Abstract
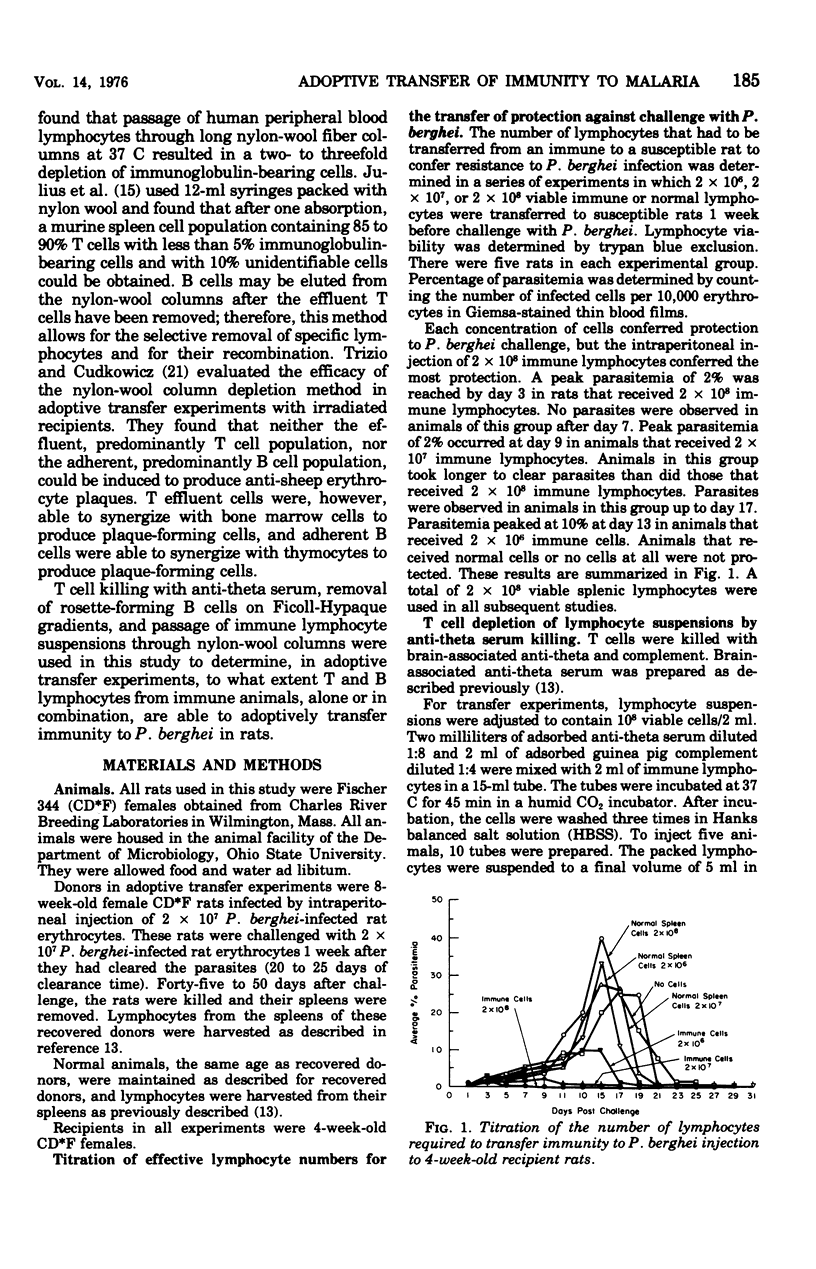
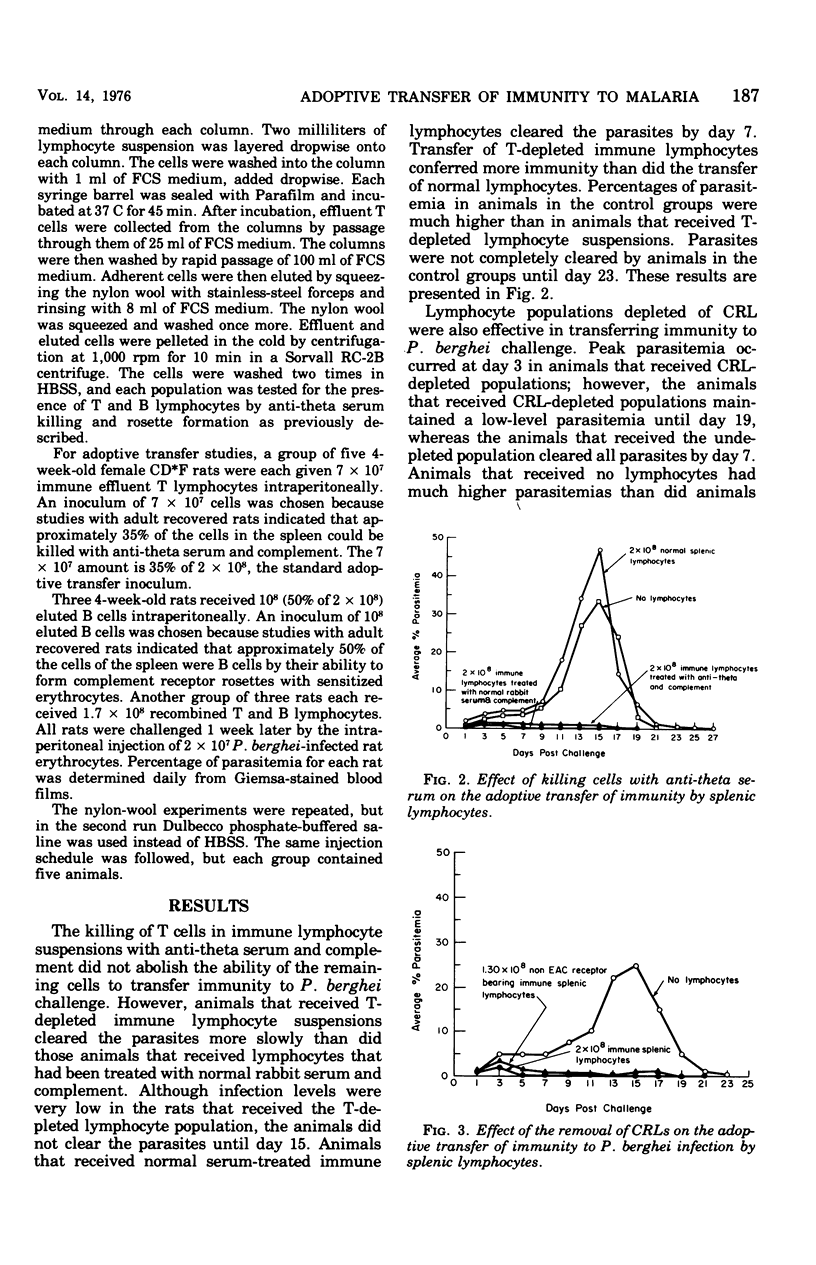
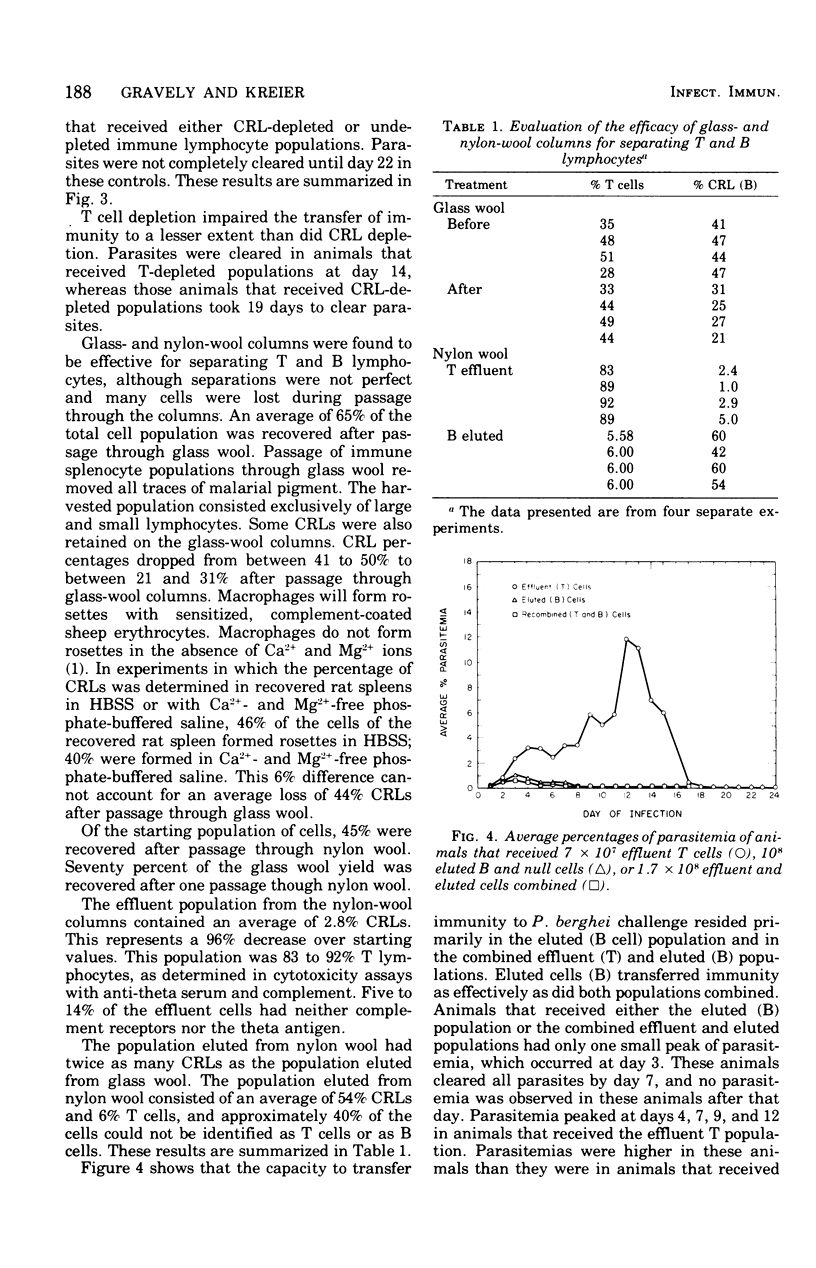
Immunity to malarial infection may be transferred with immune lymphocytes. This study was designed to determine which lymphocyte type is responsible for the adoptive transfer of immunity to malarial infection. In one set of experiments, the ability of immune T and B lymphocytes, separated by passage through nylon-wool columns, to transfer immunity to infection was determined. In another experiment, the effect of killing T lymphocytes with anti-theta serum on the transfer of immunity was determined. The effect on the ability of immune lymphocyte suspensions to transfer immunity after B lymphocytes were removed from such suspensions by centrifugation on Ficoll-Hypaque gradients, after they had formed rosettes with sensitized, complement-coated sheep erythrocytes, was also determined. The ability of lymphocyte suspensions to adoptively transfer resistance to malarial infection was greatly impaired by the removal from the suspensions of differentiated B-type lymphocytes. Our results indicate that it is the differentiated B cell, most probably an antibody-producing cell, which lacks both theta antigen and the complement receptor that is responsible for conferring immunity to malaria.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRUCE-CHWATT L. J., GIBSON F. D. Transplacental passage of Plasmodium berghei and passive transfer of immunity in rats and mice. Trans R Soc Trop Med Hyg. 1956 Jan;50(1):47–53. doi: 10.1016/0035-9203(56)90007-4. [DOI] [PubMed] [Google Scholar]
- Bianco C., Patrick R., Nussenzweig V. A population of lymphocytes bearing a membrane receptor for antigen-antibody-complement complexes. I. Separation and characterization. J Exp Med. 1970 Oct 1;132(4):702–720. doi: 10.1084/jem.132.4.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs N. T., Wellde B. T., Sadun E. H. Effects of rat antiserum on the course of Plasmodium berghei infection in mice. Mil Med. 1966 Sep;131(9 Suppl):1243–1249. [PubMed] [Google Scholar]
- Brown I. N., Allison A. C., Taylor R. B. Plasmodium berghei infections in thymectomized rats. Nature. 1968 Jul 20;219(5151):292–293. doi: 10.1038/219292a0. [DOI] [PubMed] [Google Scholar]
- COHEN S., McGREGOR I. A., CARRINGTON S. Gamma-globulin and acquired immunity to human malaria. Nature. 1961 Nov 25;192:733–737. doi: 10.1038/192733a0. [DOI] [PubMed] [Google Scholar]
- Cabrera E. J., Alger N. E. Transfer of adoptive immunity to Plasmodium berghei: a comparison of routes of injection. J Protozool. 1971 Nov;18(4):596–598. doi: 10.1111/j.1550-7408.1971.tb03381.x. [DOI] [PubMed] [Google Scholar]
- Cohen S., Butcher G. A. Properties of protective malarial antibody. Immunology. 1970 Aug;19(2):369–383. [PMC free article] [PubMed] [Google Scholar]
- Diggs C. L., Osler A. G. Humoral immunity in rodent malaria. II. Inhibition of parasitemia by serum antibody. J Immunol. 1969 Feb;102(2):298–305. [PubMed] [Google Scholar]
- Eisen S. A., Wedner H. J., Parker C. W. Isolation of pure human peripheral blood T-lymphocytes using nylon wool columns. Immunol Commun. 1972;1(6):571–577. doi: 10.3109/08820137209022965. [DOI] [PubMed] [Google Scholar]
- Epstein L. B., Cline M. J., Merigan T. C. The interaction of human macrophages and lymphocytes in the phytohemagglutinin-stimulated production of interferon. J Clin Invest. 1971 Apr;50(4):744–753. doi: 10.1172/JCI106545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gravely S. M., Hamburger J., Kreier J. P. T and B cell population changes in young and in adult rats infected with Plasmodium berghei. Infect Immun. 1976 Jul;14(1):178–183. doi: 10.1128/iai.14.1.178-183.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger J., Kreier J. P. Antibody-mediated elimination of malaria parasites (plasmodium berghei) in vivo. Infect Immun. 1975 Aug;12(2):339–345. doi: 10.1128/iai.12.2.339-345.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Julius M. H., Simpson E., Herzenberg L. A. A rapid method for the isolation of functional thymus-derived murine lymphocytes. Eur J Immunol. 1973 Oct;3(10):645–649. doi: 10.1002/eji.1830031011. [DOI] [PubMed] [Google Scholar]
- Katz D. H., Benacerraf B. The regulatory influence of activated T cells on B cell responses to antigen. Adv Immunol. 1972;15:1–94. doi: 10.1016/s0065-2776(08)60683-5. [DOI] [PubMed] [Google Scholar]
- Phillips R. S. Plasmodium berghei: passive transfer of immunity by antisera and cells. Exp Parasitol. 1970 Jun;27(3):479–495. doi: 10.1016/0014-4894(70)90052-4. [DOI] [PubMed] [Google Scholar]
- Spira D. T., Silverman P. H., Gaines C. Anti-thymocyte serum effects on Plasmodium berghei infection in rats. Immunology. 1970 Nov;19(5):759–766. [PMC free article] [PubMed] [Google Scholar]
- Stechschulte D. J. Cell-mediated immunity in rats infected with Plasmodium berghei. Mil Med. 1969 Sep;134(10):1147–1152. [PubMed] [Google Scholar]
- Stechschulte D. J. Plasmodium berghei infection in thymectomized rats. Proc Soc Exp Biol Med. 1969 Jul;131(3):748–752. doi: 10.3181/00379727-131-33968. [DOI] [PubMed] [Google Scholar]
- Trizio D., Cudkowicz G. Separation of T and B lymphocytes by nylon wool columns: evaluation of efficacy by functional assays in vivo. J Immunol. 1974 Oct;113(4):1093–1097. [PubMed] [Google Scholar]


