Abstract
Cache Valley virus (CVV) is a mosquito-borne bunyavirus (family Bunyaviridae, genus Orthobunyavirus) that is enzootic throughout much of North and Central America. White-tailed deer (Odocoileus virginianus) have been incriminated as important reservoir and amplification hosts. CVV has been found in a diverse array of mosquito species, but the principal vectors are unknown. A 16-year study was undertaken to identify the primary mosquito vectors in Connecticut, quantify seasonal prevalence rates of infection, and define the spatial geographic distribution of CVV in the state as a function of land use and white-tailed deer populations, which have increased substantially over this period. CVV was isolated from 16 mosquito species in seven genera, almost all of which were multivoltine and mammalophilic. Anopheles (An.) punctipennis was incriminated as the most consistent and likely vector in this region on the basis of yearly isolation frequencies and the spatial geographic distribution of infected mosquitoes. Other species exhibiting frequent temporal and moderate spatial geographic patterns of virus isolation within the state included Ochlerotatus (Oc.) trivittatus, Oc. canadensis, Aedes (Ae.) vexans, and Ae. cinereus. New isolation records for CVV were established for An. walkeri, Culiseta melanura, and Oc. cantator. Other species from which CVV was isolated included An. quadrimaculatus, Coquillettidia perturbans, Culex salinarius, Oc. japonicus, Oc. sollicitans, Oc. taeniorhynchus, Oc. triseriatus, and Psorophora ferox. Mosquitoes infected with CVV were equally distributed throughout urban, suburban, and rural locales, and infection rates were not directly associated with the localized abundance of white-tailed deer, possibly due to their saturation throughout the region. Virus activity in mosquitoes was episodic with no consistent pattern from year-to-year, and fluctuations in yearly seasonal infection rates did not appear to be directly impacted by overall mosquito abundance. Virus infection in mosquitoes occurred late in the season that mostly extended from mid-August through September, when adult mosquito populations were visibly declining and were comparatively low. Findings argue for a limited role for vertical transmission for the perpetuation of CVV as occurs with other related bunyaviruses.
Key Words: : Cache Valley virus, Bunyaviridae, Orthobunyavirus, Mosquito, Anopheles punctipennis
Introduction
Cache Valley virus (CVV) is a mosquito-borne bunyavirus (family Bunyaviridae, genus Orthobunyavirus) that is enzootic throughout much of North and Central America (Calisher et al. 1986, Grimstad 2001a). It infects a wide variety of wild and domestic ungulates, including deer, sheep, horses, and cattle (Kokernot et al. 1969, Buescher et al. 1970, Yuill et al. 1970, McLean et al. 1987, Campbell et al. 1989, Chung et al. 1991, Blackmore and Grimstad 1998, Sahu et al. 2002) and has been associated with fetal death, stillbirth, and congenital abnormalities in sheep (Edwards et al. 1989, Chung et al. 1990). The primary amplifying vertebrate hosts are unknown, but white-tailed deer (Odocoileus virginianus) have been incriminated on the basis of experimental infections and high prevalence rates of neutralizing antibody to CVV in wild populations (Neitzel and Grimstad 1991, McLean et al. 1996, Blackmore and Grimstad 1998).
Human infections with CVV appear to be common in areas where the virus is enzootic, with antibody prevalence rates as high as 19% (Kokernot et al. 1969, Buescher et al. 1970, Grimstad 2001a, Blitvitch et al. 2012). However, human neuroinvasive illness is rare and has been diagnosed on only three occasions, with one being fatal (Sexton et al. 1997, Campbell et al. 2006, Nguyen et al. 2013). According to Campbell et al. (2006), this rarity is partially because laboratories rarely test for the virus. CVV has also been associated with congenital defects in humans (i.e., macrocephaly in infants), but the precise role that CVV plays in inducing these abnormalities has not been determined (Calisher and Sever 1995).
The virus has been found in a diverse array of mosquito species (33 species in seven genera), most of which are mammalophilic (Calisher et al. 1986), but the primary vectors are unknown (Campbell et al. 2006). Regional differences in the presumed involvement of individual species in the transmission cycle have been inferred based on the number and frequency of viral infections detected in field collected females: Anopheles (An.) quadrimaculatus and Coquilletidia (Cq.) perturbans in the midwestern United States, Culiseta (Cs.) inornata in the northwestern United States and southern Canada, Ochlerotatus (Oc.) sollicitans and Oc. taeniorhynchus in coastal regions of the mid-Atlantic and Gulf Coast United States and central America (Calisher et al. 1986 and references therein, Farfan-Ale et al. 2010), and Oc. trivittatus in New York State (Ngo et al. 2006). The large majority of CVV isolations have been made from mosquitoes collected during the late summer and early fall (Calisher et al. 1986), further suggesting the greater involvement of late-season mosquito vectors (Grimstad 2001a). Despite these observations, no long-term investigations have examined the spatial and temporal patterns of virus activity in mosquitoes as a function of mosquito abundance, land use, or vertebrate host distribution and abundance. In this investigation, we examine the above parameters based on intensive mosquito and arbovirus surveillance conducted throughout the state of Connecticut over a 16-year period, 1997 through 2012.
Materials and Methods
Collection site descriptions
Mosquito trapping was conducted from June through October at 36 fixed collection sites during 1997–1999 and at 91 fixed collection sites (including the original 36) from 2000–2012. Approximately one-third of the sites were located in densely populated residential settings along an urban/suburban corridor in the coastal southeastern corner of the state that also extended up through the Connecticut River Valley (Fig. 1). Specific trap locations in these regions included parks, greenways, golf courses, undeveloped wood lots, sewage treatment plants, dumping stations, and temporary wetlands associated with waterways. Trapping sites in the other two-thirds of the state were established in more sparsely populated rural settings that included permanent freshwater swamps (red maple/white cedar) and bogs, coastal salt marshes, horse stables, and swamp–forest border environs.
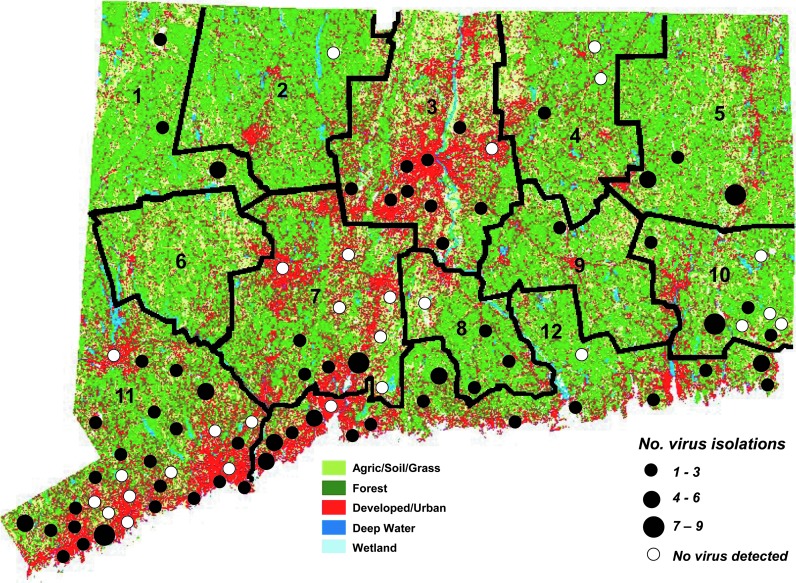
FIG. 1.
Land use map of Connecticut showing white-tailed deer management zones (1–12), geographic distribution of mosquito collection sites, and Cache Valley virus (CVV) isolations obtained from mosquitoes collected from 1997 to 2012.
Mosquito trapping and identification
Mosquito trapping was conducted with CO2 (dry ice)-baited CDC miniature light traps equipped with aluminum domes (John W. Hock Co. Gainesville, FL). Traps were suspended from a tree branch at a height of approximately 1.5 meters. They were placed in the field in the afternoon, operated overnight, and retrieved the following morning. One trap was used at each site on each trapping occasion. Trapping frequency was variable but was minimally made once every 10 days at each trap site over the course of the entire season. The yearly mean number of trap nights per site ranged from 14 to 33 (mean=22, median=18, n=16).
Adult mosquitoes were transported alive to the laboratory each morning in an ice chest lined with cool packs. Mosquitoes were immobilized with dry ice and transferred to chill tables where they were identified to species with the aid of a stereomicroscope (90×) on the basis of morphological characters and descriptive keys of Darsie and Ward (1981 and 2005) and Andreadis et al. (2005). Female mosquitoes were pooled in groups of 50 or fewer by species, collection date, and location in 2-mL microcentrifuge tubes containing a single copper BB. Mosquitoes were stored at −80°C until processed for virus.
Virus isolation and identification
Mosquito pools were prepared for virus testing by adding 1–1.5 mL of PBS-G (phosphate-buffered saline, 30% heat-inactivated rabbit serum, 0.5% gelatin, and 1× antibiotic/antimycotic) to each tube. Samples were homogenized for 4 min at 25 cycles/s on a Vibration Mill MM300 (Retsch Laboratory, Irvine, CA) and then centrifuged at 4°C for 10 min at 520×g. The unfiltered supernatant (100 μL) was inoculated onto a monolayer of Vero E6 cells (African green monkey kidney epithelial cells) growing in a 25-cm2 flask. Cell cultures were maintained at 37°C, 5% CO2 and monitored daily for cytopathic effect (CPE) days 3–7 following inoculation. Infected cell supernatants were harvested and stored at −80°C until further testing.
Viruses were initially typed by the cell-lysate antigen enzyme-linked immunosorbent assay (ELISA) through the year 2000 and by cross-neutralization tests 2001–2004, and then verified by reverse transcription polymerase chain reaction (RT-PCR) as previously described (Armstrong et al. 2005). All subsequent CVV isolates during 2005–2011 were identified by RT-PCR using primers BUNS+new (TGACCAGTAGTGTACTCCAC) and BUNS-new (CAAGCAGTAGTGTGCTCCAC) targeting the terminal ends of the Orthobunyvirus S-segment (Armstrong and Andreadis 2006).
For this procedure, RNA was isolated from infected cell supernatants using the QIAamp Viral RNA Mini Kit (Qiagen, Valencia, CA). RT-PCR was performed using the Titan One Tube RT-PCR System (Roche Diagnostics, Indianapolis, IN) in 25-μLreactions. RT-PCR reactions contained 5 μL of 5× reaction buffer, 2 μL of 10 mM deoxynucleotide triphosphates (dNTPs), 1.25 μL of 100 mM dithiothreitol (DTT), 0.5 μL of Titan enzyme mix, 0.25 μL of RNase inhibitor, 0.5 μL of each 20 μM primer, 13 μL of RNase free water, and 2 μL of template RNA. Amplification was performed as follows: One cycle of 50°C for 30 min and 94°C for 2 min, 10 cycles of 94°C for 15 s, 55°C for 30 s, and 68°C for 1 min, followed by 25 cycles of 94°C for 15 s, 55°C for 30 s, and 68 °C for 1 min+5 s per cycle, and one cycle of 68°C for 7 min. Segment amplification products of approximately 950 bp in size were identified by nucleotide sequencing or restriction enzyme digests, as previously described (Armstrong and Andreadis 2006).
Data analysis
Field infection rates for estimating CVV infection per thousand pooled mosquitoes were calculated individually for each species and collectively for each year and week of the trapping season using the bias-corrected maximum likelihood estimation (MLE) (Biggerstaff 2006). Chi-squared analysis using Yates correction for continuity was used to compare infection rates for each species, and the number of virus isolations as a function of the number of mosquitoes collected each year over the 16-year collection period was evaluated by regression analysis (SigmaPlot, Systat Software, San Jose, CA).
Associations between CVV activity and land use class were assessed by comparing CVV infection rates obtained from field-collected mosquitoes over the 15-year period with the proportion of each of the five land use categories found at each collection site where virus activity was detected. Land cover characterization was obtained for each collection site from digital Landstat satellite imagery data captured and quantified in 2006 for each municipality by the University of Connecticut's Center for Land Use Education and Research (CLEAR) (http://clear.uconn.edu/projects/landscape/category_description.htm). The 12 land cover categories were reduced into five major classes for analysis following the Anderson Level I classification system (Anderson 1976). These included: (1) Agriculture/soil/grass, (2) developed, (3) forest (deciduous and coniferous), (4) wetland (forested, nonforested, and tidal), and (5) deep water (Fig. 1). CVV MLEs and land cover categories of collection sites were compared by Pearson product-moment correlation coefficients (SigmaPlot, Systat Software, San Jose, CA).
Data on white-tailed deer populations were obtained from projected deer densities in Connecticut's 12 deer management zones (Fig. 1) on the basis of an aerial deer survey conducted from January, 2006, to February, 2007 (Gregonis 2007). CVV MLEs within each zone were calculated on the basis of virus isolations obtained from mosquitoes collected from sites located within 10 of the 12 deer management zones (no infected mosquitoes were detected in two of the zones). Relationships between deer density and CVV MLEs were analyzed by regression analysis and Pearson product moment correlation (SigmaPlot, Systat Software, San Jose, CA).
Results
Mosquito collection and virus isolation data
For the period 1997–2012, a total of 163 isolations of CVV were obtained from 16 different species of mosquitoes in seven genera representing 1,904,945 field-collected females processed as 152,216 pools (Table 1). With the exception of Cq. perturbans, all species were multivoltine. Species-specific maximum likelihood estimations (MLEs) ranged from 0.01 to 1.26, with an overall combined mean of 0.20±0.08 standard error (SE) and median of 0.08 for the entire 16-year period. New isolation records for CVV were established for An. walkeri, Cs. melanura, and Oc. cantator. Mosquito species from which no CVV isolations were obtained included: Aedes (Ae.) albopictus (n=260 mosquitoes), An. barberi (n=122), An. crucians (n=767), Cx. erraticus (n=28), Cx. pipiens (n=229,334), Cx. restuans (n=104,155), Cx. territans (n=1472), Cs. minnesotae (n=973), Cs. morsitans (n=3178), Oc. abserratus (n=34,998), Oc. atropalpus (n=23), Oc. aurifer (n=28,448), Oc. communis (n=923), Oc. diantaeus (n=5), Oc. excrucians (n=9003), Oc. fitchii (n=13), Oc. grossbecki (n=37), Oc. hendersoni (n=3), Oc. provocans (n=1175), Oc. sticticus (n=72,092), Oc. stimulans (n=20,072), Oc. thibaulti (n=64,677), Orthopodomyia signifera (n=40), Psorophora (Ps.) columbiae (n=5), Ps. howardii (n=10), and Uranotaenia sapphirina (n=43,422).
Table 1.
Isolations of Cache Valley Virus from Connecticut Mosquitoes 1997–2012
| Mosquito speciesa | Total. no. mosquitoes (pools) | No. virus isolations | MLE (95% CL) | No. years detected | No. sites detectedb |
|---|---|---|---|---|---|
| Ae. cinereus | 157,175 (10,598) | 13 | 0.08 (0.05–0.14) | 4 | 11 |
| Ae. vexans | 242,609 (13,859) | 16 | 0.07 (0.04–0.10) | 5 | 11 |
| An. punctipennis | 38,364 (8,244) | 48 | 1.26 (0.94–0.16) | 5 | 37 |
| An. quadrimaculatus | 6,872 (2,555) | 5 | 0.73 (0.27–1.61) | 4 | 5 |
| An. walkeria | 19,257 (2,062) | 1 | 0.05 (0.00–0.25) | 1 | 1 |
| Cq. perturbans | 381,371 (13,370) | 9 | 0.02 (0.01–0.04) | 6 | 9 |
| Cx. salinarius | 154,535 (9,042) | 1 | 0.01 (0.00–0.03) | 1 | 1 |
| Cs. melanuraa | 128,782 (9,685) | 1 | 0.01 (0.00–0.04) | 1 | 1 |
| Oc. canadensis | 338,963 (12,972) | 19 | 0.06 (0.03–0.09) | 4 | 11 |
| Oc. cantatora | 47,682 (43,981) | 3 | 0.06 (0.02–0.17) | 2 | 2 |
| Oc. japonicus | 10,179 (3,120) | 1 | 0.10 (0.01–0.47) | 1 | 1 |
| Oc. sollicitans | 21,673 (1,202) | 4 | 0.18 (0.06–0.44) | 2 | 2 |
| Oc. taeniorhynchus | 87,437 (2,626) | 5 | 0.06 (0.02–0.13) | 3 | 3 |
| Oc. triseriatus | 27,351 (5,574) | 7 | 0.26 (0.11–0.50) | 3 | 7 |
| Oc. trivittatus | 167,606 (8,446) | 21 | 0.13 (0.08–0.19) | 5 | 13 |
| Ps. ferox | 75,089 (4,880) | 9 | 0.12 (0.06–0.22) | 3 | 9 |
New record.
n=91 collection sites.
MLE, maximum likelihood estimation; CL, confidence level.
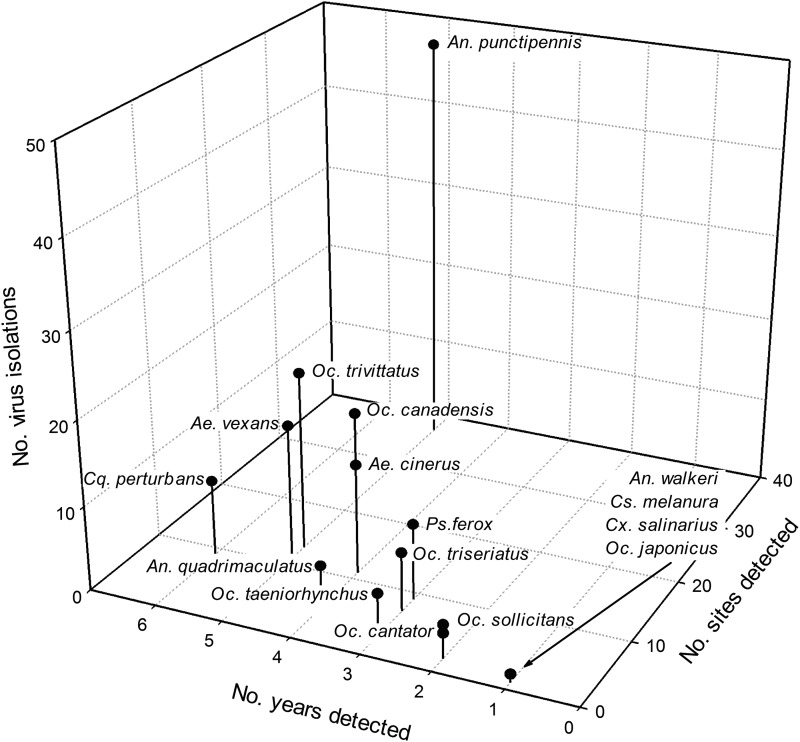
CVV was isolated from An. punctipennis more frequently and from a greater range of collection sites than any other species despite its comparatively lower frequency of collection (Fig. 2). A total of 48 CVV isolations (MLE=1.26) were made from this species, and the virus was detected in females collected from 37 of 91 (41%) trapping locations in 5 of 16 years. Other species exhibiting frequent temporal and moderate, spatial geographic patterns of virus isolation within the state included Oc. trivittatus (n=21, MLE=0.13; 13 locations), Oc. canadensis (n=19, MLE=0.06; 11 locations), Ae. vexans (n=16, MLE=0.07; 11 locations), and Ae. cinereus (n=13, MLE=0.08; 11 locations).
FIG. 2.
Cluster graph depicting the distribution and prevalence of Cache Valley virus (CVV) isolations among 16 species of mosquitoes as a function of (1) total number of virus isolations, (2) number of years virus was detected, and (3) number of sites virus was isolated from for in each species.
Spatial geographic distribution of virus and relationship to white-tailed deer populations
Over the course of the entire 16-year sampling period, CVV was isolated from mosquitoes collected from 64 of 91 (70.3%) field collection sites (Fig. 1). Locations were widely distributed throughout the state irrespective of land use. There were no significant associations between the number of virus isolations made from mosquitoes, and any of the four land cover categories that were analyzed using Pearson product moment correlation analysis: Agriculture/soil/grass (r=0.07, p=0.56), developed (r=−0.02, p=0.85), forest (r=−0.06, p=0.66), wetland (r=0.11, p=0.39), where n=64 collection sites.
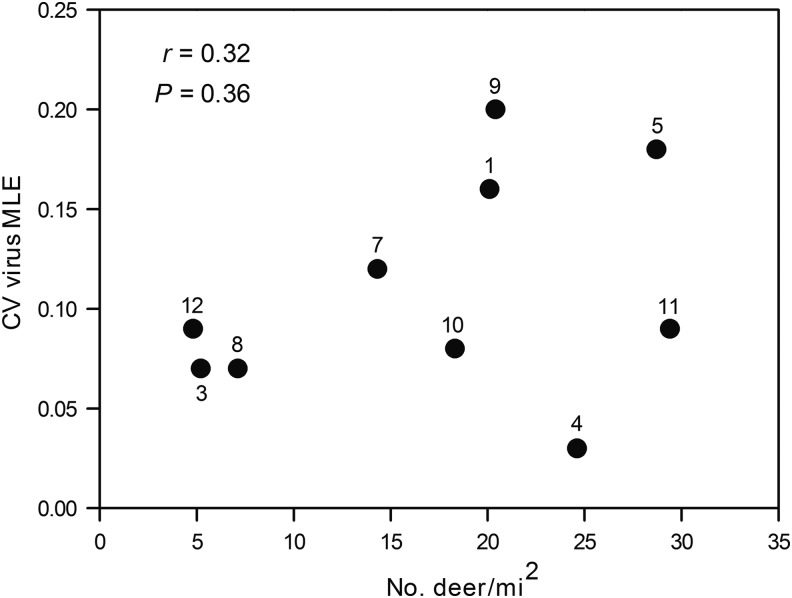
The relationship between estimated white-tailed deer populations in 10 of 12 deer management zones within the state where CVV was detected in mosquitoes (Fig. 1), and CVV MLEs from mosquitoes trapped in those zones are shown in Figure 3. Average deer densities within the state ranged from 4.8 to 29.4 deer/mile2, whereas the overall CVV MLEs ranged from 0.03 to 0.20. However, no significant relationships or associations between the two were found by regression analysis (r=0.32, p=0.36), or Pearson product moment correlation analysis (r=0.33, p=0.36).
FIG. 3.
Relationship between estimated white-tailed deer populations in 10 deer management zones in Connecticut and calculated Cache Valley virus (CVV) maximum likelihood estimations (MLEs) from mosquitoes trapped in those zones from 1997 to 2012. Numbers correspond to specific deer management zone shown in Figure 1.
Yearly and seasonal prevalence
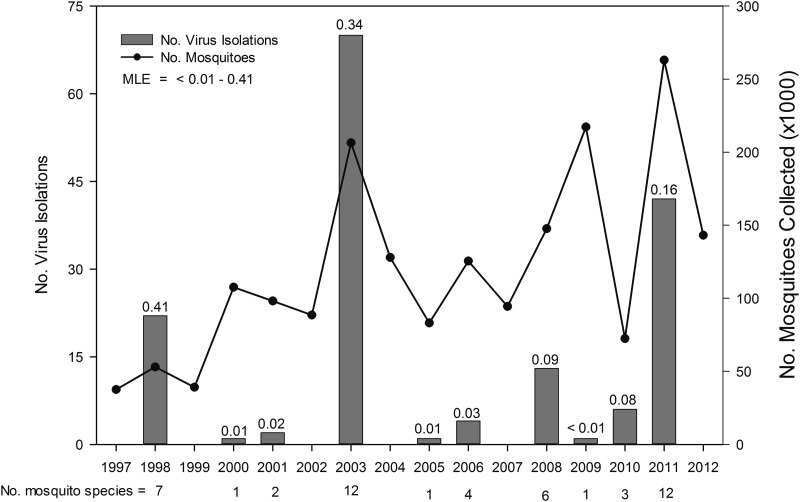
CVV was isolated from field-collected mosquitoes in 10 of the 16 years that surveillance was conducted (Fig. 4). In the years in which virus activity was detected, the number of CVV isolations made from mosquitoes varied greatly, ranging from a low of one in 2000, 2005, and 2009 to a high of 70 in 2003 (mean=16.2±7.3 standard error [SE], median=5.0) (Fig. 4). Infection rates (MLEs) ranged from <0.01 to 0.41, but there was no statistically significant association between yearly mosquito abundance and the number of CVV isolations made during that year (r=0.54, p=0.10, Pearson product moment correlation). However, the 2 years in which the greatest number of CVV isolations were made (2003 and 2011), were associated with excessively wet summers (June–September) during which rainfall amounts were 18.0 cm and 30.0 cm, respectively, above average (www.ncda.noaa.gov/temp-and-precip/climatological-rankings), resulting in markedly higher overall mosquito populations. A significant positive correlation was additionally shown between the yearly total number of CVV isolations and the overall number of mosquito species from which virus was detected in that same year (r=0.93, p<0.001), therein suggesting the involvement of multiple mosquito species during periods of heightened epizootic transmission. Although no distinct yearly patterns of activity were discernable, years in which moderately high levels of CVV activity were detected (1998, 2003, and 2011) were all followed by the absence of any virus activity in the ensuing year. However, some years in which CVV was not detected (e.g., 2002 and 2007) were not always preceded by years with high levels of virus activity (e.g., 2001 and 2006).
FIG. 4.
Yearly number of Cache Valley virus (CVV) isolations from mosquitoes and overall bias corrected minimum field infection rates (maximum likelihood estimation [MLE]) in relation to number of mosquitoes trapped and tested 1997 to 2012.
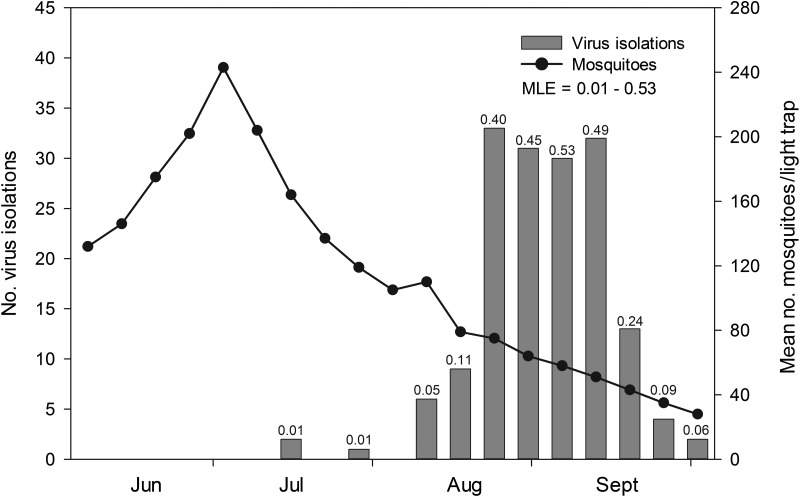
A 16-year composite summary analysis of weekly CVV isolations from mosquitoes and overall mosquito abundance (all species) revealed a clear pattern of late season virus activity that mostly extended from mid-August through September (Fig. 5). This was concomitant with a notable decline in mosquito abundance from a peak in early July (mean=243.0 mosquitoes/trap). Consistently high CVV infection rates ranging from 0.40 to 0.53 MLE were observed over a 4-week period from late August to mid-September when adult mosquito populations as determined from trap collections were comparatively low (mean=62.0±5.1 SE mosquitoes/trap).
FIG. 5.
Weekly isolations and bias corrected minimum field infection rates (MLE) of Cache Valley virus (CVV) from field-collected mosquitoes in Connecticut in relation to overall mosquito abundance, 1997 to 2012.
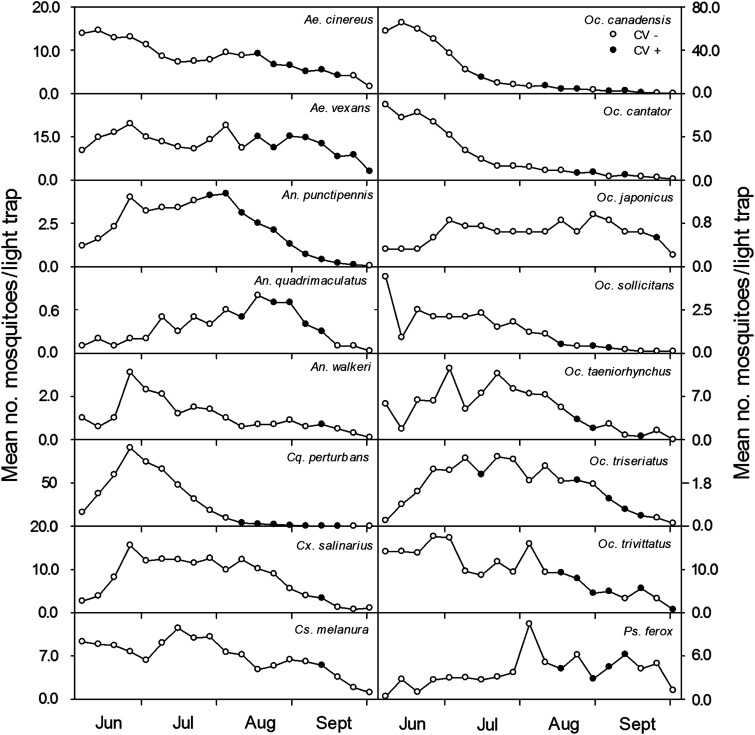
An analysis of weekly CVV isolation summaries over the entire 16-year period for each of the 16 individual mosquito species in shown in Figure 6. An. punctipennis exhibited the most consistent pattern of virus isolation that extended from late July through September. Virus activity in this species was initially detected when populations were generally at their highest level and was followed by a steady gradual decline. Similar patterns of virus isolation frequency and declining mosquito abundance were demonstrated with Ae. cinereus and Ae. vexans, albeit over a shorter period that began several weeks later in the season (mid-August). The detection of CVV in An. quadrimaculatus was more discontinuous and less frequent, but like An. punctipennis, isolations of virus in the former species were observed when populations were at their seasonal height. The detection of CVV in all other species was observed when populations were on the decline and at their lowest levels of the season.
FIG. 6.
Weekly summaries of Cache Valley virus (CVV) isolations from 16 mosquito species in relation to mosquito abundance, 1997 to 2012. (•) CVV detected; (○) CVV not detected.
Discussion
With this investigation, we have shown that CVV is widely distributed throughout Connecticut and infects a diverse array of mosquito species, but occurs sporadically with no consistent pattern of activity from one year to the next. The episodic nature of this virus has been noted in at least two other multiyear investigations, albeit for only 4 years (Buescher et al. 1970, Ngo et al. 2006), but causes for these oscillations have not been addressed. In our study, fluctuations in yearly seasonal infection rates did not appear to be directly impacted by overall mosquito abundance. However, the years in which the greatest number of CVV isolations and highest prevalence rates of infection were observed were associated with excessively wet summers, resulting in exceptionally high mosquito populations, thus suggesting a possible link. Equally perplexing, was the repeated absence of any detectible activity in mosquitoes in years immediately following those in which moderately high levels of CVV activity were seen. Buescher et al. (1970) reported a similar finding in studies conducted in the Delmarva Peninsula, where no CVV–infected mosquitoes were recovered in a year following significant activity, despite testing nearly four times more mosquitoes. While certainly speculative, it is conceivable that this may be the consequence of an increased prevalence of “herd immunity” in white-tailed deer populations, the presumed reservoir and amplifying host. Neutralizing antibody to CVV in white-tailed deer populations has been repeatedly reported to be very high (>60%) in geographic areas where the virus is enzootic (Issel et al. 1970, Neitzel and Grimstad 1991, Blackmore and Grimstad 1998, Grimstad 2001a).
Virus infection in mosquitoes occurs late in the season that mostly extends from mid-August through September, when adult mosquito populations are visibly declining and are comparatively low. This pattern of late season activity is consistent with studies in other parts of the United States and Canada, where the large majority of CVV isolations have been similarly made from mosquitoes collected during the late summer and early fall (Buescher et al. 1970, Calisher et al. 1986, Grimstad 2001a, Ngo et al. 2006). The absence of virus activity earlier in the season when mosquito populations are at their highest levels is not overtly apparent, but suggests that CVV amplification between mosquito vectors and reservoir vertebrate hosts, presumably white-tailed deer, may require several transmission cycles before it becomes prevalent enough to reach detectable levels in sampled populations. Our late season isolations of CVV from mainly multivoltine mosquito species support this view.
Our findings also argue for a limited role for vertical transmission as a principal overwintering mechanism for CVV as occurs with other related bunyaviruses such as Jamestown Canyon virus (JCV), which overwinters in mosquito eggs (Boromisa and Grimstad 1986, Grimstad 2001b). Grimstad (2001a) has hypothesized that CVV may overwinter in blood-fed Anopheles mosquitoes because no viral infections have been detected in Culicine species prior to the emergence of female Anophlines from overwintering hibernacula. However, most Anopheline mosquitoes, including An. punctipennis (Washino and Bailey 1970), normally overwinter as non-blood-fed nulliparous females (Detinova 1962, Wallace and Grimstad 2002). On the other hand, Magnarelli (1978) has reported the collection of a high percentage (29.9%) of blood-fed An. punctipennis that had undergone gonotrophic disassociation from the basement of a human dwelling in Connecticut during September and October but not November, therein demonstrating that some females may indeed blood feed prior to hibernation and potentially acquire virus.
Alternatively, acquisition of the virus would have to occur through vertical transmission. Vertical (transovarial) transmission of CVV has only been demonstrated in Cs. inornata (Corner et al. 1980), a species that also overwinters as an adult, but the observed filial infection rate was relatively low for a bunyavirus, infecting only 0.2% of progeny from experimentally infected females. In either case, if CVV overwintered to any great degree in diapusing Anopheles females, regardless of whether it was acquired horizontally or vertically, one might anticipate some early to midseason isolation of the virus from field populations, as occurs with JCV (Andreadis et al. 2008), unless of course infection rates are excessively low and below detectable levels given our trapping methodology. In the present study, we did isolate CVV from field-collected An. punctipennis earlier than nearly any other mosquito species; but the earliest isolations were still not made until late July to early August, generally 9 weeks after the first females were collected in our traps.
Mosquitoes infected with CVV are equally distributed throughout urban, suburban, and rural locales, and infection rates are not associated with the localized abundance of white-tailed deer populations in different areas of the state. Similar findings were obtained in concurrent investigations on the prevalence and distribution of JCV in the state (Andreadis et al. 2008) that also amplifies in white-tailed deer (Grimstad 2008b). This likely reflects the overall abundance and saturation of white-tailed deer throughout the region that have increased substantially from an estimated population of 49,472 in 1993 to 62,189 in 2006/2007 (Gregonis 2007). However, we note that Main (1981) recovered only a single isolation of CVV (Oc. triseriatus) from 204,753 female mosquitoes representing 36 species (including >4000 An. punctipennis) that were collected and tested in Connecticut from 1969 through 1980, when the estimated white-tailed deer population in the state was roughly 20,000 (Gregonis 2000).
Despite similar distribution patterns of many of the same mosquito vectors (n=13) and the use of white-tailed deer as primary hosts, our observations on CVV activity in the state contrast sharply with the ecology and epizootiology of JCV (Andreadis et al. 2008). From a seasonal standpoint, JCV activity essentially mirrors that of CVV. JCV infection in mosquitoes primarily occurs from June through August and closely parallels mosquito abundance, with peak infection rates extending from mid-June through mid-July when CVV is effectively absent. This partially reflects the involvement of several species of early season univoltine mosquito species (n=6) that are not involved in local transmission of CVV. Furthermore, unlike CVV, JCV prevalence rates of infection are consistent from year to year (10 year average MLE=0.27±0.2 SE) and overall virus activity, as measured by the number of virus isolations from mosquitoes, is a function of local mosquito abundance.
In our entomological surveillance conducted over the 16-year period, we isolated CVV from nearly one-third (16 of 49=32.6%) of the mosquito species known to occur in the state (Andreadis et al. 2005), establishing new virus isolation records for three species: An. walkeri, Cs. melanura, and Oc. cantator. However, on the basis of yearly isolation frequencies and the spatial geographic distribution of infected mosquitoes, An. punctipennis was incriminated as the most consistent and likely vector in this region.
An. punctipennis is a competent laboratory vector of CVV (Saliba et al. 1973), and CVV has been previously isolated from wild populations of this mosquito in several other locations in the eastern (Kentucky, New York, Tennessee) and midwestern (Illinois, Iowa, Michigan, Ohio) United States (Calisher et al. 1986, Ngo et al. 2006). It is the most frequently encountered anopheline mosquito in Connecticut and is a multivoltine species that develops in a variety of rural and suburban habitats that include semipermanent and permanent ponds, rock pools, artificial containers, and the margins of slow moving grassy ditches and streams with emergent vegetation (Means 1987, Andreadis et al. 2005). The species overwinters as inseminated nulliparous females in abandoned buildings, caves, basements of human dwellings, and other natural subterranean habitats, and emerges from these hibernacula early in the spring prior to the emergence of most “snowpool” Ochlerotatus species. Larvae of the first generation can be found in June and adults are active throughout the summer and early fall (June through October) with peak populations in late July. Local regional populations of An. punctipennis from Connecticut, New Jersey, and New York feed almost exclusively on mammalian hosts (94.8% mammalian derived blood meals, where n=271), with the overwhelming majority of mammalian hosts (91.2%) being white-tailed deer consistent with their abundance and availability throughout the region (Apperson et al. 2004, Molaei et al. 2008, 2009a). An. punctipennis females will also readily bite humans, especially outdoors in the early evening, and given their vector competence should receive strong consideration as a potentially important vector of CVV to humans.
Other likely vectors of regional importance identified in our long-term study included Oc. trivittatus, Oc. canadensis, Ae. vexans, and Ae. cinereus. The vector competence of Oc. trivittatus for CVV has not been evaluated, but this mosquito was the most frequently infected species identified in a 4-year study conducted in neighboring New York State, accounting for 42% (MLE=0.44) of all virus isolates (n=52) obtained from 10 different mosquito species (Ngo et al. 2006). CVV has also been isolated from field populations of Oc. trivittatus in several other states in the upper Midwest, most notably Ohio (also Illinois, Iowa, Michigan, South Dakota, Wisconsin) (Calisher at al. 1986). Oc. trivittatus is a multivoltine floodwater species that is widely distributed throughout southeastern Canada and the United States (Carpenter and LaCasse 1955). It was among the most abundantly collected species in our study and accounted for 12.9% of total virus isolations. Blood meal analysis of local populations from Connecticut and New York indicate that the species is strongly mammalophilic (96.7% of all blood meals, n=121) and feeds most frequently on white-tailed deer (85.5% mammalian blood meals, n=110) but also humans (5.5%) (Apperson et al. 2004, Molaei et al. 2008). Given these parameters, it should be deemed a likely enzootic as well as bridge vector of CVV to humans despite our lack of confirmation on its ability to orally transmit virus to vertebrate hosts.
Oc. canadensis was the second most commonly trapped species in our study, accounting for 11.7% of all CVV isolations. It was a species from which some of our earliest virus isolations were obtained in mid-July, consistent with its early hatch in the spring and peak adult abundance in June. Oc. canadensis was the only mosquito species from which CVV was isolated (n=3) in 6 years of mosquito surveillance (1995–2003) conducted in neighboring Rhode Island that included over 128,000 mosquitoes (Takeda et al. 2003). The virus has also been occasionally isolated from field populations of Oc. canadensis from Michigan (Calisher at al. 1986) and New York (Ngo et al. 2006), but its occurrence in these regions appears to be comparatively less common. Its vector competence for CVV has not been established. Oc. canadensis is generally considered to be univoltine (Means 1979), but a second brood may occur with heavy rain events during late summer and early fall (Magnarelli 1977). It is unclear if this phenomenon is due to delayed hatching of eggs or true multivotinism. Larvae develop in a variety of freshwater habitats, especially temporary leaf-lined pools in wooded areas, but are also found in roadside ditches, vernal pools in open fields, permanent swamps, and acid water sphagnum bogs (Andreadis et al. 2005). Oc. canadensis is a strong mammalian feeder (98.0% of blood meals, n=198), and local populations from Connecticut and New York have been shown to feed heavily on white-tailed deer (94.3%) in addition to humans (4.1%) (Apperson et al. 2004, Molaei et al. 2008). Oc. canadensis has been incriminated as the most dominant vector of JCV in Connecticut (Andreadis et al. 2008).
CVV has been frequently isolated from field populations of Ae. vexans throughout the upper Midwest (Iowa, Michigan, Minnesota, South Dakota, Wisconsin), northeastern United States (New York), and Canada (Manitoba, Ontario, Saskatchewan) (Calisher at al. 1986, Ngo et al. 2006). Ae. vexans is a cosmopolitan temporary floodwater species (Means 1979). In our study, it was commonly collected throughout the spring, summer, and early fall consistent with its multivoltine biology, and virus isolations were obtained from mid-August through early October. Females are aggressive human biters and local populations from the region exhibit a strong preference for mammals (94.0%, n=167) that includes white-tailed deer (88.8% of mammalian feeds, n=152) (Apperson et al. 2004, Molaei and Andreadis 2006). Ae. vexans can be readily infected with CVV in the laboratory, and according to Saliba et al. (1973) this likely helps to explain the high incidence of reported natural infections. However, the species is reported to be a relatively inefficient transmitter of virus and thus have less potential than either An. punctipennis or An. quadrimaculatus to serve as a biological vector (Saliba et al. 1973). Nevertheless, its biological association with CVV, aggressive human biting nature, and local abundance during periods of epizootic amplification make the species a potentially important vector.
CVV has only occasionally been isolated from Ae. cinereus (Wisconsin and New York) (Calisher at al. 1986, Ngo et al. 2006). Similar to Ae. vexans, this species is multivoltine and was commonly collected throughout the sampling period, but virus isolations were more seasonally limited, occurring from mid-August to mid-September. Local populations similarly exhibit a strong feeding preference for mammals (95.3% of all blood meals, n=107), but appear to feed less frequently on white-tailed deer than any of the aforementioned species (78.8% of mammalian blood feeds, n=99) (Apperson et al. 2004, Molaei et al. 2008). The oral susceptibility of Ae. cinereus for CVV and vector competency have not been evaluated, and thus it is not possible to reliably comment of its role in local transmission.
The comparatively fewer number of virus isolations obtained from the remaining species likely preclude their major involvement in the transmission cycle of CVV in this region. However, at least four of these species, An. quadrimaculatus, Cq. perturbans, Oc. sollicitans, and Oc. taeniorhynchus, are all capable of oral transmission (Yuill and Thompson 1970, Saliba et al. 1973, Blackmore et al. 1998) and could potentially play a role in local transmission during periods of enhanced epizootic activity. Last, we note the single isolation of CVV from the recently introduced exotic species, Oc. japonicus. This mosquito was also found to be infected for the first time in a recently completed survey in neighboring New York State (Ngo et al. 2006), thus expanding the number of arboviruses recovered from this invasive species. If proven to be a competent vector, it is not inconceivable that Oc. japonicus could contribute to local transmission as regional studies have shown that females feed exclusively on mammalian hosts that include mostly white-tailed deer and humans (Apperson et al. 2004, Molaei et al. 2008, 2009b).
Acknowledgments
We gratefully acknowledge the technical assistance of our support staff for collection and identification of mosquitoes (Terrill Goodman, Michael Misencik, John Shepard, Michael Thomas, Michael Vasil) and isolation and identification of virus (Angela Bransfield, Shannon Finan, Bonnie Hamid, Jodie Ortega, Nicholanna Price, Amanda Rahmann). This work was supported in part by grants from the Centers for Disease Control (U50/CCU116806-01-1) and the US Department of Agriculture (58-6615-1-218, CONH00768, and CONH00773).
Author Disclosure Statement
No competing financial interests exist.
References
- Anderson JR. A land use and land cover classification system for use with remote sensor data. Washington, DC: US Government Printing Office, Geological Survey Professional Paper 964, 1976 [Google Scholar]
- Andreadis TG, Thomas MC, Shepard JJ. Identification Guide to the Mosquitoes of Connecticut. Bulletin of The Connecticut Agricultural Experiment Station, No. 966, 2005:1–173 [Google Scholar]
- Andreadis TG, Anderson JF, Armstrong PM, Main AJ. Isolations of Jamestown Canyon virus (Bunyaviridae: Orthobunyavirus) from field-collected mosquitoes (Diptera: Culicidae) in Connecticut, USA: A ten-year analysis, 1997–2006. Vector-Borne Zoonotic Dis 2008; 8:175–188 [DOI] [PubMed] [Google Scholar]
- Apperson CS, Hassan HK, Harrison BA, Savage HM, et al. . Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector-Borne Zoonotic Dis 2004; 4:71–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong PM, Andreadis TG. A new genetic variant of La Crosse virus isolated from New England. Am J Trop Med Hyg 2006; 75:491–496 [PubMed] [Google Scholar]
- Armstrong PM, Andreadis TG, Anderson JF, Main AJ. Isolations of Potosi virus from mosquitoes collected in Connecticut. J Med Entomol 2005; 42:875–881 [DOI] [PubMed] [Google Scholar]
- Biggerstaff BL. PooledInfRate, Version 3.0: a Microsoft® Excel Add-In to compute prevalence estimates from pooled samples. Centers for Disease Control and Prevention, Fort Collins, CO, 2006 [Google Scholar]
- Blackmore CGM, Grimstad PR. Cache Valley and Potosi viruses (Bunyaviridae) in white-tailed deer (Odocoileus virginianus): Experimental infections and antibody prevalence in natural populations. Am J Trop Med Hyg 1998; 59:704–709 [DOI] [PubMed] [Google Scholar]
- Blackmore CGM, Blackmore MS, Grimstad PR. Role of Anopheles quadrimaculatus and Coquillettidia perturbans (Diptera: Culicidae) in the transmission cycle of Cache virus (Bunyaviridae: Bunyavirus) in the Midwest, U.S.A. J Med Entomol 1998; 35:660–664 [DOI] [PubMed] [Google Scholar]
- Blitvich BJ, Saiyasombat R, Talavera-Aguilar LG, Garcia-Rejon JE, et al. . Orthobunyavirus antibodies in humans, Yucatan Peninsula, Mexico. Emerg Infect Dis 2012; 18:1629–1632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boromisa RD, Grimstad PR. Virus-vector-host relationships of Aedes stimulans and Jamestown Canyon virus in a northern Indiana enzootic focus. Am J Trop Med Hyg 1986; 35:1285–1295 [DOI] [PubMed] [Google Scholar]
- Buescher EL, Byrne RJ, Clarke GC, Gould DJ, et al. . Cache Valley virus in the Del Mar Va Peninsula I. Virologic and serologic evidence of infection. Am J Trop Med Hyg 1970; 19:493–502 [DOI] [PubMed] [Google Scholar]
- Calisher CH, Sever JL. Are North American Bunyamwera serogroup viruses etiologic agents of human congenital defects of the central nervous system? Emerg Infect Dis 1995; 1:147–151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calisher CH, Francy DB, Smith GC, Muth DJ, et al. . Distribution of Bunyamwera serogroup viruses in North America, 1956–1984. J Trop Med Hyg 1986; 35:429–443 [DOI] [PubMed] [Google Scholar]
- Campbell GL, Eldridge BF, Hardy JL, Reeves WC, et al. . Prevalence of neutralizing antibodies against California and Bunyamwera serogroup viruses in deer from mountainous areas of California. Am J Trop Med Hyg 1989; 40:428–437 [DOI] [PubMed] [Google Scholar]
- Campbell GL, Mataczynski JD, Reisdorf ER, Powell JW, et al. . Second human case of Cache Valley virus disease. Emerg Infect Dis 2006; 12:854–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carpenter SJ, LaCasse WJ. Mosquitoes of North America (North of Mexico). Berkeley and Los Angeles: University of California Press, 1955, 360pp. [Google Scholar]
- Chung SI, Livingston CW, Jr, Edwards JF, Gauer BB, et al. . Congenital malformations in sheep resulting from in utero inoculation of Cache Valley virus. Am J Vet Res 1990; 51:1645–1648 [PubMed] [Google Scholar]
- Chung SI, Livingston CW, Jr, Jones CW, Collisson EW. Cache Valley virus infection in Texas sheep flocks. J Am Vet Med Assoc 1991; 199:337–340 [PubMed] [Google Scholar]
- Corner LC, Robertson AK, Hayles LB, Iverson JO. Cache Valley virus: Experimental infection in Culiseta inornata. Can J Microbiol 1980; 26:287–290 [PubMed] [Google Scholar]
- Darsie RF, Jr, Ward RA. Identification and geographic distribution of mosquitoes of North America, north of Mexico. Mosq Syst 1981; 1(Suppl):1–313 [Google Scholar]
- Darsie RF, Jr, Ward RA. Identification and Geographical Distribution of the Mosquitoes of North America, North of Mexico. Gainesville, FL: University Press of Florida, 2005, 383 pp. [Google Scholar]
- Detinova TS. Age-grouping Methods in Diptera of Medical Importance with Special Reference to Some Vectors of Malaria. Geneva, Switzerland: World Health Organization; 1962, 216 pp [PubMed] [Google Scholar]
- Edwards JF, Livingston CW, Chung SI, Collisson EW. Ovine arthrogryposis and central nervous system malformations associated with in utero Cache Valley virus infection: Spontaneous disease. Vet Pathol 1989; 26:33–39 [DOI] [PubMed] [Google Scholar]
- Farfan-Ale JA, Lorona-Pina MA, Garcia-Rejon JE, Soto V, et al. . Detection of flaviviruses and orthobunyavirus in mosquitoes in the Yucatan Peninsula of Mexico in 2008. Vector Borne Zoonotic Dis 2010; 10:777–783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregonis M. Aerial deer survey indicates population is increasing. Conn Wildl 2000; 20:12–13 [Google Scholar]
- Gregonis M. 2006/2007 aerial deer survey indicates stable population. Conn Wildl 2007; 27:3 [Google Scholar]
- Grimstad PR. Cache Valley virus. In: Service MW, ed. Encyclopedia of Arthropod-transmitted Infections of Man and Domestic Animals. New York: CABI Publishing, 2001a:101–104 [Google Scholar]
- Grimstad PR. Jamestown Canyon virus. In: Service MW, ed. Encyclopedia of Arthropod-Transmitted Infections of Man and Domestic Animals. New York: CABI Publishing, 2001b:235–239 [Google Scholar]
- Issel CJ, Hoff GL, Trainer DO, Thompson WH. Serologic evidence of Bunyamwera group arbovirus infections in Wisconsin and Texas deer. J Wildlife Dis 1970; 6:479–482 [DOI] [PubMed] [Google Scholar]
- Kokernot RH, Hayes J, Tempelis CH, Chan DHM, et al. . Arbovirus studies in the Ohio-Mississippi basin, 1964–1967. IV. Cache Valley virus. Am J Trop Med Hyg 1969; 18:768–773 [DOI] [PubMed] [Google Scholar]
- Magnarelli LA. Seasonal occurrence and parity of Aedes canadensis (Diptera: Culicidae) in New York state, USA. J Med Entomol 1977; 8:741–745 [DOI] [PubMed] [Google Scholar]
- Magnarelli LA. Blood-feeding sand gonotrophic dissociation in Anopheles punctipennis (Diptera: Culicidae) prior to hibernation in Connecticut. J Med Entomol 1978; 15:278–281 [Google Scholar]
- Main AJ. Arbovirus surveillance in Connecticut IV. Bunyamwera group. Mosq News 1981; 41:490–494 [Google Scholar]
- McLean RG, Calisher CH, Parham GL. Isolation of Cache Valley virus and detection of antibody for selected arboviruses in Michigan horses in 1980. Am J Vet Res 1987; 48:1039–1041 [PubMed] [Google Scholar]
- McLean RG, Kirk LJ, Shriner RB, Cook PD, et al. . The role of deer as a possible reservoir host of Potosi virus, a newly recognized arbovirus in the United States. J Wildlife Dis 1996; 32:444–452 [DOI] [PubMed] [Google Scholar]
- Means RG. Mosquitoes of New York. Part I. The Genera Aedes. NY State Mus Bull 1979; 430a:1–221 [Google Scholar]
- Means RG. Mosquitoes of New York. Part II. Genera of Culicidae other than Aedes occurring in New York. NY State Mus Bull 1987; 430b:1–180 [Google Scholar]
- Molaei G, Andreadis TG. Identification of avian- and mammalian-derived blood meals in Aedes vexans and Culiseta melanura (Diptera: Culicidae) and its implication for West Nile virus transmission in Connecticut, USA. J Med Entomol 2006; 43:1088–1093 [DOI] [PubMed] [Google Scholar]
- Molaei G, Andreadis TG, Armstrong PM, Duik-Wasser M. Host-feeding patterns of potential mosquito vectors of arboviral agents in Connecticut, USA: Molecular analysis of blood meals from twenty-three species of Aedes, Anopheles, Culex, Coquillettidia, Psorophora, and Uranotaenia. J Med Entomol 2008; 45:1143–1151 [DOI] [PubMed] [Google Scholar]
- Molaei G, Farajollahi A, Armstrong PM, Oliver J, et al. . Identification of blood meals in Anopheles quadrimaculatus and Anopheles punctipennis from eastern equine encephalitis virus foci in northeastern USA. Med Vet Entomol 2009a; 23:350–356 [DOI] [PubMed] [Google Scholar]
- Molaei G, Farajollahi A, Scott JJ, Gaugler R, et al. . Human blood feeding by the recently introduced mosquito, Aedes japonicus japonicus, and public health implications. J Am Mosq Control Assoc 2009b; 25:210–214 [DOI] [PubMed] [Google Scholar]
- Neitzel DF, Grimstad PR. Serological evidence of California group and Cache Valley virus infection in Minnesota white-tailed deer. J Wildlife Dis 1991; 27:230–237 [DOI] [PubMed] [Google Scholar]
- Ngo KA, Maffei JG, Dupuis AP, Kauffman EB, et al. . Isolation of Bunyamwera serogroup viruses (Bunyaviridae, Orthobunyavirus) in New York State. J Med Entomol 2006; 43:1004–1009 [DOI] [PubMed] [Google Scholar]
- Nguyen NL, Zhao G, Hull R, Shelly MA, et al. . Cache Valley virus in a patient diagnosed with aseptic meningitis. J Clin Microbiol 2013; 51:1966–1969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saliba EK, DeFoliart GR, Yuill TM, Hanson RP. Laboratory transmission of Wisconsin isolates of Cache Valley virus by mosquitoes. J Med Entomol 1973; 10:470–476 [DOI] [PubMed] [Google Scholar]
- Sahu SP, Pedersen DD, Ridpath HD, Ostlund EN, et al. . Serologic survey of cattle in the northeastern and north central United States, Virginia, Alaska, and Hawaii for antibodies to Cache Valley and antigenically related viruses (Bunyamwera serogroup virus). Am J Trop Med Hyg 2002; 67:119–122 [DOI] [PubMed] [Google Scholar]
- Sexton DJ, Rollin PE, Breitschwerdt EB, Corey GR, et al. . Life-threatening Cache Valley virus infection. NE J Med 1997; 336:547–549 [DOI] [PubMed] [Google Scholar]
- Takeda T, Whitehouse CA, Brewer M, Gettman AD, et al. . Arbovirus surveillance in Rhode Island: assessing potential ecologic and climatic correlates. J Am Mosq Control Assoc 2003; 19:179–189 [PubMed] [Google Scholar]
- Wallace JR, Grimstad PR. A preliminary characterization of the physiological ecology of overwintering Anopheles mosquitoes in the Midwestern USA. J Am Mosq Control Assoc 2002; 18:126–127 [PubMed] [Google Scholar]
- Washino RK, Bailey SF. Overwintering of Anopheles punctipennis (Diptera: Culicidae) in California. J Med Entomol 1970; 7:95–98 [DOI] [PubMed] [Google Scholar]
- Yuill TM, Thompson . Cache Valley virus in the Del Mar Va Peninsula IV. Biological transmission of the virus by Aedes sollicitans and Aedes taeniorhynchus. Am J Trop Med Hyg 1970; 19:513–519 [DOI] [PubMed] [Google Scholar]
- Yuill TM, Gochenour WS, Jr, Lucas FR, Collins MJ Jr, et al. . Cache Valley virus in the Del Mar Va Peninsula III. Serologic evidence for natural infection of dairy cattle. Am J Trop Med Hyg 1970; 19:506–512 [DOI] [PubMed] [Google Scholar]