Abstract
In dentistry, the maintenance of a vital dental pulp is of paramount importance, as teeth devitalized by root canal treatment may become more brittle and prone to structural failure over time. Advanced carious lesions can irreversibly damage the dental pulp by propagating a sustained inflammatory response throughout the tissue. While the inflammatory response initially drives tissue repair, sustained inflammation has an enormously destructive effect on the vital pulp, eventually leading to total necrosis of the tissue and necessitating its removal. The implications of tooth devitalization have driven significant interest in the development of bioactive materials that facilitate the regeneration of damaged pulp tissues by harnessing the capacity of the dental pulp for self-repair. In considering the process by which pulpitis drives tissue destruction, it is clear that an important step in supporting the regeneration of pulpal tissues is the attenuation of inflammation. Macrophages, key mediators of the immune response, may play a critical role in the resolution of pulpitis due to their ability to switch to a pro-resolution phenotype. This process can be driven by the resolvins, a family of molecules derived from fatty acids that show great promise as therapeutic agents. In this review, we outline the importance of preserving the capacity of the dental pulp to self-repair through the rapid attenuation of inflammation. Potential treatment modalities, such as shifting macrophages to a pro-resolving phenotype with resolvins are described, and a range of materials known to support the regeneration of dental pulp are presented.
Introduction
One of the most significant challenges in modern dentistry is the maintenance of a vital dental pulp. Due to the implications of root canal therapy, which results in a permanently devitalized tooth more susceptible to structural failure. There is an increasing desire to provide better options for endodontic therapy, effectively expanding the tools available in clinical dentistry. It is the desire to drive regeneration in the dental pulp that has prompted research into bioactive materials for use in regenerating dental pulp. True innovation in developing novel endodontic therapies will involve utilizing materials which facilitate the regeneration of the dental pulp from any remaining, vital pulp tissues, effectively harnessing the innate capacity of the tooth for self-repair. This review will explore the most prevalent cause of pulpal necrosis, bacterially induced tissue inflammation, and highlight the importance of attenuating this inflammation in order to support and facilitate dental pulp regeneration.
The Dental Pulp
The dental pulp itself is a unique and complex tissue serving to support the dentin, which provides the main structural component of the tooth organ. Its basic structure is well characterized and has been widely described (1–3). The pulp tissue itself is composed of collagen type I and type III, along with a variety of non-collagenous proteins, including a large proteoglycan component (4). There are a variety of cell types present in the pulpal tissue, including immune cells, fibroblasts, mesenchymal progenitor cells, vascular cells and nerve cells (5–11). Lining the pulp chamber is a layer of columnar odontoblasts, which have cellular projections associated with a system of fluid-filled tubules running through the dentin to the dentino-enamel junction (1, 12). These are cells directly responsible for the maintenance of the mineralized dentin during routine loading of the tooth. Fibroblasts are the most numerous cells in the dental pulp and maintain the collagen matrix of the pulpal tissue, and a population of immune cells, in particular tissue macrophages, hold themselves ready to respond to microbial incursion (2, 3, 5). A network of blood vessels runs throughout the pulp, perfusing the tissue and providing respiratory support (2, 3). Networks of nerve fibers provide enervation to the tissue, linking it with the central nervous system and providing sensory output (2, 3). This complex and dynamic environment is preserved in a delicate balance, with the odontoblasts maintaining the mineralized tissues, and the other cells types effectively positioned to support the activity of the odontoblasts. A resident population of mesenchymal progenitor cells provides a reservoir of pluripotent cells capable of differentiation into a variety of cells as required for tissue maintenance and repair (13). The progenitor cells in particular are critical to the long term function of the whole tooth and are therefore a critical target when considering the design of bioactive materials intended to encourage dental pulp regeneration.
The Capacity of the Dental Pulp for Self-Repair
It is widely accepted that the tooth organ has an innate capacity for self-repair and contains all the necessary components to regenerate both the mineralized dentin and the soft tissues of the pulpal matrix. It is established that odontoblasts respond to microbial colonization of the dentinal tubules by producing sclerotic ‘reactionary’ dentin in an attempt to block infected tubules, thus creating a barrier between the invading microbes and the pulp tissue (14, 15). In more advanced carious lesions, the inflammatory response causes cell death among odontoblasts and other cells of the pulp. Mesenchymal progenitor cells are subsequently recruited to the site of cell death and are driven by a cascade of signals, including degradation products from the dentin matrix, to differentiate into odontoblasts and begin synthesizing ‘reparative’ dentin (7, 10, 11, 16, 17). Dental pulp also contains a population of multipotent cells capable of responding to injury. In vitro, isolated human dental pulp progenitor cells have also been shown to be capable of differentiation into a variety of cell types, including osteoblasts, adipocytes, and chondrocytes (17, 18). Dental pulp derived progenitor cells have also been shown to have vasculogenic potential, both in vitro and when implanted into a murine model of hind limb ischemia (19). In vivo, the capability of the dental pulp to respond to damage has been elegantly modeled by Six and colleagues, who demonstrated that exposing rat molar pulp results in increased PCNA expression and reparative dentin formation around the site of injury, indicating increased cell proliferation and differentiation in response to dental tissue damage (20). Further, progenitor cells isolated from dental pulp and selected on the basis of their vasculogenic potential are capable of regenerating functional dental pulp with normal blood flow when encapsulated into collagen gels containing granulocyte colony stimulating factor (GCSF) and implanted into pulpectomized canine teeth (21). Embryonic mesenchymal progenitor cells from a developing tooth germ, when combined with human gingival epithelial cells in a renal capsule system, are capable of forming whole teeth, complete with developing roots and pulp structures (22).
This work clearly demonstrates that the dental pulp has the theoretical capacity to regenerate both mineralized tissues and functional, complex pulp tissues. The ability of the pulp to synthesize reparative mineralized tissue in response to the destruction of the dentin matrix is of critical importance clinically. This matrix is required for the formation of a dentin bridge between dental restorations and surrounding dentin, preventing the continuum of micro-leakage, reinfection, and ultimate failure of the restoration (23–25). In terms of pulpal soft tissue regeneration, the diversity of cell types which can arise from pulpal mesenchymal progenitor cells represent an opportunity to repair and restore functional dental pulp, even in cases where significant amounts of necrotic tissue have been extirpated.
In healthy and intact teeth, the dental pulp is highly effective at maintaining the structure of the tooth over relatively long periods of time, due to a capacity to respond to damage and initiate repair. It is when this equilibrium is disrupted by damage to the pulp tissue caused by trauma or the formation of a carious lesion, that the integrity of the tooth is undermined.
Inflammation in the Dental Pulp
Inflammation resulting from the formation of infected dental caries is a factor that commonly causes a disruption to the dynamic equilibrium of the dental pulp (Figure 1). Bacteria from the oral cavity, for example Streptococcus mutans, a key organism identified in the formation of carious lesions, attach to the enamel surface, eventually forming a biofilm which may consist of an entire mixed-population ecosystem of organisms including, lactobacilli, non-mutans streptococci and Actinomyces (26, 27). The species found in these biofilms are acidogenic and are fed by fermentable carbohydrates from the oral cavity, causing an acid-erosion of the mineralized enamel as a result of their metabolic processes, damaging the enamel matrix and enlarging the lesion (26–28). Once the bacteria erode the enamel and reach the dentin, they readily spread through the fluid-filled dentinal tubule system and rapidly cause an enlargement of the tooth area affected by the lesion. As bacteria colonize the tubules and draw nearer to the dental pulp, lipopolysaccharide (LPS) from bacterial cell walls penetrates the pulp and stimulates an inflammatory response from a variety of cells resident in the tissue (14, 29). Macrophages and neutrophils, as in many tissues, are important mediators of the innate inflammatory response in the dental pulp (5, 30). As inflammation progresses, B and T cells of the acquired immune system also infiltrate the pulp and contribute to the inflammatory response from the tissue (30). Once activated by LPS, these immune cells mediate the destruction of the pulpal tissues by secreting a range of pro-inflammatory cytokines, prime examples being Il-1β and TNFα, and tissue degrading enzymes such as matrix metalloproteinases (MMPs) (31–33). Initially inflammatory mediators are critical drivers of the repair process and stimulate reparative dentin formation by odontoblasts and the differentiation of progenitor cells into a repair phenotype (6, 34, 35). If expression of these mediators persists however, inflammation becomes sustained in the pulp, creating a maelstrom of cytotoxic and tissue disrupting effects ultimately leading to tissue necrosis (8, 29, 31, 32, 36, 37).
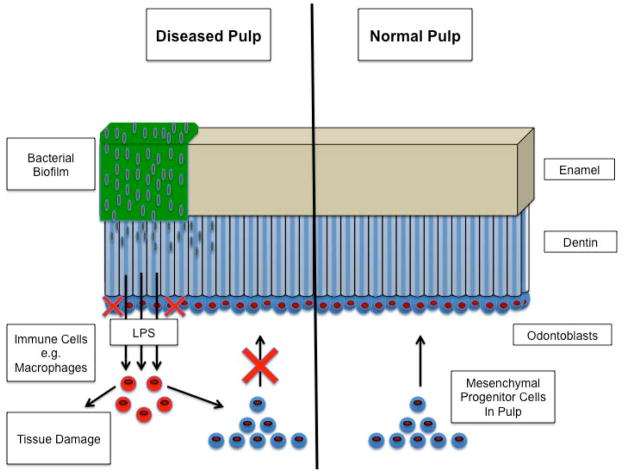
Figure 1.
Normal pulp contrasted with diseased pulp. In the normal pulp, odontoblasts are supported and renewed by a population of multipotent mesenchymal progenitor cells. This pool of cells can be drawn upon to regenerate pulp tissues and odontoblasts that are damaged by the formation of carious lesions or trauma. In the diseased pulp, bacterial biofilms form on the surface of the tooth, destroying the matrix of the enamel. When bacteria reach the fluid filled dentinal tubules, they rapidly spread through the dentin, enlarging the lesion and bringing the pulp in contact with LPS. LPS activated immune cells, for example macrophages, produce a number of inflammatory cytokines, causing widespread destruction of the pulpal tissues and impairing the recruitment and differentiation of the mesenchymal progenitor cells. Inflammation is therefore a significant barrier to dental pulp regeneration.
The process of pulpal inflammation has been modeled in a number of experimental systems. Interstitial fluid isolated from rat dental pulp stimulated with LPS contains increased levels of locally produced IL-1α, IL-1β and TNFα (38). In a study using an organotypic rat mandible section model, Roberts and colleagues demonstrated that the addition of Streptococcus anginosus group bacteria to whole dental pulp tissues upregulates the expression of IL-1β and TNFα by resident cells, leading to the destruction of pulpal tissues (39). Interestingly, odontoblasts themselves are also capable of initiating an inflammatory response (40). LPS stimulation of odontoblasts in a tooth crown organotypic culture model, caused an up-regulation of IL-1β, TNFα and IL-8 (41). When the same culture model was prepared using teeth with advanced caries, up-regulation of IL-1β and TNFα by native odontoblasts was observed (41). Odontoblasts have also been shown to induce neutrophil migration via IL-8 secretion in response to LPS stimulation (42).
In clinical terms, if there is any chance of regenerating pulpal tissues, it is clear that the lesion must be cleared of any microbial presence and pulpitis must be efficiently attenuated, preventing the destruction of the tissue, thereby facilitating the recruitment and differentiation of mesenchymal progenitor cells into repair phenotypes. The first battle is clearly against the microbial presence in a carious lesion. There is however, a second, equally critical struggle against the pro-inflammatory environment in the pulp created by these microbes, which can not only damage the tissue, but also interfere with its ability to self-repair.
Controlling Pulpitis to Create a Regenerative Environment
As acute, reversible inflammation in response to a microbial presence becomes established in the pulp, it enters a chronic phase, which can become self-sustaining and irreversible. Sustained pulpal inflammation not only damages the pulp tissue, but also actively prevents the repair response by down-regulating the recruitment and differentiation of mesenchymal progenitor cells (8, 29, 31, 32). Macrophages play a pivotal role in the innate immune response, and it is now well-established that they are capable of responding to various complex stimuli, by polarizing into either pro-inflammatory (M1) or pro-resolution (M2) phenotypes, with many subtle variations on these two broad classifications (43–46). Macrophages arise from monocytes, homing to sites of tissue damage or infection and polarizing into an M1 or M2 phenotype (45). This polarization has been shown to be reversible (46), meaning that it is quite possible for macrophages to switch between these two states, given the appropriate conditions (Figure 2). Macrophages stimulated with IL-10 and TGFβ decrease the production of inflammatory cytokines, such as TNFα, by induced M1 macrophages, inhibit the proliferation of CD4+ T cells and induce regulatory T cell formation (47).
Figure 2.
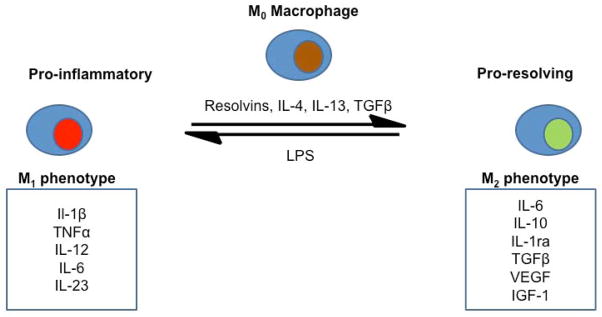
Macrophage polarization. Macrophages can switch between M1 pro-inflammatory and M2 pro-resolving phenotypes given appropriate stimuli. Bacterial LPS drives polarization into an M1 phenotype and results in the production of a variety of pro-inflammatory cytokines, ultimately leading to pulp tissue damage. By contrast, various anti-inflammatory molecules such as Il-4, Il-13 and specialized pro-resolving lipid mediators such as the resolvins stimulate M2 phenotype polarization. M2 macrophages produce anti-inflammatory cytokines and a variety of growth factors, which in turn support tissue repair.
The reversible switching of macrophages between M1 and M2 phenotypes presents a unique opportunity for the treatment of pulpitis. Relatively large numbers of M1 macrophages are present during any inflammatory response, for example in the pulp in apposition to an advancing carious lesion. If these cells could be converted to a pro-resolving M2 phenotype, they would be effectively recruited to actively create an environment favoring the resolution of inflammation, thereby restoring the balance in favor of tissue regeneration. Factors that encourage this transition are of critical interest when considering bioactive material design. The specialized pro-resolving lipid mediators (SPM), for example, are a family of molecules derived from poly-unsaturated fatty acids. This group of molecules is highly diverse and includes the resolvins, protectins, lipoxins and maresins (48). These molecules have been shown to have a substantial range of anti-inflammatory actions in diverse tissues and have been widely reviewed in the literature (48–56). The resolvins in particular have shown great promise as potential therapeutic agents in attenuating inflammation.
Resolvins for the Potent Resolution of Inflammation
Resolvins may have great efficacy in the treatment of pulpal inflammation and the preservation or regeneration of a vital dental pulp. The resolvins are a group of molecules that were first described by Serhan and colleagues (57). Two broad classes of resolvins have been characterized: the E-series, which are derived from eicosapentaenoic acid and the D-series derived from docosahexaenoic acid (48, 56). There are several key pathways for the synthesis of the resolvins in vivo, including via aspirin-acetylated COX2 enzymes for the E-series and the aspirin-triggered subgroup of the D-series (49, 58–60). Resolvins exert numerous potent anti-inflammatory effects, such as decreasing the migration and activation of neutrophils (59, 61, 62), inhibiting the production of IL-12 by dendritic cells (63), enhancing the appearance of M2 phenotype pro-resolving macrophages (62, 64) and have been shown to decrease the inflammatory response in murine models of dermal inflammation, peritoneal inflammation and colitis (57, 58, 64, 65).
Resolvin E1 (RvE1) has shown efficacy in a dental context. RvE1 acts to down-regulate NF-κβ through the ligand specific receptor Chem R23, which is expressed by a number of cell types, including monocytes/macrophages, neutrophils, dendritic cells and T cells (49, 63, 66). In a particularly interesting study, Hasturk and colleagues demonstrated that directly applied RvE1 was remarkably effective in stimulating mineralized tissue repair in a ligature-induced model of periodontitis compared to carrier treated controls (67). Further, it has been shown that bone loss can be substantially attenuated by RvE1 treatment, via increased production of osteoprotegerin (OPG) by osteoblasts, which decreases osteoclast activation via the Rank/RankL pathway (68). These studies suggest that the delivery of RvE1 stimulate regeneration by resolving the initial inflammatory response in diseased pulp, shifting macrophages from their pro-inflammatory M1 state to a pro-resolving M2 phenotype. This would allow healing of both soft and mineralized dental tissues to progress. Currently however, the potential of RvE1 to resolve pulpitis and subsequently enhance dental pulp tissue regeneration remains relatively unexplored. While showing great promise, the delivery of such endogenous molecules into a complex tissue environment must be approached with some caution, due to the possibility of an unforeseen host reactions, which could result in increased inflammation and necrosis of the tissue.
Materials to Support Pulpal Regeneration
Currently in clinical practice, restorative materials have little bioactivity and few innate anti-inflammatory properties; for example the gutta percha used in root canal procedures. While there are many materials that do not actively stimulate an immune response, the lack of active anti-inflammatory and regenerative materials currently available to endodontists significantly limits treatment options conducive to pulpal regeneration. Endodontic tissue engineering scaffolds, to be truly effective in diseased pulpal tissues, will have to address the initial microbial infection and attenuate inflammation in order to stimulate a repair response, either through their innate properties or by the delivery of antimicrobial and anti-inflammatory molecules which perform these functions. Accordingly, there have been a wide variety of materials proposed in the literature as candidates for endodontic use, which include both synthetic peptide constructs and hydrogels formed from biomimetic or naturally occurring molecules.
Numerous synthetic polymer scaffolds have been proposed as dental tissue engineering scaffolds. Polyglycolic acid (PGA) and polylactic acid (PLA) are biodegradable polyesters which can be derived from a variety of renewable sources and have been proposed for use in dental pulp tissue engineering (69–71). Scaffolds formed from PGA have been shown to support the adhesion and proliferation of pulpal fibroblasts (71). PLA scaffolds support the adherence of both isolated dental pulp progenitor cells and cells from ex vivo human pulp tissues (70, 72). When a copolymer of PGA and PLA, poly(lactic-co-glycolic acid) (PLGA), is seeded with dental pulp progenitor cells it has been shown to support the formation of pulp-like tissue in both rabbit and mouse xenograft models (73–75). Polyethylene glycol (PEG) is another commonly used polymer in tissue engineering (69, 76–78). Dental pulp progenitor cells attach to electrospun PEG scaffolds and have been transduced to form 3-D collagen structures (79). Synthetic scaffolds have been used to deliver a variety of anti-inflammatory agents such as ibuprofen, dexamethasone, diclofenac, rolipram and many others (80–84). Such scaffolds, which have been shown to support the regeneration of pulpal tissues, could also be used to deliver these or other anti-inflammatory molecules such as the resolvins in order to control pulpitis, thereby facilitating pulpal repair. From the perspective of endodontic regeneration, these polymers are attractive due to their handling properties and relative ease of production, but bear little resemblance to the native extracellular environment of the dental pulp.
There is also significant interest in using biomimetic scaffolds composed of ‘natural’ materials, although these are generally not as mechanically resilient as synthetic polymer scaffolds. Hydrogels formed from collagen have been widely used to culture dental pulp progenitor cells in vitro and as a vehicle to deliver cells, growth factors and anti-inflammatory molecules in various animal models, with some success in regenerating pulp-like tissues (21, 85–87). Collagen is, of course, prevalent in the extracellular milieu, and is therefore highly compatible with cells and tissues. Hydrogels formed from collagen are, however, often structurally weak and chemical cross-linking to improve gel stiffness often leads to decreased cyto-compatibility (69, 88, 89). Similarly, fibrin has been proposed as a candidate for tissue engineering scaffolds in various applications, due to its association with blood clotting and cell adherence in vivo, although it shares the structural disadvantages of collagen (89–91). Alginate is a naturally occurring polysaccharide that can be formed into porous hydrogels, which has been widely proposed for use in a variety of tissue engineering applications (92). These hydrogels are compatible with mesenchymal progenitor cells derived from the gingiva and periodontal ligament, and, when loaded with TGFβ1, induced odontoblast-like cell differentiation in a human tooth slice model (93, 94). Collagen and fibrin scaffolds have also been used as delivery systems for a variety of molecules with anti-inflammatory properties such as IL-10, desulfated hepatin, and neurotrophin-3 among others, and could conceivably be used to address inflammation in the context of pulpal regeneration (95–97). While these scaffolds, by definition, more closely resemble native dental pulp tissue, establishing control over their specific composition can be difficult, and can undermine both their biocompatibility and structural integrity.
Self-Assembling Peptide Hydrogels as Scaffolds in Pulpal Regeneration
The development of self-assembling peptides in the past decade has provided a new class of tissue engineering scaffolds, which have shown good biocompatibility, excellent handling properties, and strong potential as a carrier material for anti-inflammatory and bioactive molecules. Multidomain peptides (MDP), for example, are synthetic peptides that form hydrophobic ‘sandwiches’ from single β-sheets, which in turn self-assemble into multi-subunit nanofibers (Figure 3) (98, 99). These fibers can be non-covalently cross-linked to form porous, nano-fibrillar hydrogels (100–104). The non-covalent nature of the fiber cross-linking interactions within MDP hydrogels allows the material to thin into a liquid state when shear forces are applied and to recover into a solid hydrogel after shearing (102, 105, 106). MDP scaffolds are therefore injectable, allowing relatively easy application to irregularly sized defects, as found in dental caries, and also allow easy homogenization of bioactive molecules (102, 105). Furthermore, MDPs are highly customizable, as their physio-chemical properties can be manipulated by changes to their primary amino acid sequence. For example, bioactive peptide sequence motifs have also been included in MDP scaffolds, such as an RGD cell-adhesion sequence designed to increase cell attachment and an MMP-2 vulnerable cleavage site, which enhances the biodegradability of the hydrogel (100, 106, 107).
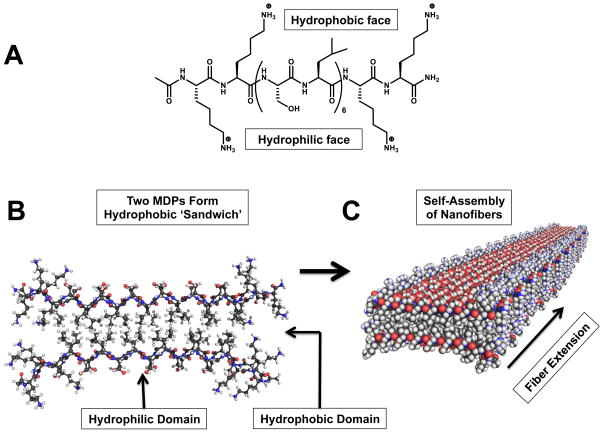
Figure 3.
A: The chemical structure of a multidomain peptide (MDP), K2(SL)6K2, which forms a facial amphiphile in water. The termini of the peptide consist of lysine residues, which are positively charged at physiological pH. B: Two MDPs align to form a ‘hydrophobic sandwich’ in order to minimize contact of the hydrophobic leucine side chains with the surrounding aqueous environment. C: Multiple hydrophobic sandwiches assemble to form nano-fibers, driven by the formation of hydrogen bonds between adjacent peptide backbones. Figures 3B and 3C created with PyMOL Molecular Graphics System, Version 1.6 Schrödinger, LLC.
There is a growing body of evidence to suggest that self-assembling peptide hydrogels are feasible as a biocompatible matrix and capable of supporting the growth and differentiation of dental pulp progenitor cells. MDP scaffolds are effective carriers for a number of growth factors, including TGFβ1, VEGF and FGF-2 and when used as a scaffold for dental pulp progenitor cells in a murine xenograft system, formed dental pulp-like tissue, complete with odontoblast-like cells (106–108). Puramatrix™, a commercially available self-assembling peptide, supports the attachment and proliferation of isolated dental pulp progenitor cells (105). These hydrogels, with their unique handling properties, biocompatibility and suitability as a carrier material may be an excellent vehicle to deliver resolvins or other anti-inflammatories to treat pulpitis, and thereby facilitate pulpal regeneration.
Conclusion
The ability to regenerate dental pulp tissue would be a significant advancement in the field of endodontics, as it would provide an alternative to root canal therapy for teeth with advanced pulp damage. One of the most significant barriers to the regeneration of diseased and substantially damaged dental pulp tissue is pulpitis. In recent years, there have been significant achievements within the field of tissue engineering, including the development of many scaffolds that support mesenchymal progenitor attachment and differentiation. It is critical however to note that attempts to regenerate dental pulp will generally take place in the context of pulpal disease, where the pulp has been damaged by microbial incursion and the resulting inflammatory response. Therefore it will be crucial to combine these materials with treatment modalities that address the underlying pathologies associated with irreversible damage to dental pulp tissues, for example the rapid attenuation of pulpal inflammation and the clearance of microbes. While challenges in dental pulp tissue engineering remain, advances in tissue engineering will hopefully continue to drive the development of targeted therapies and materials which are rationally designed to regenerate dental pulp, in even the most challenging clinical situations.
Acknowledgments
The authors acknowledge support from their institutions. The research from our laboratories described in this review was supported by grant R01-DE021798, from the NBIB/NIH.
Footnotes
They declare no conflicts of interests relating to the authorship and/or publication of this article.
References
- 1.Harris R, Griffin CJ. The fine structure of the mature odontoblasts and cell rich zone of the human dental pulp. Australian Dental Journal. 1969;14(3):168–177. doi: 10.1111/j.1834-7819.1969.tb03348.x. [DOI] [PubMed] [Google Scholar]
- 2.Berkovitz BK, Holland GR, Moxham B. Oral anatomy, embryology and histology. Mosby Edinburgh; 2002. [Google Scholar]
- 3.Nanci A. Ten Cate’s oral histology: development, structure, and function. Elsevier Health Sciences; 2007. [Google Scholar]
- 4.Linde A. The extracellular matrix of the dental pulp and dentin. Journal of dental research. 1985;64(Spec No):523–529. doi: 10.1177/002203458506400405. [DOI] [PubMed] [Google Scholar]
- 5.Iwasaki Y, Otsuka H, Yanagisawa N, Hisamitsu H, Manabe A, Nonaka N, et al. In situ proliferation and differentiation of macrophages in dental pulp. Cell Tissue Res. 2011;346(1):99–109. doi: 10.1007/s00441-011-1231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sloan AJ, Smith AJ. Stem cells and the dental pulp: potential roles in dentine regeneration and repair. Oral Diseases. 2007;13(2):151–157. doi: 10.1111/j.1601-0825.2006.01346.x. [DOI] [PubMed] [Google Scholar]
- 7.Sloan AJ, Waddington RJ. Dental pulp stem cells: what, where, how? International Journal of Paediatric Dentistry. 2009;19(1):61–70. doi: 10.1111/j.1365-263X.2008.00964.x. [DOI] [PubMed] [Google Scholar]
- 8.Schmalz G, Galler KM. Tissue Injury and Pulp Regeneration. Journal of Dental Research. 2011;90(7):828–829. doi: 10.1177/0022034511405331. [DOI] [PubMed] [Google Scholar]
- 9.Casagrande L, Cordeiro M, Nör S, Nör J. Dental pulp stem cells in regenerative dentistry. Odontology. 2011;99(1):1–7. doi: 10.1007/s10266-010-0154-z. [DOI] [PubMed] [Google Scholar]
- 10.Balic A, Aguila HL, Caimano MJ, Francone VP, Mina M. Characterization of stem and progenitor cells in the dental pulp of erupted and unerupted murine molars. Bone. 2010;46(6):1639–1651. doi: 10.1016/j.bone.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Miura M, Gronthos S, Zhao M, Lu B, Fisher LW, Robey PG, et al. SHED: Stem cells from human exfoliated deciduous teeth. Proceedings of the National Academy of Sciences. 2003;100(10):5807–5812. doi: 10.1073/pnas.0937635100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sigal MJ, Aubin JE, Ten Cate AR, Pitaru S. The odontoblast process extends to the dentinoenamel junction: an immunocytochemical study of rat dentine. Journal of Histochemistry & Cytochemistry. 1984;32(8):872–877. doi: 10.1177/32.8.6379038. [DOI] [PubMed] [Google Scholar]
- 13.Gronthos S, Mankani M, Brahim J, Robey PG, Shi S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proceedings of the National Academy of Sciences. 2000;97(25):13625–13630. doi: 10.1073/pnas.240309797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tziafas D, Smith AJ, Lesot H. Designing new treatment strategies in vital pulp therapy. Journal of Dentistry. 2000;28(2):77–92. doi: 10.1016/s0300-5712(99)00047-0. [DOI] [PubMed] [Google Scholar]
- 15.Harichane Y, Hirata A, Dimitrova-Nakov S, Granja I, Goldberg A, Kellermann O, et al. Pulpal Progenitors and Dentin Repair. Advances in Dental Research. 2011;23(3):307–312. doi: 10.1177/0022034511405322. [DOI] [PubMed] [Google Scholar]
- 16.Lin LM, Rosenberg PA. Repair and regeneration in endodontics. International Endodontic Journal. 2011;44(10):889–906. doi: 10.1111/j.1365-2591.2011.01915.x. [DOI] [PubMed] [Google Scholar]
- 17.Waddington R, Waddington S, Youde C, Lee A, Sloan Isolation of Distinct Progenitor Stem Cell Populations from Dental Pulp. Cells tissues organs. 2009;189(1–4):268–274. doi: 10.1159/000151447. [DOI] [PubMed] [Google Scholar]
- 18.d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, et al. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death & Differentiation. 2007;14(6):1162–1171. doi: 10.1038/sj.cdd.4402121. [DOI] [PubMed] [Google Scholar]
- 19.Iohara K, Zheng L, Wake H, Ito M, Nabekura J, Wakita H, et al. A Novel Stem Cell Source for Vasculogenesis in Ischemia: Subfraction of Side Population Cells from Dental Pulp. STEM CELLS. 2008;26(9):2408–2418. doi: 10.1634/stemcells.2008-0393. [DOI] [PubMed] [Google Scholar]
- 20.Six N, Tompkins K, Septier D, Veis A, Goldberg M. Recruitment and characterization of the cells involved in reparative dentin formation in the exposed rat molar pulp after implantation of amelogenin gene splice products A+ 4 and A–4. [Google Scholar]
- 21.Iohara K, Murakami M, Takeuchi N, Osako Y, Ito M, Ishizaka R, et al. A Novel Combinatorial Therapy With Pulp Stem Cells and Granulocyte Colony-Stimulating Factor for Total Pulp Regeneration. Stem Cells Translational Medicine. 2013;2(7):521–533. doi: 10.5966/sctm.2012-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Angelova Volponi A, Kawasaki M, Sharpe PT. Adult Human Gingival Epithelial Cells as a Source for Whole-tooth Bioengineering. Journal of Dental Research. 2013 doi: 10.1177/0022034513481041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Slutzky-Goldberg I, Slutzky H, Gorfil C, Smidt A. Restoration of endodontically treated teeth review and treatment recommendations. International journal of dentistry. 2010;2009 doi: 10.1155/2009/150251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kermanshahi S, Santerre J, Cvitkovitch D, Finer Y. Biodegradation of resin-dentin interfaces increases bacterial microleakage. Journal of dental research. 2010;89(9):996–1001. doi: 10.1177/0022034510372885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Başaran EG, Ayna E, Halifeoğlu M. Microleakage of endodontically treated teeth restored with 3 different adhesive systems and 4 different fiber-reinforced posts. The Journal of Prosthetic Dentistry. 2012;107(4):239–251. doi: 10.1016/S0022-3913(12)60069-9. [DOI] [PubMed] [Google Scholar]
- 26.Takahashi N, Nyvad B. The Role of Bacteria in the Caries Process: Ecological Perspectives. Journal of Dental Research. 2011;90(3):294–303. doi: 10.1177/0022034510379602. [DOI] [PubMed] [Google Scholar]
- 27.Burne RA, Zeng L, Ahn SJ, Palmer SR, Liu Y, Lefebure T, et al. Progress Dissecting the Oral Microbiome in Caries and Health. Advances in Dental Research. 2012;24(2):77–80. doi: 10.1177/0022034512449462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Torlakovic L, Klepac-Ceraj V, Ogaard B, Cotton SL, Paster BJ, Olsen I. Microbial community succession on developing lesions on human enamel. Journal of oral microbiology. 2012;4 doi: 10.3402/jom.v4i0.16125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bergenholtz G. Inflammatory response of the dental pulp to bacterial irritation. Journal of Endodontics. 1981;7(3):100–104. doi: 10.1016/S0099-2399(81)80122-7. [DOI] [PubMed] [Google Scholar]
- 30.Izumi T, Kobayashi I, Okamura K, Sakai H. Immunohistochemical study on the immunocompetent cells of the pulp in human non-carious and carious teeth. Archives of Oral Biology. 1995;40(7):609–614. doi: 10.1016/0003-9969(95)00024-j. [DOI] [PubMed] [Google Scholar]
- 31.Cooper PR, Takahashi Y, Graham LW, Simon S, Imazato S, Smith AJ. Inflammation–regeneration interplay in the dentine–pulp complex. Journal of Dentistry. 2010;38(9):687–697. doi: 10.1016/j.jdent.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 32.Cooper PR, Smith AJ. Molecular mediators of pulp inflammation and regeneration. Endodontic Topics. 2013;28(1):90–105. [Google Scholar]
- 33.Zehnder M, Wegehaupt FJ, Attin T. A First Study on the Usefulness of Matrix Metalloproteinase 9 from Dentinal Fluid to Indicate Pulp Inflammation. Journal of Endodontics. 2011;37(1):17–20. doi: 10.1016/j.joen.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 34.Goldberg M, Farges J-C, Lacerda-Pinheiro S, Six N, Jegat N, Decup F, et al. Inflammatory and immunological aspects of dental pulp repair. Pharmacological Research. 2008;58(2):137–147. doi: 10.1016/j.phrs.2008.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Paula-Silva FWG, Ghosh A, Silva LAB, Kapila YL. TNF-α promotes an odontoblastic phenotype in dental pulp cells. Journal of Dental Research. 2009;88(4):339–344. doi: 10.1177/0022034509334070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yamaguchi M, Kojima T, Kanekawa M, Aihara N, Nogimura A, Kasai K. Neuropeptides stimulate production of interleukin-1β, interleukin-6, and tumor necrosis factor-α in human dental pulp cells. Inflamm res. 2004;53(5):199–204. doi: 10.1007/s00011-003-1243-z. [DOI] [PubMed] [Google Scholar]
- 37.McLachlan JL, Sloan AJ, Smith AJ, Landini G, Cooper PR. S100 and cytokine expression in caries. Infection and immunity. 2004;72(7):4102–4108. doi: 10.1128/IAI.72.7.4102-4108.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bletsa A, Berggreen E, Fristad I, Tenstad O, Wiig H. Cytokine signalling in rat pulp interstitial fluid and transcapillary fluid exchange during lipopolysaccharide-induced acute inflammation. The Journal of Physiology. 2006;573(1):225–236. doi: 10.1113/jphysiol.2006.104711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Roberts JL, Maillard J-Y, Waddington RJ, Denyer SP, Lynch CD, Sloan AJ. Development of an Ex Vivo Coculture System to Model Pulpal Infection by Streptococcus anginosus Group Bacteria. Journal of Endodontics. 2013;39(1):49–56. doi: 10.1016/j.joen.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 40.Farges J-C, Keller J-F, Carrouel F, Durand SH, Romeas A, Bleicher F, et al. Odontoblasts in the dental pulp immune response. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 2009;312B(5):425–436. doi: 10.1002/jez.b.21259. [DOI] [PubMed] [Google Scholar]
- 41.Veerayutthwilai O, Byers MR, Pham TTT, Darveau RP, Dale BA. Differential regulation of immune responses by odontoblasts. Oral Microbiology and Immunology. 2007;22(1):5–13. doi: 10.1111/j.1399-302X.2007.00310.x. [DOI] [PubMed] [Google Scholar]
- 42.Levin LG, Rudd A, Bletsa A, Reisner H. Expression of IL-8 by cells of the odontoblast layer in vitro. European Journal of Oral Sciences. 1999;107(2):131–137. doi: 10.1046/j.0909-8836.1999.eos107209.x. [DOI] [PubMed] [Google Scholar]
- 43.Brown BN, Ratner BD, Goodman SB, Amar S, Badylak SF. Macrophage polarization: An opportunity for improved outcomes in biomaterials and regenerative medicine. Biomaterials. 2012;33(15):3792–3802. doi: 10.1016/j.biomaterials.2012.02.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Laskin DL. Macrophages and Inflammatory Mediators in Chemical Toxicity: A Battle of Forces. Chemical Research in Toxicology. 2009;22(8):1376–1385. doi: 10.1021/tx900086v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Benoit M, Desnues B, Mege J-L. Macrophage Polarization in Bacterial Infections. The Journal of Immunology. 2008;181(6):3733–3739. doi: 10.4049/jimmunol.181.6.3733. [DOI] [PubMed] [Google Scholar]
- 46.Stout RD, Suttles J. Functional plasticity of macrophages: reversible adaptation to changing microenvironments. Journal of Leukocyte Biology. 2004;76(3):509–513. doi: 10.1189/jlb.0504272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cao Q, Wang Y, Zheng D, Sun Y, Wang Y, Lee VWS, et al. IL-10/TGF-β–Modified Macrophages Induce Regulatory T Cells and Protect against Adriamycin Nephrosis. Journal of the American Society of Nephrology. 2010;21(6):933–942. doi: 10.1681/ASN.2009060592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Serhan CN, Petasis NA. Resolvins and protectins in inflammation resolution. Chemical reviews. 2011;111(10):5922–5943. doi: 10.1021/cr100396c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ariel A, Serhan CN. Resolvins and protectins in the termination program of acute inflammation. Trends in Immunology. 2007;28(4):176–183. doi: 10.1016/j.it.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 50.Zhang MJ, Spite M. Resolvins: anti-inflammatory and proresolving mediators derived from omega-3 polyunsaturated fatty acids. Annual review of nutrition. 2012;32:203–227. doi: 10.1146/annurev-nutr-071811-150726. [DOI] [PubMed] [Google Scholar]
- 51.Hong S, Lu Y. Omega-3 fatty acid-derived resolvins and protectins in inflammation resolution and leukocyte functions: targeting novel lipid mediator pathways in mitigation of acute kidney injury. Frontiers in immunology. 2013;4 doi: 10.3389/fimmu.2013.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Janakiram NB, Mohammed A, Rao CV. Role of lipoxins, resolvins, and other bioactive lipids in colon and pancreatic cancer. Cancer and Metastasis Reviews. 2011;30(3–4):507–523. doi: 10.1007/s10555-011-9311-2. [DOI] [PubMed] [Google Scholar]
- 53.Fredman G, Serhan CN. 4 Specialized Pro-resolving Lipid Derived Fatty Acid Mediators: Wiring the Circuitry of Effector Immune Homeostasis. Oral Wound Healing: Cell Biology and Clinical Management. 2012;57 [Google Scholar]
- 54.Katuri KK, Swarna C, Swamy ND. Role of Lipoxins, Resolvins and Protectins in mediating Inflammation–Review. International Journal of Dental Clinics. 2012;4(2) [Google Scholar]
- 55.Serhan CN, Yang R, Martinod K, Kasuga K, Pillai PS, Porter TF, et al. Maresins: novel macrophage mediators with potent antiinflammatory and proresolving actions. The Journal of experimental medicine. 2009;206(1):15–23. doi: 10.1084/jem.20081880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Serhan CN, Chiang N. Resolution phase lipid mediators of inflammation: agonists of resolution. Current Opinion in Pharmacology. 2013 doi: 10.1016/j.coph.2013.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Serhan CN, Clish CB, Brannon J, Colgan SP, Chiang N, Gronert K. Novel functional sets of lipid-derived mediators with antiinflammatory actions generated from omega-3 fatty acids via cyclooxygenase 2-nonsteroidal antiinflammatory drugs and transcellular processing. The Journal of experimental medicine. 2000;192(8):1197–1204. doi: 10.1084/jem.192.8.1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Arita M, Yoshida M, Hong S, Tjonahen E, Glickman JN, Petasis NA, et al. Resolvin E1, an endogenous lipid mediator derived from omega-3 eicosapentaenoic acid, protects against 2,4,6-trinitrobenzene sulfonic acid-induced colitis. Proceedings of the National Academy of Sciences of the United States of America. 2005;102(21):7671–7676. doi: 10.1073/pnas.0409271102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Bannenberg GL, Chiang N, Ariel A, Arita M, Tjonahen E, Gotlinger KH, et al. Molecular circuits of resolution: formation and actions of resolvins and protectins. Journal of immunology. 2005;174(7):4345–4355. doi: 10.4049/jimmunol.174.7.4345. [DOI] [PubMed] [Google Scholar]
- 60.Lee H-N, Surh Y-J. Therapeutic potential of resolvins in the prevention and treatment of inflammatory disorders. Biochemical Pharmacology. 2012;84(10):1340–1350. doi: 10.1016/j.bcp.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 61.Winkler JW, Uddin J, Serhan CN, Petasis NA. Stereocontrolled Total Synthesis of the Potent Anti-inflammatory and Pro-resolving Lipid Mediator Resolvin D3 and Its Aspirin-Triggered 17 R-Epimer. Organic letters. 2013;15(7):1424–1427. doi: 10.1021/ol400484u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Krishnamoorthy S, Recchiuti A, Chiang N, Yacoubian S, Lee C-H, Yang R, et al. Resolvin D1 binds human phagocytes with evidence for proresolving receptors. Proceedings of the National Academy of Sciences. 2010;107(4):1660–1665. doi: 10.1073/pnas.0907342107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, et al. Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. The Journal of experimental medicine. 2005;201(5):713–722. doi: 10.1084/jem.20042031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schif-Zuck S, Gross N, Assi S, Rostoker R, Serhan CN, Ariel A. Saturated-efferocytosis generates pro-resolving CD11blow macrophages: Modulation by resolvins and glucocorticoids. European Journal of Immunology. 2011;41(2):366–379. doi: 10.1002/eji.201040801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Spite M, Norling LV, Summers L, Yang R, Cooper D, Petasis NA, et al. Resolvin D2 is a potent regulator of leukocytes and controls microbial sepsis. Nature. 2009;461(7268):1287–1291. doi: 10.1038/nature08541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Samson M, Edinger AL, Stordeur P, Rucker J, Verhasselt V, Sharron M, et al. ChemR23, a putative chemoattractant receptor, is expressed in monocyte-derived dendritic cells and macrophages and is a coreceptor for SIV and some primary HIV-1 strains. European Journal of Immunology. 1998;28(5):1689–1700. doi: 10.1002/(SICI)1521-4141(199805)28:05<1689::AID-IMMU1689>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 67.Hasturk H, Kantarci A, Goguet-Surmenian E, Blackwood A, Andry C, Serhan CN, et al. Resolvin E1 Regulates Inflammation at the Cellular and Tissue Level and Restores Tissue Homeostasis In Vivo. The Journal of Immunology. 2007;179(10):7021–7029. doi: 10.4049/jimmunol.179.10.7021. [DOI] [PubMed] [Google Scholar]
- 68.Gao L, Faibish D, Fredman G, Herrera BS, Chiang N, Serhan CN, et al. Resolvin E1 and Chemokine-like Receptor 1 Mediate Bone Preservation. The Journal of Immunology. 2013;190(2):689–694. doi: 10.4049/jimmunol.1103688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yu N, Plachokova A, Yang F, Walboomers XF, Jansen JA. Engineering of Dental Tissues: Scaffolds and Preclinical Models. Stem Cells in Craniofacial Development and Regeneration. 2013:409–429. [Google Scholar]
- 70.Gebhardt M, Murray PE, Namerow KN, Kuttler S, Garcia-Godoy F. Cell Survival within Pulp and Periodontal Constructs. Journal of Endodontics. 2009;35(1):63–66. doi: 10.1016/j.joen.2008.09.020. [DOI] [PubMed] [Google Scholar]
- 71.Mooney DJ, Powell C, Piana J, Rutherford B. Engineering Dental Pulp-like Tissue in Vitro. Biotechnology Progress. 1996;12(6):865–868. doi: 10.1021/bp960073f. [DOI] [PubMed] [Google Scholar]
- 72.Chandrahasa S, Murray PE, Namerow KN. Proliferation of Mature Ex Vivo Human Dental Pulp Using Tissue Engineering Scaffolds. Journal of Endodontics. 2011;37(9):1236–1239. doi: 10.1016/j.joen.2011.05.030. [DOI] [PubMed] [Google Scholar]
- 73.El-Backly RM, Massoud AG, El-Badry AM, Sherif RA, Marei MK. Regeneration of dentine/pulp-like tissue using a dental pulp stem cell/poly(lactic-co-glycolic) acid scaffold construct in New Zealand white rabbits. Australian Endodontic Journal. 2008;34(2):52–67. doi: 10.1111/j.1747-4477.2008.00139.x. [DOI] [PubMed] [Google Scholar]
- 74.Moioli EK, Clark PA, Xin X, Lal S, Mao JJ. Matrices and scaffolds for drug delivery in dental, oral and craniofacial tissue engineering. Advanced Drug Delivery Reviews. 2007;59(4–5):308–324. doi: 10.1016/j.addr.2007.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Huang GT-J, Yamaza T, Shea LD, Djouad F, Kuhn NZ, Tuan RS, et al. Stem/progenitor cell–mediated de novo regeneration of dental pulp with newly deposited continuous layer of dentin in an in vivo model. Tissue Engineering Part A. 2009;16(2):605–615. doi: 10.1089/ten.tea.2009.0518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hänseler P, Jung U-W, Jung RE, Choi K-H, Cho K-S, Hämmerle CH, et al. Analysis of hydrolyzable polyethylene glycol hydrogels and deproteinized bone mineral as delivery systems for glycosylated and non-glycosylated bone morphogenetic protein-2. Acta biomaterialia. 2012;8(1):116–123. doi: 10.1016/j.actbio.2011.08.002. [DOI] [PubMed] [Google Scholar]
- 77.Thoma DS, Subramani K, Weber FE, Luder HU, Hämmerle CHF, Jung RE. Biodegradation, soft and hard tissue integration of various polyethylene glycol hydrogels: a histomorphometric study in rabbits. Clinical Oral Implants Research. 2011;22(11):1247–1254. doi: 10.1111/j.1600-0501.2010.02075.x. [DOI] [PubMed] [Google Scholar]
- 78.Thomas S, Visakh P, Mathew AP. Advances in Natural Polymers. 2013. [Google Scholar]
- 79.Rizk A, Rabie ABM. Human dental pulp stem cells expressing transforming growth factor β3 transgene for cartilage-like tissue engineering. Cytotherapy. 2013 doi: 10.1016/j.jcyt.2013.01.012. [DOI] [PubMed] [Google Scholar]
- 80.Cantón I, Mckean R, Charnley M, Blackwood KA, Fiorica C, Ryan AJ, et al. Development of an Ibuprofen releasing biodegradable PLA/PGA electrospun scaffold for tissue regeneration. Biotechnology and bioengineering. 2010;105(2):396–408. doi: 10.1002/bit.22530. [DOI] [PubMed] [Google Scholar]
- 81.Murua A, Herran E, Orive G, Igartua M, Blanco FJ, Pedraz JL, et al. Design of a composite drug delivery system to prolong functionality of cell-based scaffolds. International Journal of Pharmaceutics. 2011;407(1–2):142–150. doi: 10.1016/j.ijpharm.2010.11.022. [DOI] [PubMed] [Google Scholar]
- 82.Bae SE, Son JS, Park K, Han DK. Fabrication of covered porous PLGA microspheres using hydrogen peroxide for controlled drug delivery and regenerative medicine. Journal of Controlled Release. 2009;133(1):37–43. doi: 10.1016/j.jconrel.2008.09.006. [DOI] [PubMed] [Google Scholar]
- 83.Lamprecht A, Ubrich N, Yamamoto H, Schäfer U, Takeuchi H, Maincent P, et al. Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. Journal of Pharmacology and Experimental Therapeutics. 2001;299(2):775–781. [PubMed] [Google Scholar]
- 84.Danhier F, Ansorena E, Silva JM, Coco R, Le Breton A, Préat V. PLGA-based nanoparticles: An overview of biomedical applications. Journal of Controlled Release. 2012;161(2):505–522. doi: 10.1016/j.jconrel.2012.01.043. [DOI] [PubMed] [Google Scholar]
- 85.Kim JY, Xin X, Moioli EK, Chung J, Lee CH, Chen M, et al. Regeneration of dental-pulp-like tissue by chemotaxis-induced cell homing. Tissue engineering Part A. 2010;16(10):3023–3031. doi: 10.1089/ten.tea.2010.0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Huang GT-J, Sonoyama W, Chen J, Park SH. In vitro characterization of human dental pulp cells: various isolation methods and culturing environments. Cell Tissue Res. 2006;324(2):225–236. doi: 10.1007/s00441-005-0117-9. [DOI] [PubMed] [Google Scholar]
- 87.Nakao K, Itoh M, Tomita Y, Tomooka Y, Tsuji T. FGF-2 potently induces both proliferation and DSP expression in collagen type I gel cultures of adult incisor immature pulp cells. Biochemical and Biophysical Research Communications. 2004;325(3):1052–1059. doi: 10.1016/j.bbrc.2004.10.136. [DOI] [PubMed] [Google Scholar]
- 88.Lee KY, Mooney DJ. Hydrogels for Tissue Engineering. Chemical Reviews. 2001;101(7):1869–1880. doi: 10.1021/cr000108x. [DOI] [PubMed] [Google Scholar]
- 89.Yuan Z, Nie H, Wang S, Lee CH, Li A, Fu SY, et al. Biomaterial selection for tooth regeneration. Tissue Engineering Part B: Reviews. 2011;17(5):373–388. doi: 10.1089/ten.teb.2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Park K-H, Kim H, Moon S, Na K. Bone morphogenic protein-2 (BMP-2) loaded nanoparticles mixed with human mesenchymal stem cell in fibrin hydrogel for bone tissue engineering. Journal of bioscience and bioengineering. 2009;108(6):530–537. doi: 10.1016/j.jbiosc.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 91.Galler KM, Cavender AC, Koeklue U, Suggs LJ, Schmalz G, D’Souza RN. Bioengineering of dental stem cells in a PEGylated fibrin gel. Regenerative Medicine. 2011;6(2):191–200. doi: 10.2217/rme.11.3. [DOI] [PubMed] [Google Scholar]
- 92.Lee KY, Mooney DJ. Alginate: properties and biomedical applications. Progress in polymer science. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moshaverinia A, Chen C, Akiyama K, Ansari S, Xu X, Chee WW, et al. Alginate hydrogel as a promising scaffold for dental-derived stem cells: an in vitro study. Journal of Materials Science: Materials in Medicine. 2012;23(12):3041–3051. doi: 10.1007/s10856-012-4759-3. [DOI] [PubMed] [Google Scholar]
- 94.Dobie K, Smith G, Sloan A, Smith A. Effects of alginate hydrogels and TGF-β1 on human dental pulp repair in vitro. Connective tissue research. 2002;43(2–3):387–390. doi: 10.1080/03008200290000574. [DOI] [PubMed] [Google Scholar]
- 95.Holladay CA, Duffy AM, Chen X, Sefton MV, O’Brien TD, Pandit AS. Recovery of cardiac function mediated by MSC and interleukin-10 plasmid functionalised scaffold. Biomaterials. 2012;33(5):1303–1314. doi: 10.1016/j.biomaterials.2011.10.019. [DOI] [PubMed] [Google Scholar]
- 96.Lakshmi TSR, Shanmugasundaram N, Shanmuganathan S, Babu M. Efficacy of desulfated heparin mitigating inflammation in rat burn wound model. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2011;97(2):215–223. doi: 10.1002/jbm.b.31797. [DOI] [PubMed] [Google Scholar]
- 97.Johnson PJ, Tatara A, Shiu A, Sakiyama-Elbert SE. Controlled release of neurotrophin-3 and platelet derived growth factor from fibrin scaffolds containing neural progenitor cells enhances survival and differentiation into neurons in a subacute model of SCI. Cell transplantation. 2010;19(1):89. doi: 10.3727/096368909X477273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Cheng P-N, Pham JD, Nowick JS. The Supramolecular Chemistry of β-Sheets. Journal of the American Chemical Society. 2013;135(15):5477–5492. doi: 10.1021/ja3088407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bakota EL, Sensoy O, Ozgur B, Sayar M, Hartgerink JD. Self-assembling multidomain peptide fibers with aromatic cores. Biomacromolecules. 2013;14(5):1370–1378. doi: 10.1021/bm4000019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Galler KM, Aulisa L, Regan KR, D’Souza RN, Hartgerink JD. Self-Assembling Multidomain Peptide Hydrogels: Designed Susceptibility to Enzymatic Cleavage Allows Enhanced Cell Migration and Spreading. Journal of the American Chemical Society. 2010;132(9):3217–3223. doi: 10.1021/ja910481t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Aulisa L, Dong H, Hartgerink JD. Self-Assembly of Multidomain Peptides: Sequence Variation Allows Control over Cross-Linking and Viscoelasticity. Biomacromolecules. 2009;10(9):2694–2698. doi: 10.1021/bm900634x. [DOI] [PubMed] [Google Scholar]
- 102.Bakota EL, Wang Y, Danesh FR, Hartgerink JD. Injectable Multidomain Peptide Nanofiber Hydrogel as a Delivery Agent for Stem Cell Secretome. Biomacromolecules. 2011;12(5):1651–1657. doi: 10.1021/bm200035r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Dong H, Paramonov SE, Aulisa L, Bakota EL, Hartgerink JD. Self-Assembly of Multidomain Peptides: Balancing Molecular Frustration Controls Conformation and Nanostructure. Journal of the American Chemical Society. 2007;129(41):12468–12472. doi: 10.1021/ja072536r. [DOI] [PubMed] [Google Scholar]
- 104.Nakahara H, Misawa H, Yoshida A, Hayashi T, Tanaka M, Furumatsu T, et al. Bone repair using a hybrid scaffold of self-assembling peptide PuraMatrix and polyetheretherketone cage in rats. Cell Transplantation. 2010;19(6–7):6–7. doi: 10.3727/096368910X508906. [DOI] [PubMed] [Google Scholar]
- 105.Cavalcanti BN, Zeitlin BD, Nör JE. A hydrogel scaffold that maintains viability and supports differentiation of dental pulp stem cells. Dental Materials. 2013;29(1):97–102. doi: 10.1016/j.dental.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Galler KM, D’Souza RN, Hartgerink JD, Schmalz G. Scaffolds for Dental Pulp Tissue Engineering. Advances in Dental Research. 2011;23(3):333–339. doi: 10.1177/0022034511405326. [DOI] [PubMed] [Google Scholar]
- 107.Galler KM, Hartgerink JD, Cavender AC, Schmalz G, D’Souza RN. A customized self-assembling peptide hydrogel for dental pulp tissue engineering. Tissue engineering Part A. 2012;18(1–2):176–184. doi: 10.1089/ten.tea.2011.0222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Galler KM, D’Souza RN, Federlin M, Cavender AC, Hartgerink JD, Hecker S, et al. Dentin Conditioning Codetermines Cell Fate in Regenerative Endodontics. Journal of Endodontics. 2011;37(11):1536–1541. doi: 10.1016/j.joen.2011.08.027. [DOI] [PubMed] [Google Scholar]