Abstract
Background
Everolimus, an oral inhibitor of mammalian target of rapamycin (mTOR), has shown antitumor activity in patients with advanced pancreatic neuroendocrine tumors, in two phase 2 studies. We evaluated the agent in a prospective, randomized, phase 3 study.
Methods
We randomly assigned 410 patients who had advanced, low-grade or intermediate-grade pancreatic neuroendocrine tumors with radiologic progression within the previous 12 months to receive everolimus, at a dose of 10 mg once daily (207 patients), or placebo (203 patients), both in conjunction with best supportive care. The primary end point was progression-free survival in an intention-to-treat analysis. In the case of patients in whom radiologic progression occurred during the study, the treatment assignments could be revealed, and patients who had been randomly assigned to placebo were offered open-label everolimus.
Results
The median progression-free survival was 11.0 months with everolimus as compared with 4.6 months with placebo (hazard ratio for disease progression or death from any cause with everolimus, 0.35; 95% confidence interval [CI], 0.27 to 0.45; P<0.001), representing a 65% reduction in the estimated risk of progression or death. Estimates of the proportion of patients who were alive and progression-free at 18 months were 34% (95% CI, 26 to 43) with everolimus as compared with 9% (95% CI, 4 to 16) with placebo. Drug-related adverse events were mostly grade 1 or 2 and included stomatitis (in 64% of patients in the everolimus group vs. 17% in the placebo group), rash (49% vs. 10%), diarrhea (34% vs. 10%), fatigue (31% vs. 14%), and infections (23% vs. 6%), which were primarily upper respiratory. Grade 3 or 4 events that were more frequent with everolimus than with placebo included anemia (6% vs. 0%) and hyperglycemia (5% vs. 2%). The median exposure to everolimus was longer than exposure to placebo by a factor of 2.3 (38 weeks vs. 16 weeks).
Conclusions
Everolimus, as compared with placebo, significantly prolonged progression-free survival among patients with progressive advanced pancreatic neuroendocrine tumors and was associated with a low rate of severe adverse events.
The incidence and prevalence of pancreatic neuroendocrine tumors are increasing1-3; these tumors represent approximately 1.3% of all cases of pancreatic cancer in incidence and 10% of cases in prevalence.1-3 Pancreatic neuroendocrine tumors are frequently diagnosed at a late stage, with approximately 65% of patients presenting with unresectable or metastatic disease; as a result, these patients have a poor prognosis. The median survival time for patients with distant metastatic disease is 24 months,2 and limited treatment options are available for this population.
Streptozocin is the only approved therapy for pancreatic neuroendocrine tumors in the United States; however, the role of chemotherapy in advanced cases continues to be debated.3-12 The criteria that were used to determine the outcome measures in many earlier trials are considered unacceptable today, and a substantial number of adverse events were seen with regimens that showed improved response rates.3,10,13,14 Large, prospective, randomized trials that use validated criteria are therefore required to show the value of promising new treatment regimens for advanced pancreatic neuroendocrine tumors. A recent prospective study (reported by Raymond et al. elsewhere in this issue of the Journal) shows that sunitinib has antitumor activity.15
Everolimus (Afinitor, Novartis Pharmaceuticals) has recently shown promising antitumor activity in two phase 2 studies involving patients with pancreatic neuroendocrine tumors.3,16 Everolimus inhibits mammalian target of rapamycin (mTOR), a serine–threonine kinase that stimulates cell growth, proliferation, and angiogenesis.3,16,17 Autocrine activation of the mTOR signaling pathway, mediated through insulin-like growth factor 1, has been implicated in the proliferation of pancreatic neuroendocrine tumor cells.18 Consistent with this observation is the finding that inhibition of mTOR has a significant antiproliferative effect on pancreatic neuroendocrine tumor cell lines.19,20
The RAD001 in Advanced Neuroendocrine Tumors, third trial (RADIANT-3) study was conducted to determine whether everolimus, at a dose of 10 mg per day, as compared with placebo, would prolong progression-free survival among patients with advanced pancreatic neuroendocrine tumors.
Methods
Patients
Patients were eligible to be included in the study if they were 18 years of age or older and had low-grade or intermediate-grade advanced (unresectable or metastatic) pancreatic neuroendocrine tumors and radiologic documentation of disease progression (an unequivocal increase in the size of tumors) in the 12 months preceding randomization. Prior antineoplastic therapy was not an exclusion criterion. Other key eligibility criteria included the presence of measurable disease, as assessed according to the Response Evaluation Criteria in Solid Tumors (RECIST), version 1.0 (see the Supplementary Appendix, available with the full text of this article at NEJM.org)21; a World Health Organization (WHO) performance status of 2 or less (with 0 indicating that the patient is fully active and able to carry on all predisease activities without restriction; 1 indicating that the patient is restricted in physically strenuous activity but is ambulatory and able to carry out work of a light or sedentary nature, such as light housework or office work; and 2 indicating that the patient is ambulatory and up and about more than 50% of waking hours and is capable of all self-care but unable to carry out any work activities)22; adequate bone marrow, renal, and hepatic function; and adequately controlled lipid and glucose concentrations. Patients were ineligible if they had undergone hepatic-artery embolization within 6 months before enrollment (within 1 month if there were other sites of measurable disease) or cryoablation or radiofrequency ablation of hepatic metastasis within 2 months before enrollment, had any severe or uncontrolled medical conditions, had received prior therapy with an mTOR inhibitor, or were receiving long-term treatment with glucocorticoids or other immunosuppressive agents.
Study Oversight
The protocol was approved by the institutional review board or ethics committee at each participating center, and the study was conducted in accordance with Good Clinical Practice principles and applicable local regulations. All patients provided written informed consent.
The study was designed by the academic investigators and by representatives of the sponsor, Novartis Oncology. The data were collected with the use of the sponsor's data management systems and were analyzed by the sponsor's statistical team. All the authors contributed to the interpretation of data and the subsequent writing, reviewing, and amending of the manuscript; the first draft of the manuscript was prepared by the first author and by a medical writer employed by Novartis Oncology. The protocol, including the statistical analysis plan, is available at NEJM.org. All the authors vouch for the accuracy and completeness of the reported data and attest that the study conformed to the protocol and statistical analysis plan.
Study Design and Treatment
In this international, multicenter, double-blind, phase 3 study, patients were randomly assigned to treatment with oral everolimus, at a dose of 10 mg once daily, or matching placebo, both in conjunction with best supportive care. Patients were stratified according to status with respect to prior chemotherapy (receipt vs. no receipt) and according to WHO performance status (0 vs. 1 or 2) at baseline.
Treatment continued until progression of the disease, development of an unacceptable toxic effect, drug interruption for 3 weeks or longer, or withdrawal of consent. The study-group assignments were concealed from the investigators, but disclosure was permitted if an investigator determined that the criteria for disease progression according to RECIST had been met and if there was an intention to switch the patient to open-label therapy. Patients who had been assigned to placebo initially could then switch to open-label everolimus. This element of the study design was incorporated to address both ethical and recruitment considerations, given that the trial involved patients with a rare disease. We recognized the potential influence of this aspect of the study design on the analysis of the end point of overall survival.
Doses were delayed or reduced if patients had clinically significant adverse events that were considered to be related to the study treatment, according to an algorithm described in the protocol. In such cases, two reductions in the dose of the study drug were permitted: an initial reduction to 5 mg daily and a subsequent reduction to 5 mg every other day.
Efficacy and Safety Assessments
The primary end point was progression-free survival, documented by the local investigator according to RECIST and defined as the time from randomization to the first documentation of disease progression or death from any cause. If the disease had not progressed and the patient had not died as of the cutoff date for the analysis, data for progression-free survival were censored at the time of the last adequate tumor assessment — which was defined as the last assessment of overall lesion response that showed complete response, partial response, or stable disease — before the cutoff date or the date of initiation of other anticancer therapy.23 In the primary analysis, data for progression-free survival were censored at the time of the last adequate tumor assessment if an event occurred after two or more missing tumor assessments. Data for patients without any valid post-baseline tumor assessment were censored on day 1 (the date of randomization). Secondary end points included the confirmed objective response rate (according to RECIST, version 1.0), the duration of response, overall survival, and safety.
All randomly assigned patients were assessed for efficacy (intention-to-treat analysis). Tumor measurements (assessed by triphasic computed tomography or magnetic resonance imaging) were performed at baseline and were repeated every 12 weeks. Scans were reviewed at the local site and centrally. In cases of a discrepancy between the local investigator's assessment and the radiologic assessment at the central location with respect to the determination of progression-free survival, adjudication was performed by an independent central adjudication committee comprising a board-certified radiologist and an oncologist, both of whom had extensive experience with neuroendocrine tumors. The central adjudication committee, whose members were unaware of the patients' study-group assignments and of the source of the data (local or central), selected the assessment that in their expert opinion reflected the more accurate evaluation.
All patients who received at least one dose of the study drug and had at least one follow-up assessment were evaluated for safety. Safety assessments consisted of the monitoring and recording of all adverse events, regular monitoring of hematologic and clinical biochemical levels (laboratory evaluations) and vital signs, and physical examinations every 4 weeks. Adverse events were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 3.0 (http://ctep.info.nih.gov/protocolDevelopment/electronic_applications/docs/ctcaev3.pdf).
Statistical Analysis
The estimation of the sample size was based on the ability to detect a clinically meaningful improvement in the primary end point, which was defined as a 33% reduction in the risk of disease progression or death (a hazard ratio for progression or death of 0.67), corresponding to a 50% prolongation in median progression-free survival, from 6 months with placebo to 9 months with everolimus. We estimated that with a total of 282 progression-free survival events (i.e., disease progression or death), the study would have 92.6% power to detect a clinically meaningful improvement, with the use of an unstratified log-rank test, at a one-sided significance level of 2.5%. Taking into account the estimated rate of patient accrual and a 10% loss of the study population to follow-up, we estimated that we would have to enroll 392 patients to observe the required number of events.
Progression-free and overall survival were analyzed with the use of Kaplan–Meier methods; study groups were compared with the use of a log-rank test, stratified according to prior receipt or no prior receipt of chemotherapy and WHO performance status, and the hazard ratio was estimated with the use of a stratified Cox proportional-hazards model.
Results
Patients and Treatment
Between July 2007 and May 2009, a total of 410 patients from 82 centers in 18 countries worldwide who had advanced pancreatic neuroendocrine tumors were randomly assigned to everolimus (207 patients) or placebo (203 patients) (see the figure in the Supplementary Appendix). The baseline demographic and clinical characteristics of the patients were well balanced between the two groups (Table 1). More than 80% of the patients had well-differentiated disease, more than 90% had metastases in the liver, and approximately 60% had received a diagnosis of pancreatic neuroendocrine tumor more than 2 years before entering the study. A total of 24% of the patients had gastrinoma, glucagonoma, VIPoma, insulinoma, or somatostatinoma. The two groups were similar with respect to prior receipt of radiotherapy (23% of patients in the everolimus group and 20% in the placebo group), chemotherapy (50% in both groups), and somatostatin analogue therapy (49% in the everolimus group and 50% in the placebo group). Best supportive care included the use of somatostatin analogue therapy in approximately 40% of the patients.
Table 1. Demographic and Baseline Clinical Characteristics of the Patients.
| Characteristic | Everolimus (N = 207) |
Placebo (N = 203) |
|---|---|---|
| Age — yr | ||
| Median | 58 | 57 |
| Range | 23–87 | 20–82 |
| Sex — no. (%) | ||
| Male | 110 (53) | 117 (58) |
| Female | 97 (47) | 86 (42) |
| WHO performance status — no. (%) | ||
| 0 | 139 (67) | 133 (66) |
| 1 | 62 (30) | 64 (32) |
| 2 | 6 (3) | 6 (3) |
| Histologic status of tumor — no. (%) | ||
| Well differentiated | 170 (82) | 171 (84) |
| Moderately differentiated | 35 (17) | 30 (15) |
| Unknown | 2 (1) | 2 (1) |
| Time from initial diagnosis — no. (%) | ||
| ≤6 mo | 24 (12) | 33 (16) |
| >6 mo to ≤2 yr | 65 (31) | 43 (21) |
| >2 yr to ≤5 yr | 54 (26) | 81 (40) |
| >5 yr | 64 (31) | 46 (23) |
| Time from disease progression to randomization — no. (%) | ||
| ≤1 mo | 73 (35) | 61 (30) |
| >1 mo to ≤2 mo | 43 (21) | 53 (26) |
| >2 mo to ≤3 mo | 30 (14) | 29 (14) |
| >3 mo to ≤12 mo | 58 (28) | 54 (27) |
| >12 mo | 3 (1) | 1 (<1) |
| No. of disease sites — no. of patients (%) | ||
| 1 | 51 (25) | 62 (31) |
| 2 | 85 (41) | 64 (32) |
| ≥3 | 70 (34) | 77 (38) |
| Organ involved — no. (%) | ||
| Liver | 190 (92) | 187 (92) |
| Pancreas | 92 (44) | 84 (41) |
| Lymph nodes | 68 (33) | 73 (36) |
| Lung | 28 (14) | 30 (15) |
| Bone | 13 (6) | 29 (14) |
With a median follow-up period of 17 months, the median duration of treatment with everolimus was 8.79 months (range, 0.25 to 27.47), as compared with 3.74 months (range, 0.01 to 37.79) with placebo. A total of 31% of the patients in the everolimus group, as compared with 11% in the placebo group, were administered treatment for a minimum of 12 months. The mean relative dose intensity (the ratio of administered doses to planned doses) was 0.86 in the everolimus group and 0.97 in the placebo group. Dose adjustments (reductions or temporary interruptions) were required by 59% of the patients receiving everolimus and 28% of the patients receiving placebo.
At the time the analysis was performed for this article, treatment was ongoing for 32% of the patients in the everolimus group and 13% of the patients in the placebo group; the primary reasons for discontinuation of treatment included disease progression (in 44% of patients in the everolimus group vs. 80% in the placebo group), adverse events (17% vs. 3%), withdrawal of consent (2% in both groups), and death (2% vs. 1%).
Efficacy
The median progression-free survival (the primary end point), as assessed by the local investigators, was 11.0 months (95% confidence interval [CI], 8.4 to 13.9) in the everolimus group, as compared with 4.6 months (95% CI, 3.1 to 5.4) in the placebo group, representing a 65% reduction in the estimated risk of progression (hazard ratio for disease progression or death with everolimus, 0.35; 95% CI, 0.27 to 0.45; P<0.001) (Table 2 and Fig. 1A). The estimated proportion of patients who were alive and progression-free at 18 months was 34% (95% CI, 26 to 43) with everolimus as compared with 9% (95% CI, 4 to 16) with placebo, indicating that a sizable proportion of patients derived a prolonged benefit with everolimus.
Table 2. Progression-free Survival.
| Variable | Everolimus (N = 207) |
Placebo (N = 203) |
Difference | Hazard Ratio for Disease Progression or Death with Everolimus (95% CI) |
P Value |
|---|---|---|---|---|---|
| Assessment by local investigator | |||||
| Progression-free survival events — no. (%)* | 109 (53) | 165 (81) | |||
| Censored data — no. (%) | 98 (47) | 38 (19) | |||
| Median progression-free survival — mo | 11.0 | 4.6 | 6.4 | 0.35 (0.27–0.45) | <0.001 |
| Review by central adjudication committee | |||||
| Progression-free survival events — no. (%)* | 95 (46) | 142 (70) | |||
| Censored data — no. (%) | 112 (54) | 61 (30) | |||
| Median progression-free survival — mo | 11.4 | 5.4 | 6.0 | 0.34 (0.26–0.44) | <0.001 |
Progression-free survival events include disease progression and death.
Figure 1. Progression-free and Overall Survival.
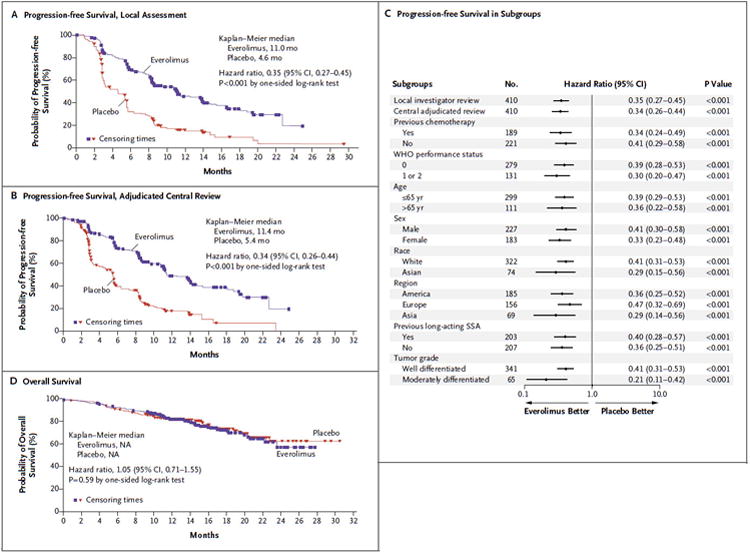
Kaplan–Meier curves are shown for progression-free survival as assessed by local investigators (Panel A) and by adjudicated central review (Panel B). A forest plot (Panel C) shows the effect of study treatment on progression-free survival in patient subgroups. Kaplan–Meier curves are also shown for overall survival (Panel D). NA denotes not available, and SSA somatostatin analogue.
The findings of the independent adjudicated central assessment of median progression-free survival were consistent with those of the assessment by local investigators. The median progression-free survival according to the central assessment was 11.4 months (95% CI, 10.8 to 14.8) with everolimus, as compared with 5.4 months (95% CI, 4.3 to 5.6) with placebo (hazard ratio for disease progression or death with everolimus, 0.34; 95% CI, 0.26 to 0.44; P<0.001) (Table 2 and Fig. 1B).
Prespecified subgroup analyses indicated that the benefit was maintained across subgroups. A benefit with everolimus was evident irrespective of status with respect to prior chemotherapy (receipt or no receipt), WHO performance status, age, sex, race, geographic region, status with respect to prior somatostatin analogue therapy (receipt or no receipt), and tumor grade (Fig. 1C).
Everolimus was associated with a superior response profile, as assessed according to RECIST (P<0.001 with the use of a two-sided Mann–Whitney U test). Confirmed objective tumor responses as assessed by local investigators (all partial responses) were observed in 10 patients receiving everolimus (5%) as compared with 4 patients receiving placebo (2%). Thus, the benefit from everolimus with respect to progression-free survival was seen primarily in the stabilization of disease or minor tumor shrinkage and in the lower incidence of progressive disease. Stable disease was evident in the case of 73% of the patients in the everolimus group as compared with 51% in the placebo group. Progressive disease as the best outcome occurred in 14% of the patients receiving everolimus and 42% of the patients receiving placebo. A total of 64% of the patients receiving everolimus, as compared with 21% receiving placebo, had some degree of tumor shrinkage (Fig. 2).
Figure 2. Percentage Change from Baseline in Size of Target Lesion.
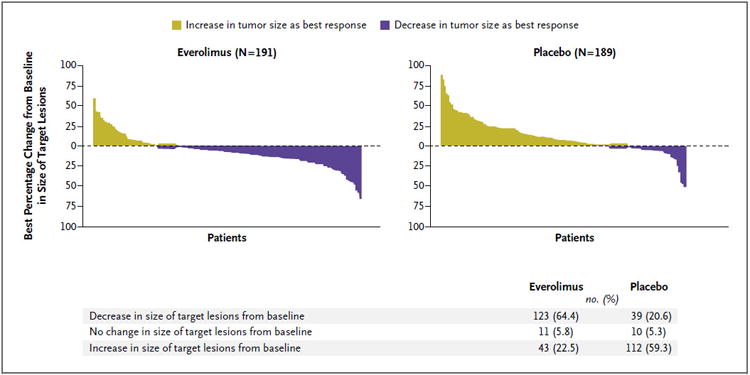
The plot shows the best percentage change from baseline in the size of the target lesion (i.e., the best response in each patient) in the everolimus group (left) and the placebo group (right). Data on 30 patients with lesions that could be evaluated in the everolimus group and 42 in the placebo group were not included in the analysis for the following reasons: 14 in the everolimus group (7.3%) and 28 in the placebo group (14.8%) showed a change in the available target lesion that contradicted the overall response of progressive disease; 1 patient in the everolimus group (0.5%) showed a change in the available target lesion, but the overall response was unknown; and the change in the target lesion could not be assessed in 15 patients in the everolimus group (7.9%) and 14 in the placebo group (7.4%).
Of the 203 patients initially assigned to receive placebo, 148 (73%) crossed over to open-label everolimus, thus confounding the detection of a treatment-related survival benefit. Median overall survival was not reached at the time of this analysis, and no significant difference between the groups was observed (hazard ratio for death with everolimus, 1.05; 95% CI, 0.71 to 1.55; P = 0.59) (Fig. 1D). The final analysis of overall survival will be performed once approximately 250 deaths have occurred.
Safety
Our findings with respect to safety were consistent with the known safety profile of everolimus, and most adverse events were grade 1 or 2. The most common drug-related adverse events occurring with a frequency of at least 10% are listed in Table 3. A total of 12 patients in the everolimus group (6%) and 4 in the placebo group (2%) died while receiving the study drug. Of these 16 deaths, 8 (5 in the everolimus group and 3 in the placebo group) were attributed to the underlying cancer or disease progression. The remaining 8 cases (7 in the everolimus group and 1 in the placebo group) were attributed to adverse events; of these, 1 in the everolimus group was related to the study drug.
Table 3. Drug-Related Adverse Events Occurring in at Least 10% of Patients.
| Adverse Event | Everolimus (N = 204) | Placebo (N = 203) | ||
|---|---|---|---|---|
| All Grades | Grade 3 or 4 | All Grades | Grade 3 or 4 | |
| no. of patients (%) | ||||
| Stomatitis* | 131 (64) | 14 (7) | 34 (17) | 0 |
| Rash | 99 (49) | 1 (<1) | 21 (10) | 0 |
| Diarrhea | 69 (34) | 7 (3) | 20 (10) | 0 |
| Fatigue | 64 (31) | 5 (2) | 29 (14) | 1 (<1) |
| Infections† | 46 (23) | 5 (2) | 12 (6) | 1 (<1) |
| Nausea | 41 (20) | 5 (2) | 37 (18) | 0 |
| Peripheral edema | 41 (20) | 1 (<1) | 7 (3) | 0 |
| Decreased appetite | 40 (20) | 0 | 14 (7) | 2 (1) |
| Headache | 39 (19) | 0 | 13 (6) | 0 |
| Dysgeusia | 35 (17) | 0 | 8 (4) | 0 |
| Anemia | 35 (17) | 12 (6) | 6 (3) | 0 |
| Epistaxis | 35 (17) | 0 | 0 | 0 |
| Pneumonitis‡ | 35 (17) | 5 (2) | 0 | 0 |
| Weight loss | 32 (16) | 0 | 9 (4) | 0 |
| Vomiting | 31 (15) | 0 | 13 (6) | 0 |
| Pruritus | 30 (15) | 0 | 18 (9) | 0 |
| Hyperglycemia | 27 (13) | 11 (5) | 9 (4) | 4 (2) |
| Thrombocytopenia | 27 (13) | 8 (4) | 1 (<1) | 0 |
| Asthenia | 26 (13) | 2 (1) | 17 (8) | 2 (1) |
| Nail disorder | 24 (12) | 1 (<1) | 2 (1) | 0 |
| Cough | 22 (11) | 0 | 4 (2) | 0 |
| Pyrexia | 22 (11) | 0 | 0 | 0 |
| Dry skin | 21 (10) | 0 | 9 (4) | 0 |
Included in this category are stomatitis, aphthous stomatitis, mouth ulceration, and tongue ulceration.
All types of infections are included.
Included in this category are pneumonitis, interstitial lung disease, lung infiltration, and pulmonary fibrosis.
The most common adverse events were stomatitis (in 64% of the patients in the everolimus group vs. 17% in the placebo group), rash (49% vs. 10%), diarrhea (34% vs. 10%), fatigue (31% vs. 14%), and infections (23% vs. 6%). Infections, as well as pneumonitis (which occurred in 12% of the patients in the everolimus group vs. 0% in the placebo group) and interstitial lung disease (2% vs. 0%), represented some of the most important clinical concerns and were primarily grade 1 or 2. The most common grade 3 or 4 drug-related adverse events were anemia, hyperglycemia, stomatitis, thrombocytopenia, diarrhea, hypophosphatemia, and neutropenia. Antibiotics were routinely prescribed for patients with infections. Glucocorticoids were administered to six of the seven patients with grade 3 or 4 noninfectious pneumonitis or interstitial lung disease; however, only 5 (2%) of these events were considered to be drug-related (Table 3). Atypical infections such as pulmonary tuberculosis, bronchopulmonary aspergillosis, and reactivation of hepatitis B (each of which occurred in one patient) were also observed in association with everolimus therapy.
The death from acute respiratory distress syndrome of one patient with insulinoma in the everolimus group (who was receiving glucocorticoid therapy) was considered to be treatment-related. Adverse events related to the study drug led to discontinuation of treatment in the case of 13% of the patients receiving everolimus (with pneumonitis, fatigue, and interstitial lung disease cited as the most common reasons) and 2% of the patients in the placebo group (as a result of cardiac failure, diarrhea, confusion and depressed level of consciousness, and elevated alanine aminotransferase concentrations). The most common drug-related adverse events necessitating dose adjustment were stomatitis (in 10% of the patients in the everolimus group vs. <1% in the placebo group), pneumonitis (7% vs. 0%), thrombocytopenia (7% vs. 0%), diarrhea (4% vs. 0%), and anemia (3% vs. 0%).
Discussion
In this trial, we compared everolimus with placebo in patients with advanced pancreatic neuroendocrine tumors in whom the disease had progressed within the previous 12 months. The majority of patients had received prior treatment with chemotherapy, radiotherapy, somatostatin analogue therapy, or some combination of those therapies. Everolimus, as compared with placebo, was associated with a 6.4-month prolongation of the median progression-free survival (an increase by a factor of 2.4). The patients in our study, who otherwise had a poor prognosis, had a 65% reduction in the relative risk of progression with everolimus therapy as compared with placebo (P<0.001). This study confirmed the prolonged progression-free survival that had been observed with everolimus in earlier phase 2 studies.3,16
Although the molecular pathogenesis of sporadic pancreatic neuroendocrine tumors is unknown, several genetic cancer syndromes involving the mTOR pathway, including tuberous sclerosis, neurofibromatosis, and von Hippel–Lindau disease, are linked to the development of pancreatic neuroendocrine tumors.24 In sporadic pancreatic neuroendocrine tumors, down-regulation of tuberin (TSC2) and phosphatase and tensin homologue (PTEN) leads to dysregulation of the mTOR pathway. Low TSC2 and PTEN are linked to progression of the cancer, an increased rate of proliferation (as assessed by Ki 67 labeling), and shortened progression-free and overall survival.20 In a study of paired biopsy specimens, treatment with everolimus reduced tumor proliferation in neuroendocrine tumors, as evidenced by a decreasing percentage of cells with Ki 67 labeling.16 The magnitude of the clinical benefit observed in our study confirms the importance of the mTOR pathway in pancreatic neuroendocrine tumors.
Sunitinib, an oral inhibitor of a number of tyrosine kinases (but not an inhibitor of mTOR), also shows activity against advanced pancreatic neuroendocrine tumors.15 It is not yet clear whether sunitinib and everolimus can be combined and, if so, whether antitumor activity would be further increased with combined treatment.
We have previously shown that everolimus can be safely administered to patients with neuroendocrine tumors either with or without concurrent octreotide long-acting release (LAR) therapy.3 The safety profile of everolimus in the current study was consistent with that in previous phase 2 studies. Despite a significantly longer duration of exposure in the population of patients with pancreatic neuroendocrine tumors, the rate of adverse events was similar to that in phase 3 trials involving patients with renal-cell carcinoma.25 The most common drug-related adverse event in our trial was stomatitis or aphthous ulceration, characterized by sporadic occurrences of discrete white ulcerations that frequently appeared and resolved during treatment. Everolimus therapy can also be associated with mild lymphopenia and neutropenia. Although in our trial, infections were more common among patients receiving everolimus than among those receiving placebo, grade 3 or 4 drug-related infections occurred in only 2% of the patients in the everolimus group. The most commonly reported infections were mild upper respiratory infections. Adverse events were generally manageable, as evidenced by the low rate of discontinuation of treatment. Noninfectious pneumonitis and interstitial lung disease, potentially serious adverse events associated with sirolimus (previously called rapamycin) derivatives, were also observed, but these events can be effectively managed according to existing treatment guidelines.
In summary, our study shows that everolimus, as compared with placebo, improves progression-free survival in patients with advanced pancreatic neuroendocrine tumors. The adverse events seen with everolimus were mainly grade 1 and 2 events, thus allowing for long-term daily administration.
Supplementary Material
Acknowledgments
Supported by Novartis Oncology.
We thank all the investigators and their patients for their participation in the study, Peter Berry (Novartis Oncology) for assistance in writing the first draft of the manuscript, and Kathy Covino (ApotheCom) for assistance with the preparation of the manuscript.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Yao JC, Eisner MP, Leary C, et al. Population-based study of islet cell carcinoma. Ann Surg Oncol. 2007;14:3492–500. doi: 10.1245/s10434-007-9566-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28:69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Broder LE, Carter SK. Pancreatic islet cell carcinoma. II. Results of therapy with streptozotocin in 52 patients. Ann Intern Med. 1973;79:108–18. doi: 10.7326/0003-4819-79-1-108. [DOI] [PubMed] [Google Scholar]
- 5.Chernicoff D, Bukowski RM, Groppe CW, Jr, Hewlett JS. Combination chemotherapy for islet cell carcinoma and metastatic carcinoid tumors with 5-fluorouracil and streptozotocin. Cancer Treat Rep. 1979;63:795–6. [PubMed] [Google Scholar]
- 6.Moertel CG, Hanley JA, Johnson LA. Streptozocin alone compared with streptozocin plus fluorouracil in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1980;303:1189–94. doi: 10.1056/NEJM198011203032101. [DOI] [PubMed] [Google Scholar]
- 7.Moertel CG, Lefkopoulo M, Lipsitz S, Hahn RG, Klaassen D. Streptozocin–doxorubicin, streptozocin–fluorouracil, or chlorozotocin in the treatment of advanced islet-cell carcinoma. N Engl J Med. 1992;326:519–23. doi: 10.1056/NEJM199202203260804. [DOI] [PubMed] [Google Scholar]
- 8.Cheng PNM, Saltz LB. Failure to confirm major objective antitumor activity for streptozocin and doxorubicin in the treatment of patients with advanced islet cell carcinoma. Cancer. 1999;86:944–8. [PubMed] [Google Scholar]
- 9.Bajetta E, Procopio G, Ferrari L, et al. Update on the treatment of neuroendocrine tumors. Expert Rev Anticancer Ther. 2003;3:631–42. doi: 10.1586/14737140.3.5.631. [DOI] [PubMed] [Google Scholar]
- 10.Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762–71. doi: 10.1200/JCO.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 11.McCollum AD, Kulke MH, Ryan DP, et al. Lack of efficacy of streptozocin and doxorubicin in patients with advanced pancreatic endocrine tumors. Am J Clin Oncol. 2004;27:485–8. doi: 10.1097/01.coc.0000135343.06038.eb. [DOI] [PubMed] [Google Scholar]
- 12.Ramanathan RK, Cnaan A, Hahn RG, Carbone PP, Haller DG. Phase 2 trial of dacarbazine (DTIC) in advanced pancreatic islet cell carcinoma: study of the Eastern Cooperative Oncology Group-E6282. Ann Oncol. 2001;12:1139–43. doi: 10.1023/a:1011632713360. [DOI] [PubMed] [Google Scholar]
- 13.Jensen RT, Berna MJ, Bingham DB, Norton JA. Inherited pancreatic endocrine tumor syndromes: advances in molecular pathogenesis, diagnosis, management, and controversies. Cancer. 2008;113(Suppl):1807–43. doi: 10.1002/cncr.23648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Delaunoit T, Ducreux M, Boige V, et al. The doxorubicin-streptozotocin combination for the treatment of advanced well-differentiated pancreatic endocrine carcinoma: a judicious option? Eur J Cancer. 2004;40:515–20. doi: 10.1016/j.ejca.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 15.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–13. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 16.Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311–8. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Donnell A, Faivre S, Burris HA, III, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–95. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 18.von Wichert G, Jehle PM, Hoeflich A, et al. Insulin-like growth factor-I is an autocrine regulator of chromogranin A secretion and growth in human neuroendocrine tumor cells. Cancer Res. 2000;60:4573–81. [PubMed] [Google Scholar]
- 19.Moreno A, Akcakanat A, Munsell MF, Soni A, Yao JC, Meric-Bernstam F. Antitumor activity of rapamycin and octreotide as single agents or in combination in neuroendocrine tumors. Endocr Relat Cancer. 2008;15:257–66. doi: 10.1677/ERC-07-0202. [DOI] [PubMed] [Google Scholar]
- 20.Missiaglia E, Dalai I, Barbi S, et al. Pancreatic endocrine tumors: expression profiling evidences a role for AKT-mTOR pathway. J Clin Oncol. 2010;28:245–55. doi: 10.1200/JCO.2008.21.5988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 22.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5:649–55. [PubMed] [Google Scholar]
- 23.Bushnell W. An overview of independent review of PFS and proposal for an audit methodology; Presented at the Conference on Clinical Cancer Research; Washington, DC. September 14, 2009; http://www.brookings.edu/∼/media/Files/events/2009/0914_clinical_cancer_research/Panel2ApresFINAL.pdf. [Google Scholar]
- 24.Yao JC, Rindi G, Evans DB. Pancreatic endocrine tumors. In: DeVita VT, Lawrence TS, Rosenberg SA, editors. Cancer: principles and practice of oncology. 8th. Philadelphia: Wolters Kluwer/Lippin-cott Williams & Wilkins; 2008. pp. 1702–21. [Google Scholar]
- 25.Motzer RJ, Escudier B, Oudard S, et al. Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet. 2008;372:449–56. doi: 10.1016/S0140-6736(08)61039-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


