Abstract
Empirical testing of candidate vaccines has led to the successful development of a number of lifesaving vaccines. The advent of new tools to manipulate antigens and new methods and vectors for vaccine delivery has led to a veritable explosion of potential vaccine designs. As a result, selection of candidate vaccines suitable for large-scale efficacy testing has become more challenging. This is especially true for diseases such as dengue, HIV, and tuberculosis where there is no validated animal model or correlate of immune protection. Establishing guidelines for the selection of vaccine candidates for advanced testing has become a necessity. A number of factors could be considered in making these decisions, including, for example, safety in animal and human studies, immune profile, protection in animal studies, production processes with product quality and stability, availability of resources, and estimated cost of goods. The “immune space template” proposed here provides a standardized approach by which the quality, level, and durability of immune responses elicited in early human trials by a candidate vaccine can be described. The immune response profile will demonstrate if and how the candidate is unique relative to other candidates, especially those that have preceded it into efficacy testing and, thus, what new information concerning potential immune correlates could be learned from an efficacy trial. A thorough characterization of immune responses should also provide insight into a developer's rationale for the vaccine's proposed mechanism of action. HIV vaccine researchers plan to include this general approach in up-selecting candidates for the next large efficacy trial. This “immune space” approach may also be applicable to other vaccine development endeavors where correlates of vaccine-induced immune protection remain unknown.
Introduction
Advances in molecular modeling and recombinant technology have greatly expanded the number of candidate vaccines that could potentially be tested in diseases where the absence of a predictive animal model or known correlates of protection would necessitate empiric efficacy testing. This is especially true for diseases such as dengue, HIV, malaria, and tuberculosis where there is no validated animal model or correlate of immune protection. Establishing criteria that will help select unique vaccine candidates that not only have the potential for technical success, but will also help guide future vaccine design is an imperative. One approach, summarized here, is to systematically profile the immunological response induced by candidate vaccines, thereby providing a potential approach to rationally compare vaccine platforms, distinguish those that are most likely to advance the field, and provide insight into potential correlates of immune protection.
HIV Vaccine Development in 2014
Development of a safe and effective HIV vaccine will be central to any global strategy to slow and one day end the HIV epidemic. Yet development of that vaccine faces enormous scientific challenges. Although some individuals can control HIV infection for many years without antiretroviral therapy,1,2 HIV successfully evades and escapes the natural immune response to infection in most infected persons.3,4 HIV's global variability, the lack of a validated correlate of protective immunity, and the lack of an animal model that reliably predicts vaccine efficacy in humans remain key obstacles to vaccine development. Novel antigens and vector delivery systems are expanding the depth, breadth, and durability of measured immune responses and animal models are being refined with a view to establishing meaningful correspondence to observed efficacy data in humans.5 In addition, new adjuvants have been heralded as an advance for HIV, malaria, and TB vaccines, and unique adjuvants may help induce unique immune responses.6–8
In the almost three decades since the epidemic began, six human efficacy trials that evaluated four different vaccine strategies have been completed (Table 1). The RV144 Thai trial, which evaluated a canarypox prime followed by boosts with canarypox and gp120 envelope protein in a community-based trial in Thailand, was the only trial to demonstrate that a vaccine candidate can protect against HIV acquisition, although protection was modest (31.2% efficacy).9 A case-controlled evaluation of specimens from RV144 generated specific hypotheses regarding correlates of risk10; these hypotheses, which will be evaluated in future trials, may or may not prove to be valid in other populations (with different host genetics, circulating HIV subtypes, prior antigenic exposures, environmental factors, and/or transmission routes), or when other vaccine designs are evaluated. For example, efficacy trials that build directly on RV144 are planned for South Africa and Thailand. The immune profile of clade C-based vaccines similar to that used in the RV144 trial will serve as the basis for determining if that vaccine should also advance into a licensure trial. In addition, multiarm trials to help select candidates to advance into a phase 2b “correlates” study will include a number of different primes (e.g., canary pox, NYVAC, DNA, or DNA then NYVAC) followed by readministration of the prime combined with a gp120 envelope boost, which may be formulated on one of two different adjuvants. Characterization of the immune response profiles will demonstrate how each candidate is unique and determine if new immune correlate hypotheses could be explored in an efficacy trial.
Table 1.
Overview of HIV-1 Vaccine Efficacy Trials Performed to Date
| Trial Name | VAX 004a | VAX 003b | HVTN 502 (STEP)c | HVTN 503 (Phambili)d | RV144e | HVTN 505f |
|---|---|---|---|---|---|---|
| Year | 1998–2002 | 1999–2003 | 2004–2007 | 2006–2007 | 2003–2009 | 2009–2013 |
| Vaccine approach | Recombinant gp120 protein (AIDSVAX B/B) | Recombinant gp120 protein (AIDSVAX B/E) | Adenovirus serotype 5 vector (MRKAd5 HIV-1) | Pox prime (ALVAC-HIV) Recombinant gp120 protein boost (AIDSVAX B/E) | DNA prime (VRC-HIVDNA016-00-VP) Adenovirus serotype 5 boost (VRC-HIVADV014-00-VP) | |
| Population | Men who have sex with men and women at high risk for heterosexual transmission of HIV-1 | Injection drug users | Men who have sex with men and women at high risk for heterosexual transmission of HIV-1 | Heterosexual community-based | Heterosexual community-based | Ad5 negative, circumcised men who have sex with men and transgender women |
| Age eligibility | 18–60 years | 20–60 years | 18–45 years | 18–35 years | 18–30 years | 18–50 years |
| Location | North America, the Netherlands, and Puerto Rico | Bangkok, Thailand | North America, the Caribbean, South America, and Australia | South Africa | Rayong and Chon Buri Provinces, Thailand | United States |
The rgp120 HIV Vaccine Study Group: placebo-controlled phase 3 trial of a recombinant glycoprotein 120 vaccine to prevent HIV-1 infection. J Infect Dis 2005;191:654–665.
Pitisuttithum P, et al.: Randomized, double-blind, placebo-controlled efficacy trial of a bivalent recombinant glycoprotein 120 HIV-1 vaccine among injection drug users in Bangkok, Thailand. J Infect Dis 2006;194:1661–1671.
Buchbinder SP, et al.: Efficacy assessment of a cell-mediated immunity HIV-1 vaccine (the Step Study): a double-blind, randomised, placebo-controlled, test-of-concept trial. Lancet 2008;372:1881–1893.
Gray G, et al.: Safety and efficacy assessment of the HVTN 503/Phambili Study: a double-blind randomized placebo-controlled test-of-concept study of a clade B-based HIV-1 vaccine in South Africa. Lancet Infect Dis 2011;11:507–515.
Rerks-Ngarm S, et al.: Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009;361:2209–2220.
Hammer, SM, et al.: Efficacy trial of a DNA/rAd5 HIV-1 preventive vaccine. N Engl J Med 2013;369(22):2083–2092.
In addition to the planned RV144 follow-up studies, a number of new vaccine strategies are in earlier stages of the evaluation. These include novel nonreplicating pox, chimpanzee and simian adenoviruses, replicating pox and other replicating vectors, and new forms of HIV envelope protein, including those designed to engage specific germ line B cell receptors or present a “native” envelope trimer.11–14
The Immune Space Template
Evaluation of a vaccine concept from inception to test-of-concept trial requires tens of millions of dollars, time, and significant resource commitments. Community engagement and education need to be initiated well before efficacy trial initiation. Trial site preparations may require a year or longer depending on existing infrastructure and the level of training and expertise at the site. Continued training of personnel, monitoring of participants, and ongoing resource-intensive quality management and assurance processes are required. Finally, trial volunteers need to be followed for up to 5 years after the trial concludes.
Recent advances in vaccine immunology and design have led to far more vaccine candidates than can be evaluated in test-of-concept trials given the limitations in funding. Trial developers will thus need to judiciously select among vaccine candidate designs, and funders will need to prioritize which designs will move forward into test-of-concept or efficacy trials. A number of factors are likely to be considered (Table 2). These include, for example, (1) an induced immunological profile in early clinical trials that is distinct in character and/or durability from the candidates that preceded it, (2) significant protection in a NHP model that closely parallels human experience, and/or (3) a product profile that indicates the final cost of production and delivery will be suitable for those who most need a preventive vaccine, etc.
Table 2.
Potential Considerations for Advancement to Efficacy Trials
| Immune profile in early clinical trials (immune space filled; potential to help decipher correlates; relationship to circulating strains; durability of response) |
| Safety profile |
| Protection data in nonhuman primate trials |
| Product/production considerations (GMP scalable; route of administration; administration schedule; dose requirements; stability, cost of goods, etc.) |
| Availability of resources (funding; clinical trial site capacity) |
| Acknowledged public and personal health need and buy-in by the local community |
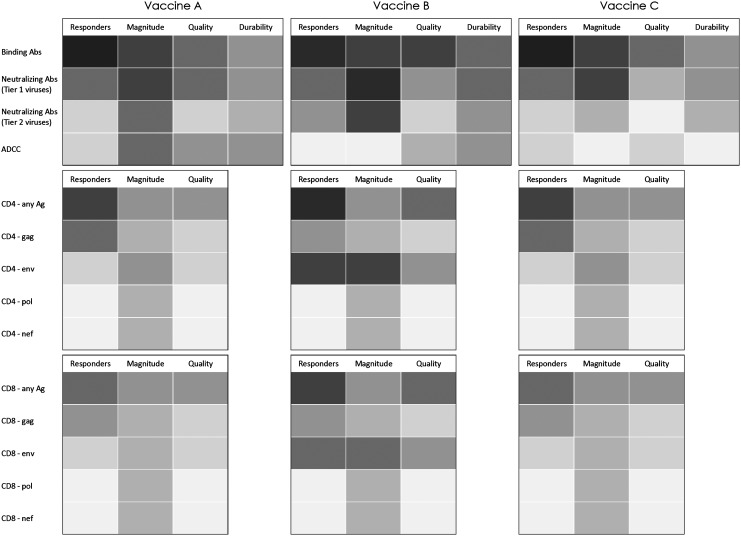
Until a correlate of immune protection or an animal model is validated, one factor in deciding which candidates should advance to efficacy testing would be to advance candidates that elicit distinct immunological profiles in early clinical trials and, in doing so, enable evaluation of a spectrum of potential correlate(s) of immune protection in the context of phase 2b vaccine efficacy trials. Components of a distinct profile could be, for example, the type, specificity, and ratios of antibody or T cell responses, the anatomic compartment(s) of the responses, and/or the durability or breadth of the responses. The “immune space template” proposed here (www.vaccineenterprise.org/immunespace) provides an approach by which the character, quantity, and durability of immune responses elicited by a candidate HIV vaccine in early human trials can be described (Fig. 1). The immune space template aims to provide vaccine developers with a tool to describe the immunological characteristics of a vaccine candidate and its potential mechanism(s) of action.
FIG. 1.
A portion of the immune space filled by three hypothetical vaccine candidates (A–C). All three candidates elicit humoral and cellular immune responses through distinct vaccination approaches. However, vaccines A and C induce similar immune profiles. Vaccine funders would use this information together with results from protection in NHP trials and product and production considerations and move forward with the candidates that fill a different profile. Abs, antibodies.
Similarly, the frequency, magnitude, and variability of elicited immune responses will further define what immune responses are sufficiently robust to enable an evaluation of whether that response correlates with the biological outcome. The template will allow comparison of immune profiles induced by different candidates. Vaccine developers will be able to better describe how their approach differs from other approaches, and funders can consider this information in determining whether the vaccine concept warrants additional investment.
Assays Included in the Immune Space Template
The immune space template provides a practical list of standardized assays that are recommended in the early clinical evaluation of all HIV candidate vaccines and is divided into “core” assays and “nice-to-have” assays. The list of assays was developed by a group of expert laboratory researchers* and refined based on feedback from numerous other experts and stakeholders in the HIV vaccine field. “Core” refers to those assays that describe the functional nature and breadth of immune responses that may be elicited through vaccination and that could prove to be relevant for protection (Table 3). Core assays do not necessarily relate to formal trial endpoints that are used to assess immunogenicity. Although specific assays, labs, reagents, vaccine strains, etc. are not described in the template, vaccine developers are strongly encouraged to utilize assays that are either performed in a central laboratory established for the purpose of conducting comparative assays or that are at least standardized if not fully validated.15 A number of groups, including the Duke Central Reference Laboratory, the Vaccine Research Center, the HIV Vaccine Trials Network central core laboratories, and others, have made their standardized and often also validated protocols available.16–20 “Core” assays are recommended to be carried out on every candidate that a vaccine developer hopes to advance to phase IIb/III trials.
Table 3.
Currently Suggested “Core” Assays
| Humoral assays |
| 1. Plasma or serum anti-Env IgG binding antibody (if envelope is included in the vaccine) |
| • Percent responders |
| • Magnitude of response to the vaccine strain(s) |
| • Magnitude of response to circulating strains in proposed phase IIb trial population, preferably transmitted/founder strains |
| • Durability of response |
| • Specificity of response at peak immunogenicity time point [clade specificity, transmitted/founder vs. chronic, epitopes (linear and conformational, including V2)] |
| 2. Plasma or serum anti-Env IgA binding antibody (if envelope is included in the vaccine) |
| • Percent responders |
| • Magnitude of response to vaccine strain(s) |
| • Durability of response |
| • Calculation of ratio anti-env IgG to anti-env IgA |
| 3. HIV-1 neutralization |
| • Percent responders |
| • Magnitude of response to vaccine strain(s) |
| • Durability of neutralization against vaccine strain(s) and against circulating strains in proposed trial population; standard tier 1 and tier 2 panels of molecularly cloned viruses are recommended |
| 4. ADCC against virus-infected cell targets (preferably the same subtype as circulating strains if not transmitted/founder strains from the proposed test regions) |
| • Assays that utilize virus-infected cell targets are preferred |
| Cellular assays |
| 1. ICS or both ICS and ELISpot |
| • Percent responders: total, CD4+ T cells, CD8+ T cells |
| • Magnitude of response to vaccine |
| • Number and types of cytokines (minimum of three, preferably four) |
| 2. Cellular proliferation in response to vaccine antigen(s) (CFSE cell staining) |
| • Percent responders |
ADCC, antibody-dependent cellular cytotoxicity; ICS, intracellular cytokine staining; ELISpot, enzyme-linked immunosorbent spot; CFSE, carboxyfluoresceinsuccinimidyl ester.
Assays included under “nice to have” are encouraged based on the immune responses the vaccine was designed to elicit and should be standardized, but are not considered core at this time due to practical issues such as difficulties in qualification, specimen collection, and assay parameters.
All assays should be performed at a minimum on quality specimens collected at the peak of immune response, at ∼6 months after the last immunization, and perhaps one or more time points in between to characterize peak and contracted responses. Assays that are not qualified or validated with low false positivity would also require specimen collection at baseline. Vaccine developers are often frustrated by the timeline of candidate vaccine development and evaluation so that durability of the immune response is relegated to a secondary evaluation level until the candidate is shown to have a unique immunological profile or other advantage.
As additional knowledge is gained and new assays are developed and made available, this list will evolve; assays may shift from “nice to have” to “core” and assays may be added or replaced. For example, the quality and quantity of vaccine-induced immune responses at the initial mucosal site(s) of HIV entry are presumed to be of importance for vaccine design. Mucosal assays, currently listed under “nice to have,” could become “core” when issues around collection, storage methods, and timing of collection are addressed, and when assays become standardized if not qualified, and sufficient quantities of specimens can be acquired more routinely. The immune space template will be a “living” document maintained by the Global HIV Vaccine Enterprise (www.vaccineenterprise.org/immunespace), which is an alliance of independent organizations committed to accelerating the development of an HIV vaccine through collaboration and coordination, and will be reviewed regularly and updated as needed.
Conclusions and Next Steps
Test-of-concept and efficacy trials of prophylactic vaccines provide a unique opportunity to gain information on potential correlates of immune protection in humans. Identifying such correlates would greatly accelerate the design and development of improved safe and effective vaccine candidates. However, limited resources demand careful selection of what candidates should advance. While the immune space should not be interpreted as synonymous with criteria for advancement to an efficacy study, together with preclinical studies and production and community considerations, understanding the immune profile induced by each candidate will help inform a more rational pathway for the selection of candidates to advance. Although there is no certainty that the core assays have any relationship to protection, or that vaccines occupying different immune spaces as defined by these assays are necessarily independent, by testing candidates with unique immunological profiles the identification of immune correlates should be accelerated. And when potential correlates are identified, subsequent trials to further evaluate those hypothetical correlates of protection, as is planned for the RV144 pox-protein combination, would be justified.
The HVTN is leading the design and implementation of efficacy trials in southern Africa to build on the RV144 trial results. To help up-select candidates for inclusion in a multiarm efficacy trial designed to identify potential correlates of protection, the HVTN is developing an algorithm to measure the unique immune profile of several vaccine candidates and combinations that they are evaluating in smaller trials (Peter Gilbert, personal communication).
This method of defining an immune space could be utilized by other vaccine developers and funders struggling with challenges similar to those facing the HIV vaccine field. The list of assays describing the immune space could be adapted to include what is viewed as the immune responses that may be important for protection against each disease and evolve together with the science to ensure that the most relevant immune space is captured.
Acknowledgments
We thank Marcus Altfeld, Barton Haynes, Rick Koup, John Mascola, Julie McElrath, Bali Pulendran, and Georgia Tomaras who helped develop an initial list of assays to be included in the immune space template; we also thank Peter Gilbert and Julie McElrath for insights into the HVTN plans for efficacy studies in southern Africa. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the U.S. Government or any of the other affiliate organizations. IAVI's work is made possible by generous support from many donors. A full list of IAVI donors is available at www.iavi.org
Author Disclosure Statement
No competing financial interests exist.
The initial expert group included Marcus Altfeld, Barton Haynes, Jerome Kim (co-chair), Rick Koup, John Mascola, Julie McElrath, Bali Pulendran, and Georgia Tomaras, facilitated by Margaret I. Johnson (co-chair).
References
- 1.Blankson JN, Bailey JR, Thayil S, et al. : Isolation and characterization of replication-competent human immunodeficiency virus type 1 from a subset of elite suppressors. J Virol 2007;81(5):2508–2518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Miura T, Brumme CJ, Brockman MA, et al. : HLA-associated viral mutations are common in human immunodeficiency virus type 1 elite controllers. J Virol 2009;83(7):3407–3412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lackner AA, Lederman MM, and Rodriguez B: HIV pathogenesis: The host. Cold Spring Harb Perspect Med [Internet] 2012. [cited 2013 Jul 18];2(9). Available from http://perspectivesinmedicine.cshlp.org/content/2/9/a007005 [DOI] [PMC free article] [PubMed]
- 4.Swanstrom R. and Coffin J: HIV-1 pathogenesis: The virus. Cold Spring Harb Perspect Med [Internet] 2012. [cited 2013 July18];2(12). Available from http://perspectivesinmedicine.cshlp.org/content/2/12/a007443 [DOI] [PMC free article] [PubMed]
- 5.Evans DT. and Silvestri G: Nonhuman primate models in AIDS research. Curr Opin HIV AIDS 2013;8(4):255–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffman RL, Sher A, and Seder RA: Vaccine adjuvants: Putting innate immunity to work. Immunity 2010;33(4)492–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dey AK. and Srivastava IK: Novel adjuvants and delivery systems for enhancing immune responses induced by immunogens. Expert Rev Vaccines 2011;10(2):227–251 [DOI] [PubMed] [Google Scholar]
- 8.Harandi AM, Medaglini D, Shattock RJ, Working Group convened by EUROPRISE: Vaccine adjuvants: A priority for vaccine research. Vaccine 2010;28(12):2363–2366 [DOI] [PubMed] [Google Scholar]
- 9.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, et al. : Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009;361(23):2209–2220 [DOI] [PubMed] [Google Scholar]
- 10.Haynes BF, Gilbert PB, McElrath MJ, et al. : Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med 2012;366(14):1275–1286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jardine J, Julien J-P, Menis S, et al. : Rational HIV immunogen design to target specific germline B cell receptors. Science 2013;340(6133):711–716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGuire AT, Hoot S, Dreyer AM, et al. : Engineering HIV envelope protein to activate germline B cell receptors of broadly neutralizing anti-CD4 binding site antibodies. J Exp Med 2013;210(4):655–663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Klasse PJ, Depetris RS, Pejchal R, et al. : Influences on trimerization and aggregation of soluble, cleaved HIV-1 SOSIP envelope glycoprotein. J Virol 2013;87(17):9873–9885 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Haynes BF, Kelsoe G, Harrison SC, and Kepler TB. B-cell-lineage immunogen design in vaccine development with HIV-1 as a case study. Nat Biotechnol 2012;30(5):423–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guidance for Industry: Q2B Validation of Analytical Procedures: Methodology [Internet]. [cited 2014March4]. Available from www.fda.gov/downloads/Regulator%20yInformation/Guidances/UCM128049.pdf
- 16.Lamoreaux L, Roederer M, and Koup R: Intracellular cytokine optimization and standard operating procedure. Nat Protoc 2006;1(3):1507–1516 [DOI] [PubMed] [Google Scholar]
- 17.Dubey S, Clair J, Fu T-M, et al. : Detection of HIV vaccine-induced cell-mediated immunity in HIV-seronegative clinical trial participants using an optimized and validated enzyme-linked immunospot assay. J Acquir Immune Defic Syndr 1999 2007;45(1):20–27 [DOI] [PubMed] [Google Scholar]
- 18.Russell ND, Hudgens MG, Ha R, Havenar-Daughton C, and McElrath MJ: Moving to human immunodeficiency virus type 1 vaccine efficacy trials: Defining T cell responses as potential correlates of immunity. J Infect Dis 2003;187(2):226–242 [DOI] [PubMed] [Google Scholar]
- 19.Horton H, Thomas EP, Stucky JA, et al. : Optimization and validation of an 8-color intracellular cytokine staining (ICS) assay to quantify antigen-specific T cells induced by vaccination. J Immunol Methods 2007;323(1):39–54 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Montefiori DC: Standardized Assessments of Neutralizing Antibodies for HIV/AIDS Vaccine Development [Internet]. [cited 2014May15]. Available from www.hiv.lanl.gov/content/nab-reference-strains/html/home.htm