J, Anversa P, and Leri A. Stem cell niches in the adult mouse heart. Proc Natl Acad Sci U S A 103: 9226–9231, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Urbanek K, Quaini F, Tasca G, Torella D, Castaldo C, Nadal-Ginard B, Leri A, Kajstura J, Quaini E, and Anversa P. Intense myocyte formation from cardiac stem cells in human cardiac hypertrophy. Proc Natl Acad Sci U S A 100: 10440–10445, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Urbanek K, Rota M, Cascapera S, Bearzi C, Nascimbene A, De Angelis A, Hosoda T, Chimenti S, Baker M, Limana F, Nurzynska D, Torella D, Rotatori F, Rastaldo R, Musso E, Quaini F, Leri A, Kajstura J, and Anversa P. Cardiac stem cells possess growth factor-receptor systems that after activation regenerate the infarcted myocardium, improving ventricular function and long-term survival. Circ Res 97: 663–673, 2005 [DOI] [PubMed] [Google Scholar]
- 159.Urbanek K, Torella D, Sheikh F, De Angelis A, Nurzynska D, Silvestri F, Beltrami CA, Bussani R, Beltrami AP, Quaini F, Bolli R, Leri A, Kajstura J, and Anversa P. Myocardial regeneration by activation of multipotent cardiac stem cells in ischemic heart failure. Proc Natl Acad Sci U S A 102: 8692–8697, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Vacanti NM. and Metallo CM. Exploring metabolic pathways that contribute to the stem cell phenotype. Biochim Biophys Acta 1830: 2361–2369, 2013 [DOI] [PubMed] [Google Scholar]
- 161.Wang X, Hu Q, Nakamura Y, Lee J, Zhang G, From AH, and Zhang J. The role of the sca-1+/CD31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells 24: 1779–1788, 2006 [DOI] [PubMed] [Google Scholar]
- 162.Williams AR. and Hare JM. Mesenchymal stem cells: biology, pathophysiology, translational findings, and therapeutic implications for cardiac disease. Circ Res 109: 923–940, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Williams AR, Hatzistergos KE, Addicott B, McCall F, Carvalho D, Suncion V, Morales AR, Da Silva J, Sussman MA, Heldman AW, and Hare JM. Enhanced effect of combining human cardiac stem cells and bone marrow mesenchymal stem cells to reduce infarct size and to restore cardiac function after myocardial infarction. Circulation 127: 213–223, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yalcin S, Marinkovic D, Mungamuri SK, Zhang X, Tong W, Sellers R, and Ghaffari S. ROS-mediated amplification of AKT/mTOR signalling pathway leads to myeloproliferative syndrome in Foxo3(-/-) mice. EMBO J 29: 4118–4131, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Yalcin S, Zhang X, Luciano JP, Mungamuri SK, Marinkovic D, Vercherat C, Sarkar A, Grisotto M, Taneja R, and Ghaffari S. Foxo3 is essential for the regulation of ataxia telangiectasia mutated and oxidative stress-mediated homeostasis of hematopoietic stem cells. J Biol Chem 283: 25692–25705, 2008 [DOI] [PubMed] [Google Scholar]
- 166.Yaniz-Galende E, Chen J, Chemaly E, Liang L, Hulot JS, McCollum L, Arias T, Fuster V, Zsebo KM, and Hajjar RJ. Stem cell factor gene transfer promotes cardiac repair after myocardial infarction via in situ recruitment and expansion of c-kit+cells. Circ Res 111: 1434–1445, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Zhang Y, Du Y, Le W, Wang K, Kieffer N, and Zhang J. Redox control of the survival of healthy and diseased cells. Antioxid Redox Signal 15: 2867–2908, 2011 [DOI] [PubMed] [Google Scholar]