Abstract
Epidemiological studies suggest that prevalent herpes simplex virus type 2 (HSV-2) infection increases the risk of HIV acquisition, underscoring the need to develop coinfection models to evaluate promising prevention strategies. We previously established a single high-dose vaginal coinfection model of simian human immunodeficiency virus (SHIV)/HSV-2 in Depo-Provera (DP)-treated macaques. However, this model does not appropriately mimic women's exposure. Repeated limiting dose SHIV challenge models are now used routinely to test prevention strategies, yet, at present, there are no reports of a repeated limiting dose cochallenge model in which to evaluate products targeting HIV and HSV-2. Herein, we show that 20 weekly cochallenges with 2–50 TCID50 simian human immunodeficiency virus reverse transcriptase (SHIV-RT) and 107 pfu HSV-2 results in infection with both viruses (4/6 SHIV-RT, 6/6 HSV-2). The frequency and level of vaginal HSV-2 shedding were significantly greater in the repeated exposure model compared to the single high-dose model (p<0.0001). We used this new model to test the Council's on-demand microbicide gel, MZC, which is active against SHIV-RT in DP-treated macaques and HSV-2 and human papillomavirus (HPV) in mice. While MZC reduced SHIV and HSV-2 infections in our repeated limiting dose model when cochallenging 8 h after each gel application, a barrier effect of carrageenan (CG) that was not seen in DP-treated animals precluded evaluation of the significance of the antiviral activity of MZC. Both MZC and CG significantly (p<0.0001) reduced the frequency and level of vaginal HSV-2 shedding compared to no gel treatment. This validates the use of this repeated limiting dose cochallenge model for testing products targeting HIV and HSV-2.
Repeated limiting dose simian human immunodeficiency virus (SHIV) challenge in cycling macaques mimics human exposure better than single high-dose exposure in Depo-Provera (DP)-treated macaques and is increasingly being used in the field to test promising microbicides. Because herpes simplex virus type 2 (HSV-2) infection increases the risk of HIV acquisition,1,2 we are developing microbicides that target both viruses and thus sought to develop a repeated limiting dose cochallenge model of simian human immunodeficiency virus reverse transcriptase (SHIV-RT) and HSV-2 in naturally cycling macaques. We had previously shown that cochallenge of DP-treated macaques with ∼103 TCID50 SHIV-RT and 2×108 pfu HSV-2 resulted in 67% of the animals becoming infected with SHIV-RT and 100% with HSV-2.3 Notably, HSV-2 shedding was infrequent and sporadic, making it difficult to evaluate the impact of a product on HSV-2 shedding.
For development of a repeated cochallenge model in non-DP-treated animals, we initiated weekly atraumatic vaginal cochallenges of six rhesus macaques (housed at the Tulane National Primate Research Center) with either ∼2 or ∼10 TCID50 SHIV-RT (n=3 each) combined with 107 pfu HSV-2. SHIV-RT, possessing the HIV-1HXB2 reverse transcriptase (RT) gene in the background of SIVmac239, was originally provided by Disa Böttinger (Medivir, AB) and was grown and titered in rhesus macaque peripheral blood mononuclear cells (PBMCs).4 HSV-2 strain G was obtained from ATCC and grown and titered on Vero cells.5 Because only one animal in each group was infected after 11 challenges (Fig. 1 and Table 1), we increased the dose of SHIV-RT to 50 TCID50 in all animals while preserving the HSV-2 inoculum at 107 pfu from challenge 12 on. After 20 weekly cochallenges, 4/6 animals were infected with SHIV-RT, a 66.7% infection frequency overall (Fig. 1 and Table 1), which is similar to what we observed after a single high-dose exposure to ∼103 TCID50 SHIV-RT alone6–11 or ∼103 TCID50 SHIV-RT and 2×108 pfu HSV-23 in DP-treated macaques.
FIG. 1.
Simian human immunodeficiency virus reverse transcriptase (SHIV-RT) infection of macaques after repeated limiting dose cochallenge with SHIV-RT and herpes simplex virus type 2 (HSV-2). Rhesus macaques in the no gel group were cochallenged weekly with ∼2 TCID50 SHIV-RT and 107 pfu HSV-2 or ∼10 TCID50 SHIV-RT and 107 pfu HSV-2 (n=3 each). At challenge 12, the SHIV-RT dose was increased to 50 TCID50 in all animals for the last nine cochallenges. Macaques treated intravaginally with gels (n=6 CG, n=8 MZC) 8 h before each cochallenge were challenged weekly for 20 weeks with 50 TCID50 SHIV-RT and 107 pfu HSV-2. SHIV infection was measured as previously described.33 (A) Survival curve showing the fraction of animals remaining SHIV plasma virus RNA negative at each challenge. (B) SHIV plasma viral loads are shown over time with each symbol representing an animal. (C) SIV-specific antibody (Ab) responses were determined against lysed simian immunodeficiency virus (SIV) by enzyme-linked immunosorbent assay (ELISA).3 Symbols are the same as in B.
Table 1.
Summary of Rhesus Macaques
| SHIV-RT | |||||
|---|---|---|---|---|---|
| Animal ID | Gel | 20 cochallenges | 26 cochallenges | SIV Ab | HSV-2 |
| EJ65 | None | ch 13a | nab | ch 15c | + |
| FE13 | None | — | na | — | + |
| FG85 | None | ch 9 | na | ch 11 | + |
| FR90 | None | ch 17 | na | ch 19 | + |
| FN39 | None | ch 3 | na | ch 5 | + |
| FK56 | None | — | na | — | + |
| HG90 | CG | ch 8 | na | ch 10 | − |
| HT08 | CG | ch 3 | na | ch 5 | +d |
| HA05 | CG | — | — | — | + |
| FC50 | CG | — | wk 1 | wk 6e | − |
| FF66 | CG | — | — | — | + |
| DH91 | CG | — | — | — | + |
| EK22 | MZC | ch 12 | na | ch 14 | + |
| DE58 | MZC | ch 4 | na | ch 6 | + |
| CV37 | MZC | — | ch 24 | ch 26 | + |
| EJ82 | MZC | — | ch 25 | wk 1 | − |
| DV13 | MZC | — | — | — | + |
| GI57 | MZC | — | — | — | + |
| IB26 | MZC | — | ch 23 | wk 1e | +d |
| HG86 | MZC | — | ch 25 | wk 8e | + |
Blood drawn immediately prior to this challenge (ch) or week post last challenge (wk) had first detectable plasma virus RNA.
na, not applicable.
Blood drawn immediately prior to this time point had first detectable SIV-specific Ab.
HSV-2 PCR positive in tissues only.
Samples not available for testing: FC50 wk 3–5, IB26 ch 24–26, HG86 wk 1–7.
SHIV-RT, simian human immunodeficiency virus reverse transcriptase; HSV-2, herpes simplex virus type 2; CG, carrageenan.
We followed the animals for 6 months, over which time their average plasma viremia (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/aid) also paralleled that observed in animals infected with a single high-dose challenge,3,6–11 confirming published results from both vaginal12,13 and rectal14 studies. SIV-specific antibodies (Abs) developed in all infected animals (Fig. 1), consistently arising 2 weeks after plasma viremia as expected (this could not be confirmed in FC50 because samples were not available immediately after infection was first detected; Table 1). No Ab response was detected in animals remaining uninfected, suggesting that repeated SHIV/HSV-2 exposure did not prime immune responses to SHIV in accordance with data acquired from repeated limiting dose rectal exposure.14–16
The study was performed in the summer when rhesus macaque menstrual cycles tend to be irregular and anovulatory.17 However, five of the six animals appeared to be cycling based on fluctuating serum estradiol-17 and progesterone levels and luteal phase characterized by progesterone peaks >1 ng/ml17 while one animal (FG85) was not cycling based on lack of periodic hormone fluctuation (Supplementary Fig. S2). This did not appear to affect susceptibility to SHIV-RT infection since three cycling animals and the noncycling animal became infected, and two cycling animals remained uninfected. Consistent with published reports,17 the cycling animals exhibited irregularity in the times of progesterone dominance, unlike pigtail macaques and humans, which have more regular, lunar cycles.18
HSV-2 infection was assessed by nested PCR (nPCR) performed on DNA isolated from vaginal swabs.3,19 Using HSV-2 plasmid DNA (Advanced Biotechnologies, Columbia, MD), we first determined the limit of detection of this nPCR to be 1–10 copies of HSV-2 DNA per reaction (Supplementary Fig. S3). Input of 100 copies or more per reaction resulted in >79% of the replicates being positive while 10 copies gave 23% positive, 1 copy gave 6.6% positive, and 0.1 copies or less gave 0% positive. The number of PCR replicates in which HSV-2 DNA was detected was proportional to the amount of viral DNA present in the sample.
Just as we observed in DP-treated animals challenged with a single SHIV-RT/HSV-2 inoculum3 (Kenney et al., unpublished observations), 100% of the animals became infected with HSV-2 after repeated cochallenge (Fig. 2 and Table 1). Because residual inoculum virus can be detected in swabs for up to 2 weeks,3 HSV-2 shedding was measured at weeks 4, 6, 8, 12, 16, and 20 post last challenge (postchallenge), preventing correlation analysis between the timing of acquisition of HSV-2 and SHIV infection. Vaginal shedding is sporadic and short-lived in humans20–23 and macaques.3,19 Thus, to gain more insight on HSV-2 infection levels, we also biopsied (vagina and cervix) all animals 8 weeks postchallenge to stress the local environment and reactivate virus, collecting swabs at 2, 6, 24, 30, 48, 54, and 72 h postbiopsy.3,19
FIG. 2.
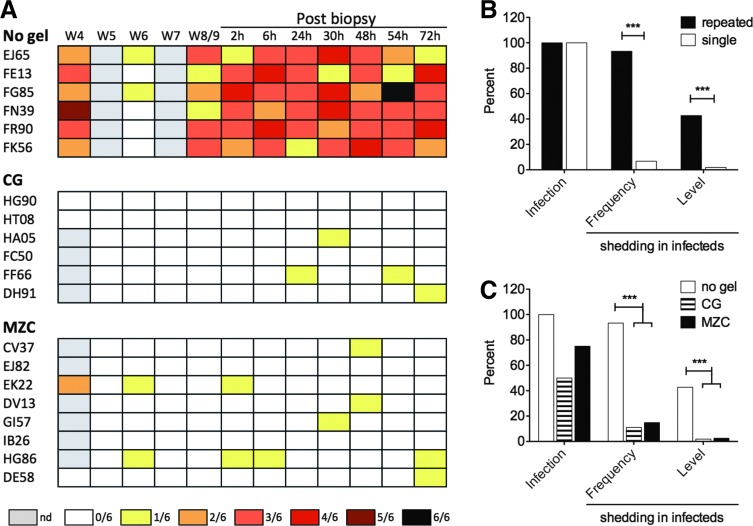
High levels of HSV-2 shedding after repeated SHIV-RT/HSV-2 cochallenge are reduced by carrageenan (CG)-containing gels. HSV-2 was measured by nested polymerase chain reaction (PCR)3,19 performed on vaginal swabs and vaginal and cervical biopsies from non-Depo-Provera (DP)-treated animals challenged weekly for 20 weeks with 2–50 TCID50 SHIV-RT and 107 pfu HSV-2 without (repeated, no gel) or with CG or MZC gel treatment 8 h prior to cochallenge or DP-treated animals challenged once with 103 TCID50 SHIV-RT and 2×108 pfu HSV-2 (single).3 (A) Heat map depicting HSV-2 shedding in vaginal swabs observed over time for the repeatedly challenged no gel, CG, and MZC-treated groups (summarized in C). Each row represents an animal. Shedding was measured at weeks 4, 5, 6, 7, and 8 or 9 postchallenge, and then again 2, 6, 24, 30, 48, 54, and 72 h following biopsy. The legend shows the colors representing the fraction of replicates positive with gray indicating that no sample was available at that time point (nd, not done). HSV-2 infection and shedding frequency and level were compared for repeated vs. single cochallenged animals (B) and repeatedly cochallenged animals treated with no gel, CG, or MZC (C). In (B) and (C), the frequency of infection was reported based on any positive PCR result in swabs or tissues. In infected animals, the frequency and level of shedding in swabs were determined from the ratio of the number of time points positive to the number of time points tested (frequency) and the ratio of the number of independent PCR replicates (of six total per swab) positive to the number of total replicates tested (level). Significant differences were determined using Fisher's exact and Chi square tests where appropriate.
Shedding was apparent in all animals at varying levels before biopsy between 4 and 8 weeks postchallenge, was reliably detected at all sampled time points postbiopsy, and dropped off thereafter during weeks 12–20 (Fig. 2A and not shown). The frequency of shedding overall was 93% (56 time points positive of 60 tested, Fig. 2B). As an indicator of relative viral load (based on the relationship derived from plasmid DNA testing), the level of shedding in HSV-2-infected animals was 42.8% (154 replicates positive of 360 replicates; Fig. 2B and Table 2) across all time points tested and 45.8% (154 of 336) when only the shedding time points were considered (Table 2). This suggests that the average number of copies per sample was in the range of 103–104 (10–100 in 10 μl of a 1,000-μl swab sample), though at times there were closer to 105 copies or more (Supplementary Fig. S3 and Fig. 2A). Both the frequency and level of shedding in the repeated limiting dose model were significantly greater than those measured in DP-treated animals cochallenged once with 103 TCID50 SHIV-RT and 2×108 pfu HSV-2 (Fig. 2B).
Table 2.
Carrageenan-Containing Gels Significantly Reduce the High Levels of Herpes Simplex Virus Type 2 Shedding After Repeated Simian Human Immunodeficiency Virus Reverse Transcriptase/Herpes Simplex Virus Type 2 Cochallenge
| HSV-2 sheddingb | ||||
|---|---|---|---|---|
| Leveld | ||||
| Gel | HSV-2 infectiona | Frequencyc | Of total time pointse | Of shedding time pointsf |
| None | 6/6 (100%) | 56/60 (93.3%) | 154/360 (42.8%) | 154/336 (45.8%) |
| CG | 4/6 (66.7%) | 4/36 (11.1%) p<0.0001g |
4/216 (1.85%) p<0.0001 |
4/24 (16.7%) p=0.005 |
| MZC | 7/8 (87.5%) | 11/74 (14.9%) p<0.0001 |
12/444 (2.70%) p<0.0001 |
12/66 (18.2%) p<0.0001 |
Infection determined by PCR on vaginal swabs and vaginal and cervical biopsies.
Shedding in swabs; reported for infected animals only.
Number of time points PCR positive/number of time points tested (%).
Number of PCR replicates (of six) positive/number of replicates tested (%).
All time points are included.
Only time points are included in which shedding was detected.
p-values are all with respect to the no gel group.
When we considered only time points during which shedding was detected, the difference in shedding level between repeated limiting dose and single high-dose models still approached significance (p=0.051), suggesting that the viral load at times of shedding was higher in the repeated limiting dose model. Detection of HSV-2-specific Abs in plasma could provide more information about the time of HSV-2 infection; however, sufficient plasma samples were not available during the cochallenges to test this. Moreover, the assay had poor sensitivity (as previously reported in the single high-dose model3) and proved an unreliable measure of infection, even when plasmas were tested on a commercially purified virus lysate (Advanced Biotechnologies, Columbia, MD). Responses in plasma after completion of the cochallenges were low to undetectable and were detected only sporadically over time (not shown). Altogether, the repeated limiting dose cochallenge model affords a system in which macaques can be infected with both SHIV-RT and HSV-2 at levels similar to the single high-dose cochallenge model but in which the impact of HSV-2 is more apparent, thereby providing a way to test products for their ability to prevent both infections, as well as to assess the impact of a product on HSV-2 shedding.
We used this model to test MZC gel, a promising microbicide candidate in development with activity against three important viral sexually transmitted infections—HIV, HSV-2, and HPV.9 MZC is composed of the nonnucleoside reverse transcriptase inhibitor (NNRTI) MIV-150, zinc acetate (ZA), and carrageenan (CG). In previous studies, MZC gel protected DP-treated macaques against SHIV-RT infection when they were challenged with a single SHIV-RT6,8,9 or SHIV-RT/HSV-23 inoculum administered 8 h after the gel (compared to the CG controls in which there was no impact of CG on SHIV-RT infection). In these animals, HSV-2 infection was reduced by only ∼30%, and no impact of the gel on HSV-2 shedding could be assessed due to the low levels of shedding.3 MZC gel with the same composition as that used herein (Supplementary Table S1) is now in Phase 1 clinical testing and has demonstrated safety and efficacy against HIV, SHIV-RT, and HSV-2 in mucosal explants (unpublished) as well as safety and efficacy against HSV-2 and human papillomavirus (HPV) in mice.9
To evaluate MZC in the repeated limiting dose model, MZC (n=8) or CG (n=6) gels were applied 8 h before each of 20 weekly cochallenges with 50 TCID50 SHIV-RT and 107 pfu HSV-2. Blood was collected to measure SHIV RNA levels and SIV-specific Ab responses immediately prior to each challenge.6,8,9 Cycling was observed in gel-treated animals as in the no gel animals (Supp. Fig. 2). The cycle length, calculated for each animal by averaging the number of progesterone dominant periods24 (P/E >1) over the weeks studied, was 3.67±0.76 (weeks, mean±SEM) in the no gel group, 4.86±0.83 in the CG group, and 5.03±0.65 in the MZC group. These were not statistically different (Kruskal–Wallis and Mann–Whitney U tests) and similar to what has been previously reported for rhesus macaques.17
After 20 cochallenges, 2/6 animals that received CG and 2/8 that received MZC were SHIV infected in contrast to the 4/6 challenged in the absence of gels (Fig. 1 and Table 1). MZC reduced SHIV infection better than CG (62.5% and 50.1% protection, respectively, vs. no gel), but this was not significant with this number of animals. The reduction in infection frequency by CG as well as MZC suggests that CG provided a barrier effect to infection in the repeated limiting dose cochallenge model. CG prevents immunodeficiency virus infection both by coating the epithelium and blocking cell–virus interactions through a characteristic polyanion effect.25 Blocking by CG was not seen in DP-treated animals when they were challenged with ∼20 times more SHIV-RT 8 h after CG dosing3,6,8 but was observed if animals were treated close to the time of SHIV challenge (e.g., 30 min before),11 indicating that there is a time-dependent and dose-dependent effect of CG.
At this inoculum and timing of challenge relative to gel application in a small number of non-DP-treated animals, the CG barrier effect masked the anti-SHIV activity of MZC. It is possible that MIV-150 levels remaining in the tissues and blood after 8 h were lower than what was observed previously in DP-treated macaques,6,9 but samples were not available for testing in this study to address this question. DP impacts the absorption of APIs26 including MIV-150,10 but recent PK studies verified that MIV-150 is readily absorbed in non-DP-treated macaques (Kenney et al., unpublished observations).
The animals that became infected over the course of these 20 challenges exhibited viremia similar to the no gel-treated animals and animals previously challenged with a single high dose of SHIV-RT or SHIV-RT and HSV-23,6,8–10 (Supplementary Fig. S1). Infected animals developed SIV-specific Ab responses similar to the no gel-treated animals (Fig. 1 and Table 1). In one uninfected animal from the MZC group, we detected a low-level Ab response beginning at week 11 and persisting throughout the remainder of the study, which may suggest low-level priming of immune responses. No other uninfected animals mounted an anti-SIV Ab response.
To confirm that gel-treated animals remaining uninfected after 20 challenges were indeed susceptible to SHIV infection and to see whether we could overcome the barrier effect of CG, we performed six additional weekly cochallenges at the conclusion of the 20-challenge study using 150 TCID50 SHIV-RT combined with 107 pfu HSV-2. Only the animals remaining SHIV negative were challenged and received the same gel regimen as during the first phase of the study (Table 1). One of four CG-treated animals became infected with its first positive plasma virus RNA detected 1 week after the last challenge, and 4/6 MZC-treated animals became infected, with viremia first detected in blood drawn just before challenges 23, 24, and 25 (two animals). SIV-specific Abs developed in all animals upon becoming infected (Table 1).
While a larger fraction of the MZC-treated animals became infected in this phase of the study vs. CG-treated animals, the difference was not statistically significant (Fisher's exact test) and may reflect a number of contributing factors including the number of animals available for infection, HSV-2 infection status at the time of dose escalation (which could not be determined due to the presence of virus DNA from residual inoculum), and possible impact of the gels on the vaginal epithelium during an extended use regimen. However, previous long-term testing revealed no indication of safety concerns,6 and rabbit vaginal irritation studies showed that daily MZC gel application for 14 days resulted in no irritation at any gel dose tested (unpublished). Additional vaginal and rectal safety testing of the MZC gel in the pigtail macaque model is ongoing; however, extended chronic safety studies in humans are still needed. Data to that end will be gathered from the Phase 1 trial of vaginal MZC gel currently underway.
As five animals were still uninfected at the conclusion of these last six challenges, we confirmed their susceptibility to infection in vitro, infecting phytohemagglutinin/interleukin-2 (PHA/IL-2)-activated PBMCs (isolated from the animals by Ficoll Hypaque centrifugation19,27) with 100 TCID50/ml SHIV-RT.4 By qPCR and p27 ELISA,4 PBMCs from four of five of these animals (all except HA05, a CG-treated animal) became infected with SHIV-RT as did control 174xCEM cells.
It has been suggested that the luteal phase of the menstrual cycle, characterized by high levels of progesterone, constitutes a “window of vulnerability” to infection.18,28 However, the actual concentrations of estrogens and progesterone observed here could not be associated with susceptibility to SHIV-RT (not shown). This may be due to the infrequent sampling (weekly), the time of year the study was performed, and the uncertainty associated with identifying the exact time of infection. However, the ratio of progesterone to estradiol-17 (P/E)24 in animals from any of the groups (no gel, CG, or MZC) that became SHIV infected during the 20- or 26-challenge regimen in the weeks leading up to virus positivity (Supplementary Fig. S2, the red symbols indicate the first positive plasma viral load) significantly declined (p=0.048, Wilcoxon rank sum test) from the time point 3 weeks before the first positive plasma viral load (−3) to the time point 2 weeks before (−2) and then trended upward again over the following 2 weeks (Supplementary Fig. S4). In one outlier animal (DE58), the P/E increased from week −3 to week −2 but then declined thereafter following the same trend.
These results suggest that although the concentrations of progesterone and estrogens could not be correlated with SHIV susceptibility, the time of infection with respect to the menstrual cycle was synchronized. Greater susceptibility coincided with the end of the luteal phase when progesterone levels are rapidly decreasing compared to more stable lower levels of estrogens, consistent with reports in rhesus and pigtail macaques as well as humans.18,28 Synchronicity was also observed in another cohort of repeatedly challenged non-DP-treated macaques (Kenney et al., unpublished observations), in which P/E declined in the weeks preceding systemic infection. Validating an eclipse period of up to 4 weeks in this model, systemic infection was detected 4 weeks post the last challenge in one animal in that study. However, on noninfecting challenges in animals that eventually did become infected herein as well as those that remained SHIV negative, no correlation was evident between protection and cycling, lack thereof, or the concentrations/ratios of endogenous sex hormones (Supplementary Fig. S2).
All gel-treated animals were assayed for HSV-2 infection in vaginal swabs at weeks 4, 5, 6, 7, and 9 after 20–26 cochallenges and again 2, 6, 24, 30, 48, 54, and 72 h post week 9 biopsy (relative to the last cochallenge). Infection was also measured in the vaginal and cervical biopsy tissues collected from these animals. A similar fraction of CG-treated and MZC-treated animals became HSV-2 infected (4/6 CG and 7/8 MZC, 66.7% and 87.5%, respectively, p=0.539 Fisher's exact test; Fig. 2 and Table 1), comparable to the MZC effect on HSV-2 infection after a single cochallenge of DP-treated animals.3 One animal from each group had no detectable shedding and was only positive in the tissues (Table 1). There was no obvious correlation between SHIV and HSV-2 infections, but analysis is hampered by not being able to pinpoint when the animals were infected with HSV-2.
Protection from HSV-2 in mice has been shown to depend on both the time of gel application relative to challenge and also the viral dose.9 Thus, challenging macaques with a lower inoculum at a time closer to gel dosing would likely be more effective. The HSV-2 inoculum used herein (∼107 DNA copies per challenge3) was selected relative to the single high-dose model to ensure that we observed vaginal shedding,19 but it is still orders of magnitude greater than that observed in infected humans (102–105 DNA copies/ml).20,21,29 Given that the repeated limiting dose model produces higher levels of shedding, we plan to test a more relevant dose in future studies, which may better enable us to detect protection from outright HSV-2 infection. Despite only slightly reducing HSV-2 infection, both CG and MZC significantly decreased HSV-2 shedding frequencies and levels compared to those seen in the no gel group of animals (Fig. 2). Even when only shedding time points were included in the analysis, both gels significantly reduced shedding levels (16.7% CG, 18.2% MZC; p=0.005 for CG vs. no gel and p<0.0001 for MZC vs. no gel, Fisher's exact test). This level translates to approximately 102 copies of HSV-2 DNA per sample (1 copy in 10 μl of a 1,000-μl sample), which is ∼1–2 logs reduction in viral load. Reduced shedding, suggesting a less severe infection, may have resulted from the physical barrier of CG allowing less virus across the epithelium.
Although in mice, the combination of ZA and CG is more potent and durable against HSV-2,9 CG has been shown to prevent low-dose vaginal HSV-2 infection of mice when applied close to the time of challenge.30 In this model when cochallenging 8 h after each gel dose, there appeared to be no additional effect of MZC over CG.
The barrier effects of CG in this repeated cochallenge model confounded evaluation of the MZC gel, which, compared to untreated (no gel) animals, did reduce infections (though not significantly) and significantly diminished HSV-2 shedding. Importantly, we have shown in a stringent single high-dose model that forces one application of microbicide to protect against a large viral inoculum that MZC gel indeed significantly protects against SHIV-RT (with no CG barrier effect) when applied up to 8 h before challenge.
These data confirm the relevance of testing MZC gel clinically for the prevention of HIV acquisition. Potent activity of MZC gel vs. CG control was also confirmed in vaginal explants in vitro even when tissues were challenged up to 4 days post gel exposure.31 However, when tissues from MZC-treated vs. CG-treated macaques (DP treated) were challenged with SHIV-RT ex vivo, both MZC and CG reduced infection. Thus, for certain models, including the repeated limiting dose model described here, the data suggest that CG is not an appropriate control. Other control gels have no activity close to the time of challenge and thus might be more suitable in the model described here. The universal placebo, hydroxyethylcellulose (HEC), has shown no activity 30 min before repeated vaginal challenge in non-DP-treated macaques,32 and we have shown that methyl cellulose has no activity in DP-treated macaques 30 min before high-dose SHIV-RT challenge.11 Despite the durability of MZC gel in the single high-dose model, MZC is being developed for on-demand use and would be indicated for use closer to the time of potential viral exposure. In that setting, CG might contribute to the activity even more—as we have observed in the single high-dose model 30 min before challenge11 and in tissues from treated macaques31—even though the CG gel, Carraguard, was not effective against HIV in a Phase 3 clinical trial.33
To better deliver all three components of the MZC combination with improved control over release of each, we have developed an intravaginal ring (IVR). Initial studies have revealed that an MZC-containing IVR significantly protects macaques (67%) against SHIV-RT infection and also significantly reduces HSV-2 shedding after repeated cochallenge (Kenney et al., unpublished observations). Nonetheless, both gels and IVRs have an important role to play in public health as the MZC gel also exhibits potential for rectal use. We have previously demonstrated that the MZC gel is isoosmolar and safe rectally as well as vaginally8,9 and protects against HSV-2 and HPV in rectally challenged mice.9
In conclusion, we established a novel, repeated limiting dose cochallenge model of SHIV and HSV-2 coinfection in macaques that can be utilized to evaluate the ability of candidate microbicides to prevent or reduce both HIV and HSV-2 infections.
Supplementary Material
Acknowledgments
We thank the veterinary staff at TNPRC for continued support. Hormone analysis by the Endocrine Technology and Support Core was supported by the Oregon National Primate Research Center Core Grant P51 OD011092. This work was supported by the United States Agency for International Development (USAID) Cooperative Agreement GPO-A-00-04-00019-00, the TNPRC base grant P51-OD011104-52, and federal funds from the National Cancer Institute, NIH, under contract HHSN261200800001E. This research is made possible by the generous support of the American people through the USAID.
The contents of this article are the sole responsibility of the Population Council and do not necessarily reflect the views or policies of the funding agencies. The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the article. None of the material in this article has been published or is under consideration elsewhere, including the Internet. M.R. is a 2001 Elizabeth Glaser scientist.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Freeman EE, Weiss HA, Glynn JR, et al. : Herpes simplex virus 2 infection increases HIV acquisition in men and women: Systematic review and meta-analysis of longitudinal studies. AIDS 2006;20(1):73–83 [DOI] [PubMed] [Google Scholar]
- 2.Lingappa JR, Baeten JM, Wald A, et al. : Daily acyclovir for HIV-1 disease progression in people dually infected with HIV-1 and herpes simplex virus type 2: A randomised placebo-controlled trial. Lancet 2010;375(9717):824–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu M, Aravantinou M, Menon R, et al. : A combination microbicide gel protects macaques against vaginal SHIV-RT infection, but only partially reduces HSV-2 infection after a single high-dose co-challenge. AIDS Res Hum Retroviruses 2014;30(2):174–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Singer R, Derby N, Rodriguez A, et al. : The nonnucleoside reverse transcriptase inhibitor MIV-150 in carrageenan gel prevents rectal transmission of simian/human immunodeficiency virus infection in macaques. J Virol 2011;85(11):5504–5512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aurelian L: Herpes simplex virus. In: Clinical Virology Manual, 3rd ed. (Specter S, Hodinka RL, Young SA, eds). ASM Press, Washington, DC, 2000, pp. 384–409 [Google Scholar]
- 6.Kenney J, Aravantinou M, Singer R, et al. : An antiretroviral/zinc combination gel provides 24 hours of complete protection against vaginal SHIV infection in macaques. PLos One 2011;6(1):e15835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kenney J, Rodriguez A, Kizima L, et al. : A modified zinc acetate gel, a potential nonantiretroviral microbicide, is safe and effective against simian-human immunodeficiency virus and herpes simplex virus 2 infection in vivo. Antimicrob Agents Chemother 2013;57(8):4001–4009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kenney J, Singer R, Derby N, et al. : A single dose of a MIV-150/zinc acetate gel provides 24 h of protection against vaginal simian human immunodeficiency virus reverse transcriptase infection, with more limited protection rectally 8–24 h after gel use. AIDS Res Hum Retroviruses 2012;28(11):1476–1484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kizima L, Rodriguez A, Kenney J, et al. : A potent combination microbicide that targets SHIV-RT, HSV-2 and HPV. PLoS One 2014;9(4):e94547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Singer R, Mawson P, Derby N, et al. : An intravaginal ring that releases the NNRTI MIV-150 reduces SHIV transmission in macaques. Sci Transl Med 2012;4(150):150ra123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Turville SG, Aravantinou M, Miller T, et al. : Efficacy of Carraguard-based microbicides in vivo despite variable in vitro activity. PLoS One 2008;3(9):e3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harouse JM, Gettie A, Eshetu T, et al. : Mucosal transmission and induction of simian AIDS by CCR5-specific simian/human immunodeficiency virus SHIV(SF162P3). J Virol 2001;75(4):1990–1995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng-Mayer C, Huang Y, Gettie A, et al. : Delay of simian human immunodeficiency virus infection and control of viral replication in vaccinated macaques challenged in the presence of a topical microbicide. AIDS 2011;25(15):1833–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.McDermott AB, Mitchen J, Piaskowski S, et al. : Repeated low-dose mucosal simian immunodeficiency virus SIVmac239 challenge results in the same viral and immunological kinetics as high-dose challenge: A model for the evaluation of vaccine efficacy in nonhuman primates. J Virol 2004;78(6):3140–3144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kersh EN, Luo W, Adams DR, et al. : Repeated rectal SHIVSF162P3 exposures do not consistently induce sustained T cell responses prior to systemic infection in the repeat-low dose preclinical macaque model. AIDS Res Hum Retroviruses 2009;25(9):905–917 [DOI] [PubMed] [Google Scholar]
- 16.Regoes RR: The role of exposure history on HIV acquisition: Insights from repeated low-dose challenge studies. PLoS Comput Biol 2012;8(11):e1002767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Walker ML, Gordon TP, and Wilson ME: Menstrual cycle characteristics of seasonally breeding rhesus monkeys. Biol Reprod 1983;29(4):841–848 [DOI] [PubMed] [Google Scholar]
- 18.Vishwanathan SA, Guenthner PC, Lin CY, et al. : High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. J Acquir Immune Defic Syndr 2011;57(4):261–264 [DOI] [PubMed] [Google Scholar]
- 19.Crostarosa F, Aravantinou M, Akpogheneta OJ, et al. : A macaque model to study vaginal HSV-2/immunodeficiency virus co-infection and the impact of HSV-2 on microbicide efficacy. PLoS One 2009;4(11):e8060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tronstein E, Johnston C, Huang ML, et al. : Genital shedding of herpes simplex virus among symptomatic and asymptomatic persons with HSV-2 infection. JAMA 2011;305(14):1441–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mark KE, Wald A, Magaret AS, et al. : Rapidly cleared episodes of herpes simplex virus reactivation in immunocompetent adults. J Infect Dis 2008;198(8):1141–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sacks SL, Griffiths PD, Corey L, et al. : Introduction: Is viral shedding a surrogate marker for transmission of genital herpes? Antiviral Res 2004;63(Suppl 1):S3–9 [DOI] [PubMed] [Google Scholar]
- 23.Sacks SL, Griffiths PD, Corey L, et al. : HSV shedding. Antiviral Res 2004;63(Suppl 1):S19–26 [DOI] [PubMed] [Google Scholar]
- 24.Hadzic SV, Wang X, Dufour J, et al. : Comparison of the vaginal environment of Macaca mulatta and Macaca nemestrina throughout the menstrual cycle. Am J Reprod Immunol 2014;71(4):322–329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fletcher PS, Wallace GS, Mesquita PM, and Shattock RJ: Candidate polyanion microbicides inhibit HIV-1 infection and dissemination pathways in human cervical explants. Retrovirology 2006;3(1):46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Malcolm RK, Veazey RS, Geer L, et al. : Sustained release of the CCR5 inhibitors CMPD167 and maraviroc from vaginal rings in rhesus macaques. Antimicrob Agents Chemother 2012;56(5):2251–2258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Frank I, Piatak M, Jr, Stoessel H, et al. : Infectious and whole inactivated simian immunodeficiency viruses interact similarly with primate dendritic cells (DCs): Differential intracellular fate of virions in mature and immature DCs. J. Virol 2002;76(6):2936–2951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sodora DL, Gettie A, Miller CJ, and Marx PA: Vaginal transmission of SIV: Assessing infectivity and hormonal influences in macaques inoculated with cell-free and cell-associated viral stocks. AIDS Res Hum Retroviruses 1998;14(Suppl 1):S119–123 [PubMed] [Google Scholar]
- 29.Gianella S, Strain MC, Rought SE, et al. : Associations between virologic and immunologic dynamics in blood and in the male genital tract. J Virol 2012;86(3):1307–1315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zacharopoulos VR. and Phillips DM: Vaginal formulations of carrageenan protect mice from herpes simplex virus infection. Clin Diagn Lab Immunol 1997;4(4):465–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barnable P, Calenda G, Ouattara L, et al. : A MIV-150/zinc acetate gel inhibits SHIV-RT infection in macaque vaginal explants. PLoS One 2014;9(9):e108109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Parikh UM, Dobard C, Sharma S, et al. : Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. J Virol 2009;83(20):10358–10365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Skoler-Karpoff S, Ramjee G, Ahmed K, et al. : Efficacy of Carraguard for prevention of HIV infection in women in South Africa: A randomised, double-blind, placebo-controlled trial. Lancet 2008;372(9654):1977–1987 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.