Abstract
Background. Immunization of healthy volunteers by bites from Plasmodium falciparum–infected mosquitoes during chloroquine chemoprophylaxis (hereafter, chemoprophylaxis and sporozoites [CPS] immunization) induces sterile protection against malaria. CPS-induced protection is mediated by immunity against pre-erythrocytic stages, presumably at least partially by cytotoxic cellular responses. We therefore aimed to investigate the association of CPS-induced cytotoxic T-cell markers with protection.
Methods. In a double-blind randomized controlled trial, we performed dose titration of CPS immunization followed by homologous challenge infection in 29 subjects. Immune responses were assessed by in vitro restimulation of peripheral blood mononuclear cells and flow cytometry.
Results. Dose-dependent complete protection was obtained in 4 of 5 volunteers after immunization with bites from 45 P. falciparum–infected mosquitoes, in 8 of 9 volunteers with bites from 30, and in 5 of 10 volunteers with bites from 15 (odds ratio [OR], 5.0; 95% confidence interval [CI], 1.5–17). Completely protected subjects had significantly higher proportions of CD4 T cells expressing the degranulation marker CD107a (OR, 8.4; 95% CI, 1.5–123; P = .011) and CD8 cells producing granzyme B (OR, 11; 95% CI, 1.9–212; P = .004) after P. falciparum restimulation.
Conclusions. These data underline the efficiency of CPS immunization to induce sterile protection and support a possible role for cytotoxic CD4 and CD8 T-cell responses in pre-erythrocytic immunity.
Clinical Trials Registration. NCT01218893.
Keywords: malaria, Plasmodium, immunization, protection, immunity, chloroquine, T cells, degranulation, granzyme B, cytotoxicity
Malaria remains a major public health problem, with an estimated incidence of 207 million clinical cases and approximately 627 000 deaths every year [1]. Plasmodium falciparum is the most severe and lethal of 5 species that can cause malaria in humans. Availability of an effective vaccine will be critical to fight this disease, but currently there is no licensed vaccine available, despite decades of research. Most efforts have focused on the development of subunit vaccines, unfortunately showing only limited protective efficacy [2, 3]. Immunization strategies based on whole parasites, however, have repeatedly induced high levels of protection in experimental settings [4–7]. Previously, we showed that immunization of healthy, malaria-naive subjects with live sporozoites delivered by 36–45 mosquito bites during chloroquine chemoprophylaxis (hereafter, chemoprophylaxis and sporozoites [CPS] immunization) induces robust, long-lasting sterile protection against P. falciparum malaria [8, 9]. CPS immunization is about 20 times more efficient than the only alternative approach for complete sterile protection against malaria in humans, immunization with radiation-attenuated P. falciparum sporozoites (RAS), which requires bites from >1000 infected and irradiated mosquitoes [4] or intravenous administration of 675 000 sporozoites [10].
CPS-induced protective immunity targets the earliest stages of the parasite life cycle (ie, sporozoites and/or liver stages), rather than the subsequently developing asexual blood stages [11]. The immune pathways responsible for this pre-erythrocytic protection, however, remain unknown. In murine malaria models, cytotoxic killing of Plasmodium-infected hepatocytes appears to play a role in protection, but the exact contribution and mechanism of cytotoxicity remain elusive [12, 13]. Also, in humans, a role for both cytotoxic CD4 T cells and CD8 T cells has been suggested, but evidence is scarce and largely circumstantial [14]. We conducted a double-blind, randomized, controlled CPS immunization dose titration and challenge study. Subjects, while receiving chloroquine prophylaxis, were immunized by bites from 45, 30, or 15 infected mosquitoes, followed by a challenge infection, resulting in dose-dependent protection. Next, we explored markers of cytotoxic T-cell responses induced by CPS immunization and identified 2 cytotoxic markers associated with protection.
MATERIALS AND METHODS
Human Ethics Statement
All subjects provided written informed consent before screening. The study was approved by the Central Committee for Research Involving Human Subjects of the Netherlands (NL33904.091.10) and complied with the Declaration of Helsinki and good clinical practice, including monitoring of data.
Clinical Trial Design and Procedures
A single-center, double-blind study was conducted at the Leiden University Medical Center from April 2011 until April 2012. Healthy subjects aged 18–35 years with no history of malaria were screened as described previously [11]. Thirty subjects were randomly divided into 4 groups, using a computer-generated random-number table. Subjects, investigators, and primary outcome assessors were blinded to the allocation. All subjects received 3 CPS immunizations at monthly intervals, as described previously [8, 11], but the number of NF54 P. falciparum–infected versus uninfected mosquitoes varied per group: 5 subjects were each exposed to bites from 15 infected mosquitoes 3 times (group 1), 10 subjects were each exposed to bites from 10 infected and 5 uninfected mosquitoes on three occasions (group 2), 10 subjects were each exposed to bites from 5 infected and 10 uninfected mosquitoes on three occasions (group 3), and 5 control subjects were exposed to bites from 15 uninfected mosquitoes on three occasions (group 4). Nineteen weeks after the last immunization (15 weeks after the last chloroquine dose), all subjects were challenged by exposure to bites from 5 mosquitoes infected with the homologous NF54 P. falciparum strain, according to previous protocols [8, 15]. The primary outcome was prepatent period, defined as the time between challenge and first P. falciparum–positive thick blood smear. Thick blood smears were prepared and read as described previously [11]. For more details about the immunization and challenge procedures and follow-up, see the Supplementary Materials.
Immunological Methods
Peripheral blood mononuclear cells (PBMCs) were collected at the following time points: before initiation of chloroquine prophylaxis (baseline [B]), 27 days after immunization 1 (I1; 1 day before the second immunization), 27 days after immunization 2 (I2; 1 day before the third immunization), 27 days after immunization 3 (I3), the day before the challenge infection (C − 1), and 20 weeks after the challenge infection (C + 140). For the assessment of P. falciparum–specific immune responses, PBMCs were restimulated in vitro with P. falciparum–infected red blood cells (RBCs) as described before [16]. Expression of the degranulation marker CD107a, the cytotoxic molecule granzyme B, and the cytokine interferon γ (IFN-γ) by CD4, CD8, and γδ T cells was assessed by flow cytometry. For a detailed description of these methods, see the Supplementary Materials.
Statistical Analysis
The dose-dependent induction of protection was tested by logistic regression, using SPSS 20. Comparison of CD107a expression and granzyme B and IFN-γ production by T-cell subsets between immunized unprotected and protected volunteers after CPS immunization was done per selected cellular response by means of Firth penalized logistic regression [17, 18], resulting in P values, odds ratios (ORs) related to a change of 1 interquartile range (IQR), and 95% profile likelihood confidence intervals (CIs) for the OR. Analyses were performed using R, version 3.0.1 [19], with the packages logistf, version 1.21 [20]; rms, version 4.1–3 [21]; and penalized, version 0.9–42 [22, 23]. The ability of (a combination of) markers to discriminate between protected and unprotected volunteers was assessed with the area under the receiver operator curve (ROC), based on leave-one-out cross-validation, using R and pROC, version 1.7.1 [24]. For further details about these methods, see the Supplementary Materials.
RESULTS
CPS Immunization
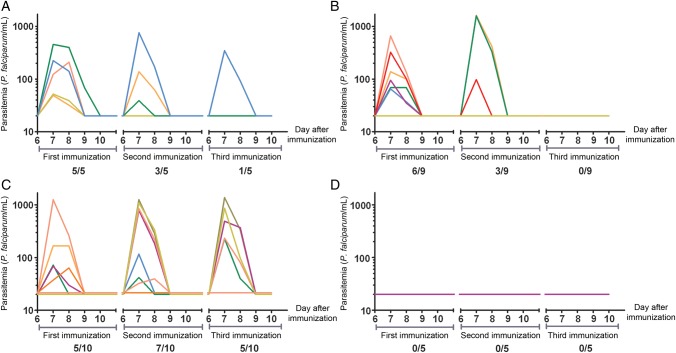
Thirty volunteers were included (median age, 21 years; range, 19–31 years), out of 63 subjects screened for eligibility (Supplementary Figure 1). Volunteers were randomly assigned to one of the 4 groups described in Materials and Methods. After each consecutive immunization, the number of subjects with parasitemia, as retrospectively detected by quantitative polymerase chain reaction (qPCR) analysis, steadily decreased in groups 1 and 2. In group 3, however, 5 volunteers still showed parasitemia after the second and third immunization (Figure 1). Remarkably, in 4 immunized subjects (3 in group 2 and 1 in group 3), parasitemia was never detectable by qPCR. One subject from group 2 withdrew consent after the first immunization for reasons unrelated to the trial and was excluded from the analysis.
Figure 1.
Parasitemia after the first, second and third chemoprophylaxis and sporozoites immunization. Parasitemia was determined once daily by quantitative polymerase chain reaction (qPCR) analysis from day 6 until day 10 after each immunization. Each line represents an individual subject. Panels show data for volunteers from group 1 (A; exposure to bites from 15 infected mosquitoes on three occasions), group 2 (B; exposure to bites from 10 infected and 5 uninfected mosquitoes on three occasions), group 3 (C; exposure to bites from 5 infected and 10 uninfected mosquitoes on three occasions), and group 4 (D; exposure to bites from 15 uninfected mosquitoes on three occasions; control). Values shown as 10 on the log scale were negative (ie, half the lower detection limit of the qPCR, 20 parasites/mL). Proportions along the x-axes denote subjects with a positive qPCR result/total number of subjects immunized.
Challenge Infection
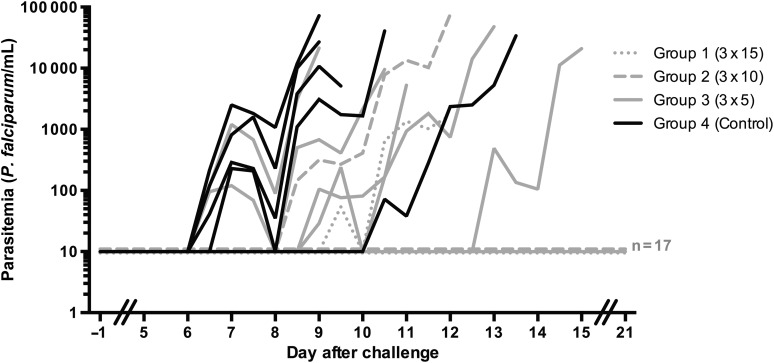
Nineteen weeks after the last immunization, volunteers were challenged by standard exposure to bites from 5 homologous strain NF54-infected mosquitoes [5]. Protection by CPS immunization was dose-dependently induced in 4 of 5 subjects in group 1, 8 of 9 subjects in group 2, and 5 of 10 subjects in group 3, while all control subjects became thick smear positive (OR, 5.0; 95% CI, 1.5–17; P = .01). The median prepatent period was 2.5 days longer in CPS-immunized unprotected subjects, compared with controls, both by thick smear and qPCR. Although these differences were not statistically significant (P = .22 for thick smear findings and P = .31 for qPCR findings), this delay suggests the presence of partial protection at least in some of the unprotected CPS-immunized subjects (Figure 2 and Table 1). In retrospect, all 6 volunteers with detectable parasitemia by qPCR after the third immunization were not completely protected from challenge infection, while 17 of 18 subjects with a negative qPCR result after the third immunization were fully protected.
Figure 2.
Parasitemia after challenge infection. Parasitemia was assessed retrospectively by real-time quantitative PCR (qPCR) from day 5 after challenge onward, until day 21, at 2 time points per day for thick-smear-positive volunteers and at 1 time point per day for protected volunteers. Each line represents an individual subject. Gray dotted lines show chemoprophylaxis and sporozoites–immunized volunteers from group 1 (exposed to bites from 15 infected mosquitoes on three occasions; n = 5), gray dashed lines subjects from group 2 (exposed to bites from 10 infected and 5 uninfected mosquitoes on three occasions; n = 9), gray solid lines represent subjects from group 3 (exposed to bites from 5 infected and 10 uninfected mosquitoes on three occasions; n = 10), and black lines represent malaria-naive control subjects (exposed to bites from 15 uninfected mosquitoes on three occasions; n = 5). Values shown as 10 on the log scale were negative. The 2 thick-smear-positive subjects from groups 1 and 2 became qPCR positive on days 9.5 and 8.5 respectively; both became thick smear positive on day 12.0.
Table 1.
Protection Against Challenge Infection After Chemoprophylaxis and Sporozoites Immunization
| Group | Protection |
Day of Positivity After Challenge, Median (Range)c |
||
|---|---|---|---|---|
| Proportiona | Percentage (95% CIb) | Thick Smear | qPCR | |
| Group 1 | 4/5 | 80 (36.0–98.0) | 12.0 | 9.5 |
| Group 2 | 8/9 | 89 (54.3 to >99.9) | 12.0 | 8.5 |
| Group 3 | 5/10 | 50 (23.7–76.3) | 11.0 (9.0–15.0) | 9.0 (6.5–13.0) |
| Group 4 | 0/5 | 0 (0.0–48.9) | 9.5 (9.0–13.5) | 6.5 (6.5–10.5) |
Subjects were exposed to bites from 15 infected mosquitoes on three occasions (group 1), to bites from 10 infected and 5 uninfected mosquitoes on three occasions (group 2), to bites from 5 infected and 10 uninfected mosquitoes on three occasions (group 3), or to bites from 15 uninfected mosquitoes on three occasions (group 4; control).
a Data are no. of protected subjects/total no. of subjects.
b 95% confidence intervals (CIs) were calculated by the modified Wald method.
c Data are for thick-smear-positive subjects.
Platelet levels decreased below the reference value (150 × 109 platelets/L) in 8 of 12 thick-smear-positive subjects (ie, both controls and CPS-unprotected) at any point after challenge (median for all thick-smear-positive subjects, 134 × 109 platelets/L; range, 79 × 109–213 × 109 platelets/L). D-dimer levels were elevated in all thick-smear-positive subjects after challenge (median peak concentration, 2431 ng/mL; range, 1014–5000 ng/mL). Parameters normalized in all subjects after treatment without complications. All thick-smear-positive subjects experienced solicited adverse events (AEs) during challenge infection consistent with uncomplicated malaria (median number/subject, 9.5 AEs [range, 4–14 AEs); median duration of each AE, 1.1 days [range, 0.0–12.3 days]). As expected, protected subjects presented with fewer AEs: 15 of 17 subjects experienced solicited AEs possibly or probably related to the challenge (median number/subject, 2 AEs [range, 0–15 AEs]; median duration, 0.7 days [range, 0.00–15.9 days]). One subject from group 2 was preliminarily treated with atovaquone/proguanil on day 10.5 after challenge because of unrelated exertional rhabdomyolysis after extensive sports activity (weightlifting) followed by sauna visits. No other severe AEs occurred. One volunteer from group 1 was treated for reasons unrelated to the trial at day 19. Both of these volunteers remained parasite negative by qPCR analysis after the third immunization and at any time point after challenge and were considered protected in further analysis.
Analysis of Cytotoxic T-Cell Markers After In Vitro P. falciparum Stimulation
Next, we tested a panel of representative cytotoxic T-cell markers, including surface expression of degranulation marker CD107a and granzyme B and IFN-γ production, in CD4, CD8, and γδ T cells after in vitro restimulation with P. falciparum–infected RBCs in all immunized subjects (Table 2). CPS immunization induced a significant increase in both the percentage and integrated geometric mean fluorescence intensity (iMFI) of CD107a+ CD4 and γδ T cells from the first immunization until challenge. Similarly, CD8 T cells expressed a significantly higher CD107a iMFI after the second immunization. The proportion of granzyme B–producing cells did not change after immunization, but the granzyme B iMFI was significantly increased in both CD8 and γδ T cells, returning to baseline on C − 1. Production of IFN-γ was induced in all T-cell subsets, but induction was most pronounced in CD4 and γδ T cells. There were only weak correlations between cellular responses on C − 1 and total blood-stage parasite exposure, as calculated by the number of parasites per milliliter after all 3 immunizations (data not shown; Spearman rho for all, <0.5). None of the responses in the control group changed significantly from baseline at any point of time (Table 2), suggesting that chloroquine alone did not affect P. falciparum–specific T-cell responses.
Table 2.
Cytotoxic T-Cell Markers, Relative to Immunization Time, for 24 Volunteers Who Received Chemoprophylaxis and Sporozoites (CPS) Immunizations and 5 Controls
| Study Group, Markera | CD4 T Cells |
CD8 T Cells |
γδ T Cells |
||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B | I1 | P Value | I2 | P Value | I3 | P Value | C−1 | P Value | B | I1 | P Value | I2 | P Value | I3 | P | C−1 | P Value | B | I1 | P Value | I2 | P Value | I3 | P Value | C−1 | P Value | |
| CPS | |||||||||||||||||||||||||||
| CD107a | |||||||||||||||||||||||||||
| Expression, %, mean | 0.26 | 0.53 | <.001 | 0.64 | <.001 | 0.59 | <.001 | 0.53 | <.001 | 0.08 | 0.09 | 0.15 | 0.14 | 0.19 | 26.6 | 36.5 | <.001 | 41.2 | <.001 | 40.3 | <.001 | 33.8 | <.01 | ||||
| iMFI | 15.7 | 38.5 | <.001 | 46.0 | <.001 | 41.0 | <.001 | 37.0 | <.001 | 7.6 | 14.5 | 19.2 | <.05 | 17.5 | <.05 | 20.9 | <.01 | 4472 | 6925 | <.001 | 7925 | <.001 | 7594 | <.001 | 6262 | <.01 | |
| Granzyme B | |||||||||||||||||||||||||||
| Production, %, mean | 0.25 | 0.49 | 0.41 | 0.38 | 0.43 | 0.50 | 0.89 | 0.92 | 1.03 | 0.94 | 6.06 | 7.35 | 8.27 | 8.96 | 8.79 | ||||||||||||
| iMFI | 1.40 | 9.80 | 11.3 | 7.50 | 3.24 | 5.11 | 71.3 | <.05 | 40.5 | 26.7 | 19.9 | 2.54 | 15.3 | <.001 | 15.0 | <.001 | 12.4 | <.001 | 8.18 | ||||||||
| IFN-γ | |||||||||||||||||||||||||||
| Production, %, mean | 0.08 | 0.43 | <.001 | 0.54 | <.001 | 0.42 | <.001 | 0.47 | <.001 | 0.04 | 0.11 | 0.13 | <.05 | 0.08 | 0.13 | <.01 | 6.01 | 13.6 | <.001 | 16.3 | <.001 | 14.9 | <.001 | 12.0 | <.01 | ||
| iMFI | 5.16 | 32.2 | <.001 | 37.7 | <.001 | 25.7 | <.05 | 37.2 | <.001 | 2.96 | 9.8 | 9.3 | 6.4 | 12.5 | <.01 | 407 | 1020 | <.01 | 1173 | <.001 | 1029 | <.01 | 982 | <.05 | |||
| Control | |||||||||||||||||||||||||||
| CD107a | |||||||||||||||||||||||||||
| Expression, %, mean | 0.25 | 0.27 | 0.16 | 0.20 | 0.42 | 0.02 | 0.06 | 0.02 | 0.00 | 0.05 | 26.3 | 22.6 | 22.6 | 21.1 | 29.2 | ||||||||||||
| iMFI | 15.6 | 17.2 | 10.6 | 13.4 | 27.3 | 3.38 | 7.04 | 3.28 | 1.56 | 7.92 | 4438 | 4439 | 4191 | 4512 | 5608 | ||||||||||||
| Granzyme B | |||||||||||||||||||||||||||
| Production, %, mean | 0.31 | 0.18 | 0.20 | 0.10 | 0.60 | 0.95 | 0.43 | 0.96 | 0.72 | 1.16 | 5.68 | 2.41 | 1.00 | 1.73 | 8.56 | ||||||||||||
| iMFI | 1.06 | 4.04 | 2.98 | −0.36 | 11.1 | −23.2 | −3.02 | 14.6 | −25.2 | 25.3 | 45.1 | −77.4 | −258 | −326 | 302 | ||||||||||||
| IFN-γ | |||||||||||||||||||||||||||
| Production, %, mean | 0.09 | 0.02 | 0.00 | 0.02 | 0.12 | 0.02 | 0.02 | 0.01 | 0.01 | 0.07 | 5.58 | 3.51 | 3.40 | 3.13 | 7.06 | ||||||||||||
| iMFI | 4.62 | 0.86 | 0.36 | 0.70 | 6.98 | 1.44 | 0.38 | 0.22 | 0.26 | 5.44 | 356 | 161 | 146 | 143 | 486 | ||||||||||||
Plasmodium falciparum–infected red blood cell (RBC)–specific responses were corrected for the value for the uninfected RBC background. P values were calculated using 1-way analysis of variance with the Dunnett post hoc test, with the B value serving as a control.
Abbreviations: B, baseline; C − 1, 1 day before challenge; I1, 27 days after immunization 1; I2, 27 days after immunization 2; I3, 27 days after immunization 3.
a The geometric mean fluorescence intensity (iMFI) is calculated as the percentage of positive cells multiplied by the MFI of this positive population.
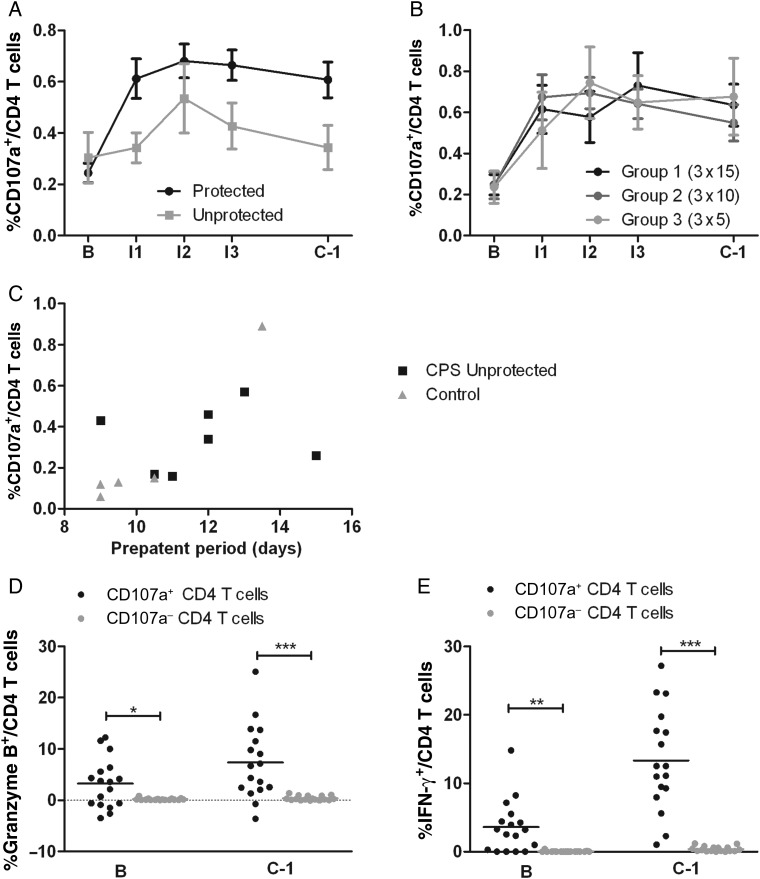
We next assessed the association of these markers with protection after challenge (Figure 3). Indeed, complete protection associated with the proportion of CD107a+ CD4 T cells (OR, 8.4; 95% CI, 1.5–123; P = .011; Figure 3A), the iMFI of CD107a on CD4 T cells (OR, 11; 95% CI, 1.6–188; P = .011; data not shown), and production of granzyme B by CD8 T cells (OR, 11; 95% CI, 1.9–212; P = .004; Figure 3E) at C − 1. A subgroup analysis of data from group 3 confirmed these findings: the only markers with higher levels in protected subjects were the proportion of both CD107a+ CD4 T cells (OR, 4.2; 95% CI, .9–140; P = .081) and granzyme B–producing CD8 T cells (OR = 27; 95% CI, 1.5–27 687; P = .019). While expression of CD107 on CD4 T cells and granzyme B in CD8 T cells predicted protection, with areas under the ROC of 0.73 (95% CI, .48–.98) and 0.81 (95% CI, .63–.99), respectively, combining both markers resulted in only a slight improvement of the area under the ROC (0.82; 95% CI, .61–1).
Figure 3.
Cytotoxic immune responses upon in vitro Plasmodium falciparum–infected red blood cell stimulation 1 day before challenge infection (C − 1). Each symbol represents a single protected (black symbols) or chemoprophylaxis and sporozoites–immunized unprotected (gray symbols) individual from group 1 (exposed to bites from 15 infected mosquitoes on three occasions; dots), group 2 (exposed to bites from 10 infected and 5 uninfected mosquitoes on three occasions; triangles), or group 3 (exposed to bites from 5 infected and 10 uninfected mosquitoes on three occasions; squares). Horizontal bars and whiskers represent means and standard errors of the mean (SEM). Panels show CD107a+ CD4 T cells (A), CD8 T cells (B), and γδ T cells (C); granzyme B expression by CD4 T cells (D), CD8 T cells (E), and γδ T cells (F); and interferon γ (IFN-γ) expression by CD4 T cells (G), CD8 T cells (H), and γδ T cells (I). Values are corrected for uninfected red blood cell (uRBC) background and for baseline response before immunization. Mean background responses (±SEM) to uRBC stimulation for CD4, CD8, and γδ T cells were 0.19 ± 0.01, 0.41 ± 0.02, and 0.61 ± 0.05, respectively, for CD107a; 1.65 ± 0.50, 15.34 ± 1.46, and 64.56 ± 1.74, respectively, for granzyme B; and 0.09 ± 0.00, 0.07 ± 0.00, and 0.14 ± 0.01, respectively, for IFN-γ (calculated for all volunteers at both baseline and C − 1). High uRBC granzyme B responses in CD8 and γδ T cells indicate that a significant percentage of these cells contain granzyme B even in a resting situation. uRBC responses did not change significantly from baseline for any of the readouts. The differences between responses of protected and unprotected volunteers that are not indicated with a P value are nonsignificant. The differences between protected and unprotected volunteers were evaluated using logistic regression.
P. falciparum–specific IFN-γ production by CD4, CD8, or γδ T cells could not distinguish protected volunteers (Figure 3G, 3H, and 3I). Also pluripotent (IFN-γ+IL-2+) effector memory T-cell (CD4+CD62L−CD45RO+) responses, previously shown to be significantly increased by CPS immunization [8], were again induced (P = .013) but did not differentiate between protected and unprotected volunteers (OR, 1.6; 95% CI, .5–4.9; P = .41; data not shown).
CD107a+ CD4 T cells presented as the clearest marker associated with protection, with values consistently higher in fully protected subjects from I1 onward (Figure 4A), and was independent of immunization dose (Figure 4B). A significant correlation was found between CD107a expression by CD4 T cells after 1 immunization and prepatent period after challenge infection in all thick-smear-positive subjects (Spearman rho, 0.69; P = .013; Figure 4C). The proportion CD107a+ CD4 T cells in the control subject who developed parasitemia significantly later than the other controls (ie, day 13.5 vs days 9–10.5) was, at baseline, on average 2.8-fold higher than in the other subjects. Possibly, the inherently higher response in this volunteer contributed to delayed patency after challenge.
Figure 4.
Cytotoxic profile of chemoprophylaxis and sporozoites (CPS)–induced CD4 T cells. A and B, Induction of Plasmodium falciparum–specific CD107a+ CD4 T cells was determined in protected and unprotected CPS-immunized subjects (A) and in protected subjects separated for each immunization dose (B) over the course of immunization. Horizontal bars and whiskers represent mean responses and standard errors of the mean. C, The relationship between P. falciparum–specific CD107a CD4 T cells on day 27 after immunization 1 (I1) and the prepatent period after challenge for all thick-smear-positive volunteers (CPS-immunized individuals and controls). D and E, Among protected CPS-immunized subjects, granzyme B (D) and interferon γ (IFN-γ) production (E) by CD107a+ (black dots) and CD107a− (gray dots) CD4 T cells were analyzed at baseline (B) and after CPS immunization (on the day before the challenge infection [C − 1]). Horizontal bars show the mean response. All data were corrected for uninfected red blood cell background for every volunteer at each time point. *P < .05, **P < .01, and ***P < .001. I2, 27 days after immunization 2; I3, 27 days after immunization 3.
CD107a+ CD4 T cells expressed proportionally more granzyme B (7.4% vs 0.39% on C − 1; P < .0008) in protected subjects, indicative of their cytotoxic phenotype, and proportionally more IFN-γ (13.3% vs 0.39% on C − 1; P < .0001) than CD107a− CD4 T cells (Figure 4D and 4E). CD8 T cells, traditionally considered the cytotoxic subclass of T cells, contained a larger proportion of CD107a+ cells at baseline than CD4 T cells when uninfected (0.39% vs 0.19%; P < .0001 [all volunteers]). However, the proportion of P. falciparum–specific degranulation of CD8 T cells was not notably increased by CPS immunization (P = .44), in contrast to CD4 T cells (P < .0001; Supplementary Figure 2A and 2B).
Both CD107a expression by CD4 T cells and granzyme B production by CD8 T cells remained significantly elevated up to 20 weeks after the challenge infection (C + 140; P < .05 and P < .01, respectively; Figure 5A and 5B), demonstrating longevity of the CPS-induced T-cell response.
Figure 5.
Longevity of cellular immune responses after chemoprophylaxis and sporozoites (CPS) immunization. Plasmodium falciparum–specific cellular immune responses (corrected for uninfected red blood cell background) were assessed in protected (black dots) and unprotected (gray squares) CPS-immunized volunteers before CPS immunization (B), and before (C − 1) and 20 weeks after (C + 140) challenge infection. Data are shown as mean ± standard error of the mean for CD107a expression on CD4 T cells (A) and granzyme B production by CD8 T cells (B). Tests are performed separately for protected and immunized unprotected volunteers, by repeated-measures analysis of variance (including all time points before and after immunizations) and the Dunnett multiple comparison post test, using B as control column. Only the test results for C + 140, compared with those for baseline, for protected volunteers are displayed. For immunized unprotected volunteers, all results were nonsignificant. *P < .05 and **P < .01.
DISCUSSION
We show that CPS immunization reproducibly and dose-dependently induces protection against a homologous challenge infection. With exposure to a total number of P. falciparum–infected mosquito bites as low as 30, CPS immunization still induces 89% protection in healthy volunteers. We furthermore demonstrate that markers of cytotoxic T-cell responses are associated with protection against malaria after whole-sporozoite immunization.
This study provides further support for the remarkable potency of the CPS-protocol to induce complete protection by using even lower numbers of P. falciparum–infected mosquitoes than before [8]. The observed dose-dependent protection is in line with results from RAS immunization trials with sporozoites administered either intravenously by needle and syringe [10] or by bites from irradiated infected mosquitoes [4]. Although the delay of patency in unprotected CPS-immunized subjects was not statistically significant, the patterns of parasitemia indicate partial protection in some subjects. The unexpectedly delayed control subject hampered statistical significance but could be considered an outlier, possibly because of the inherently high baseline immune response. The establishment of a suboptimal CPS immunization regimen inducing protection in 50% of the volunteers immunized with bites from 5 mosquitoes on three occasions will facilitate further studies of protective immune mechanisms against P. falciparum malaria.
Our data provide evidence for a role of cytotoxic T-cell responses in pre-erythrocytic immunity in humans. Because of obvious practical limitations, we only assessed immune cells in the peripheral blood, which may not necessarily reflect responses in the liver but rather represent a surrogate. The results of this exploratory analysis will have to be confirmed in future trials, and the functional relevance remains to be investigated.
So-called classic cytotoxic CD8 T cells can be activated by malaria parasite antigen on infected hepatocytes via major histocompatibility complex (MHC) class I [25] and are associated with protection in a number of (animal) models [13, 14, 26]. CD8 T cells are involved in protection in the murine CPS and RAS models [27–29], but their precise effector mechanisms remain subject of debate. They might either require direct contact with infected hepatocytes [13] or be independent of granzyme B and/or other cytotoxic molecules, suggesting a more indirect cytokine-mediated effect by CD8 T cells [12] or other hepatic immune cells [30]. In addition, a functional role for cytotoxic CD4 T cells is also conceivable because these cells can use cytolytic pathways, such as those involving granulysin, perforin, granzymes, and FAS-L, as shown mostly in viral infections [31, 32]. The protective role of CD4 T cells in murine malaria has been suggested, using in vitro experiments [33] and in vivo depletion [12] or passive transfer [34]. Furthermore, functional cytotoxic CD4 T cells derived from RAS-immunized or synthetic-peptide-immunized volunteers are able to lyse autologous B cells pulsed with a peptide from the circumsporozoite protein [35–37]. We used surface expression of CD107a (LAMP-1), a marker for cytotoxic degranulation, to phenotypically identify cytotoxic CD4 T cells [31]. To directly kill a P. falciparum–infected hepatocyte, parasite antigens should be presented in the context of MHC class II to the cytotoxic CD4 T cells. Although hepatocytes do not express MHC class II in noninflammatory circumstances, the presence of MHC class II on human hepatocytes has been shown in a small number of patients with chronic hepatitis [38] and immune-mediated liver disorders [39, 40]. Functionally, overexpression of MHC class II on hepatocytes in a transgenic mice model showed their capacity for costimulation, antigen presentation, and CD4 T-cell activation [41]. Only indirect evidence suggests that MHC class II expression on mice hepatocytes may play a role in murine malaria [33, 42], and the presence of MHC class II on hepatocytes in human malaria has never been studied. Here, we show for the first time that degranulating CD4 T cells are associated with protection in human malaria and are significantly induced after 1 immunization.
The observed lack of boosting by the second and third immunizations may reflect a saturated response of antigen-specific memory cells. This raises the possibility that fewer immunizations may be sufficient to induce protection, supported by the increased proportion of volunteers without parasitemia after the second and third immunizations in groups 1 and 2. Moreover, the observed longevity of the immune response is in line with long-term protection after CPS immunization in a previous study [9].
The T-helper type 1 (Th1) cytokine IFN-γ has been repeatedly shown to be an important effector molecule in protection against malaria parasites [43], and the clear induction of Th1 responses in our study corroborates earlier findings in both animals and humans after whole-sporozoite immunization [8, 10, 12, 26, 27]. We previously showed that a broad range of both innate and adaptive cellular subsets contribute to CPS-induced P. falciparum–specific IFN-γ production [16], which is sustained at least up to 2.5 years after immunization [9]. IFN-γ production alone, however, does not correlate with protection in the RAS model [10] or our CPS model. Also, production of both IFN-γ and IL-2 by effector memory CD4 T cells and production of IFN-γ by γδ T cells, although clearly increased in immunized volunteers [8, 16], were not different between protected and unprotected volunteers.
During CPS immunization, 4 protected subjects did not show parasitemia by qPCR at any measured time point, not even after the first immunization. A possible explanation is that the number of merozoites released from the liver is too low for qPCR detection. A strong primary innate immune response may be responsible for clearing sporozoites and/or killing infected hepatocytes upon first encounter. Previous studies in mice indeed showed that the inflammatory cytokines interleukin 1 (IL-1) and interleukin 6 (IL-6) block pre-erythrocytic development in mice [16, 44]. Alternatively, chloroquine may have contributed to the decreased (ie undetectable) number of parasites released from the liver either by direct killing or, indirectly, by stimulating the immune system.
Antigen recognition and immune cell activation are essential for an effective response. To investigate pre-erythrocytic cellular immune responses, stimulation with cultured liver-stage P. falciparum would be preferred, but this is currently impossible. We therefore used asexual blood-stage parasites for our experiments, and although responses to purely pre-erythrocytic antigens may be missed, the majority of potential memory responses are likely detected upon P. falciparum–infected RBC stimulation, given the large overlap between liver-stage and blood-stage antigens [45]. Future antigen screening by stimulation with a comprehensive library of pre-erythrocytic and cross-stage proteins or peptides, and subsequent functional studies focusing on cytotoxic T cells, will further identify and delineate the specificity of protective responses [33, 46].
In conclusion, we identified 2 in vitro cellular cytotoxic immune markers that are associated with protection against malaria in a controlled clinical setting. Furthermore, this study confirms the robustness of CPS immunization as a highly efficient and reproducible immunization strategy for complete homologous protection. Further exploration of immune responses induced by CPS immunization will make important contributions to pre-erythrocytic malaria vaccine development and clinical testing.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online (http://jid.oxfordjournals.org/). Supplementary materials consist of data provided by the author that are published to benefit the reader. The posted materials are not copyedited. The contents of all supplementary data are the sole responsibility of the authors. Questions or messages regarding errors should be addressed to the author.
Notes
Acknowledgments. We thank all of the trial volunteers, for their participation in this study; K. Suijk-Benschop, J. Fehrmann-Naumann, C. Prins, E. Jonker, G. Hardeman, and S. ten Velden-Schipper, for blood collection and their good care of the volunteers; the LUMC Department of Medical Microbiology, for facilitating parasitological diagnosis; M. Erkens, T. Arens, J. van der Slot, H. Gerritsma, F. van de Sande, J. van Schie, E. Brienen, J. Schelfaut, J. Verweij, J.Kromhout, E. van Oorschot, and M. Beljon, for reading many thick smears; C. Janse, for his unlimited hospitality; M. Bootsma, for her cardiac monitoring of the trial volunteers; W. Graumans and R. Siebelink-Stoter, for culturing parasites; J. Klaassen, L. Pelser-Posthumus, J. Kuhnen, and A. Pouwelsen, for generating infected mosquitoes and for assistance with immunization of and providing challenge infection to the volunteers; G. Bastiaens and J. Wiersma, for assistance with the immunizations and the challenge infections; P. Houzé, for chloroquine measurements; members of the Safety Monitoring Committee—J. A. Romijn, M. de Boer, and M. Laurens—for their participation, guidance, and safety recommendations throughout the trial; J. Verweij, for the set up and supervision of the DNA isolation and qPCR activities; E. Brienen, for performing DNA isolation and qPCR activities; CromSource, for clinical monitoring; the staff from the LUMC Central Clinical Biochemistry and Haematology Laboratories and the staff from the LUMC Pharmacy, for making this study possible; A. Jansens, and D. Zimmerman, for their administrative support; K. Teelen, for help with sample logistics; M. Vos and B. van Schaijk, for molecular analysis of the parasite batches; and W. Nahrendorf, for critically reading the manuscript.
Financial support. This work was supported by The Netherlands Organisation for Health Research and Development (ZonMw) (project 95110086), the Dioraphte Foundation (project 12010100), and a European Malaria Vaccine Development Association fellowship (to A.C.T.). The research leading to these results has furthermore received funding from the European Union Seventh Framework Programme (FP7/2007-2013; grant 242095 [EviMalar]).
Potential conflicts of interest. All authors: No reported conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.WHO. WHO; 2013. World malaria report 2013. [Google Scholar]
- 2.Crompton PD, Pierce SK, Miller LH. Advances and challenges in malaria vaccine development. J Clin Invest. 2010;120:4168–78. doi: 10.1172/JCI44423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Agnandji ST, Lell B, et al. RTS'S Clinical Trials Partnership. A phase 3 trial of RTS,S/AS01 malaria vaccine in African infants. N Engl J Med. 2012;367:2284–95. doi: 10.1056/NEJMoa1208394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hoffman SL, Goh LM, Luke TC, et al. Protection of humans against malaria by immunization with radiation-attenuated Plasmodium falciparum sporozoites. J Infect Dis. 2002;185:1155–64. doi: 10.1086/339409. [DOI] [PubMed] [Google Scholar]
- 5.Sauerwein RW, Roestenberg M, Moorthy VS. Experimental human challenge infections can accelerate clinical malaria vaccine development. Nat Rev Immunol. 2011;11:57–64. doi: 10.1038/nri2902. [DOI] [PubMed] [Google Scholar]
- 6.McCarthy JS, Good MF. Whole parasite blood stage malaria vaccines: a convergence of evidence. Hum Vaccin. 2010;6:114–23. doi: 10.4161/hv.6.1.10394. [DOI] [PubMed] [Google Scholar]
- 7.Butler NS, Vaughan AM, Harty JT, Kappe SH. Whole parasite vaccination approaches for prevention of malaria infection. Trends Immunol. 2012;33:247–54. doi: 10.1016/j.it.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 8.Roestenberg M, McCall M, Hopman J, et al. Protection against a malaria challenge by sporozoite inoculation. N Engl J Med. 2009;361:468–77. doi: 10.1056/NEJMoa0805832. [DOI] [PubMed] [Google Scholar]
- 9.Roestenberg M, Teirlinck AC, McCall MBB, et al. Long-term protection against malaria after experimental sporozoite inoculation: an open-label follow-up study. Lancet. 2011;377:1770–6. doi: 10.1016/S0140-6736(11)60360-7. [DOI] [PubMed] [Google Scholar]
- 10.Seder RA, Chang LJ, Enama ME, et al. Protection against malaria by intravenous immunization with a nonreplicating sporozoite vaccine. Science. 2013;341:1359–65. doi: 10.1126/science.1241800. [DOI] [PubMed] [Google Scholar]
- 11.Bijker EM, Bastiaens GJ, Teirlinck AC, et al. Protection against malaria after immunization by chloroquine prophylaxis and sporozoites is mediated by preerythrocytic immunity. Proc Natl Acad Sci U S A. 2013;110:7862–7. doi: 10.1073/pnas.1220360110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doolan DL, Hoffman SL. The complexity of protective immunity against liver-stage malaria. J Immunol. 2000;165:1453–62. doi: 10.4049/jimmunol.165.3.1453. [DOI] [PubMed] [Google Scholar]
- 13.Overstreet MG, Cockburn IA, Chen YC, Zavala F. Protective CD8 T cells against Plasmodium liver stages: immunobiology of an ‘unnatural’ immune response. Immunol Rev. 2008;225:272–83. doi: 10.1111/j.1600-065X.2008.00671.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsuji M. A retrospective evaluation of the role of T cells in the development of malaria vaccine. Exp Parasitol. 2010;126:421–5. doi: 10.1016/j.exppara.2009.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Verhage DF, Telgt DS, Bousema JT, et al. Clinical outcome of experimental human malaria induced by Plasmodium falciparum-infected mosquitoes. Neth J Med. 2005;63:52–8. [PubMed] [Google Scholar]
- 16.Teirlinck AC, McCall MBB, Roestenberg M, et al. Longevity and composition of cellular immune responses following experimental Plasmodium falciparum malaria infection in humans. PLoS Pathog. 2011;7:e1002389. doi: 10.1371/journal.ppat.1002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Firth D. Bias reduction of maximum likelihood estimates. Biometrika. 1993;80:27–38. [Google Scholar]
- 18.Heinze G. A comparative investigation of methods for logistic regression with separated or nearly separated data. Stat Med. 2006;25:4216–26. doi: 10.1002/sim.2687. [DOI] [PubMed] [Google Scholar]
- 19.R Foundation for Statistical Computing. Vienna, Austria: R: A language and environment for statistical computing. http://www.R-project.org/ [Google Scholar]
- 20.Heinze G, Ploner M, Dunkler D, Southworth H. logistf: Firth's bias reduced logistic regression. R package version 1.21, 2013http://CRAN.R-project.org/package=logistf . Accessed 2 April 2014.
- 21.Harrell FE., Jr rms: Regression Modeling Strategies. 2014. R package version 4.1–3 http://CRAN.R-project.org/package=rms . Accessed 2 April 2014.
- 22.Goeman JJ. L-1 Penalized Estimation in the Cox Proportional Hazards Model. Biom J. 2010;52:70–84. doi: 10.1002/bimj.200900028. [DOI] [PubMed] [Google Scholar]
- 23.Goeman JJ. Penalized R package, version 0.9–42. 2012.
- 24.Robin X, Turck N, Hainard A, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77. doi: 10.1186/1471-2105-12-77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma J, Trop S, Baer S, et al. Dynamics of the major histocompatibility complex class I processing and presentation pathway in the course of malaria parasite development in human hepatocytes: implications for vaccine development. PLoS One. 2013;8:e75321. doi: 10.1371/journal.pone.0075321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Epstein JE, Tewari K, Lyke KE, et al. Live attenuated malaria vaccine designed to protect through hepatic CD8(+) T cell immunity. Science. 2011;334:475–80. doi: 10.1126/science.1211548. [DOI] [PubMed] [Google Scholar]
- 27.Belnoue E, Costa FT, Frankenberg T, et al. Protective T cell immunity against malaria liver stage after vaccination with live sporozoites under chloroquine treatment. J Immunol. 2004;172:2487–95. doi: 10.4049/jimmunol.172.4.2487. [DOI] [PubMed] [Google Scholar]
- 28.Nganou-Makamdop K, van Gemert GJ, Arens T, Hermsen CC, Sauerwein RW. Long term protection after immunization with P. berghei sporozoites correlates with sustained IFNgamma responses of hepatic CD8+ memory T cells. PLoS One. 2012;7:e36508. doi: 10.1371/journal.pone.0036508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Cockburn IA, Amino R, Kelemen RK, et al. In vivo imaging of CD8+ T cell-mediated elimination of malaria liver stages. Proc Natl Acad Sci U S A. 2013;110:9090–5. doi: 10.1073/pnas.1303858110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kimura K, Kimura D, Matsushima Y, et al. CD8+ T cells specific for a malaria cytoplasmic antigen form clusters around infected hepatocytes and are protective at the liver stage of infection. Infect Immun. 2013;81:3825–34. doi: 10.1128/IAI.00570-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marshall NB, Swain SL. Cytotoxic CD4 T cells in antiviral immunity. BioMed Research International. 2011;2011:1–8. doi: 10.1155/2011/954602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brown DM. Cytolytic CD4 cells: direct mediators in infectious disease and malignancy. Cell Immunol. 2010;262:89–95. doi: 10.1016/j.cellimm.2010.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Renia L, Marussig MS, Grillot D, et al. In vitro activity of CD4+ and CD8+ T lymphocytes from mice immunized with a synthetic malaria peptide. Proc Natl Acad Sci U S A. 1991;88:7963–7. doi: 10.1073/pnas.88.18.7963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsuji M, Romero P, Nussenzweig RS, Zavala F. CD4+ cytolytic T cell clone confers protection against murine malaria. J Exp Med. 1990;172:1353–7. doi: 10.1084/jem.172.5.1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moreno A, Clavijo P, Edelman R, et al. Cytotoxic Cd4+ T-Cells from a Sporozoite-Immunized Volunteer Recognize the Plasmodium falciparum Cs Protein. Int Immunol. 1991;3:997–1003. doi: 10.1093/intimm/3.10.997. [DOI] [PubMed] [Google Scholar]
- 36.Frevert U, Nardin E. Cellular effector mechanisms against Plasmodium liver stages. Cell Microbiol. 2008;10:1956–67. doi: 10.1111/j.1462-5822.2008.01211.x. [DOI] [PubMed] [Google Scholar]
- 37.Frevert U, Moreno A, Calvo-Calle JM, Klotz C, Nardin E. Imaging effector functions of human cytotoxic CD4+ T cells specific for Plasmodium falciparum circumsporozoite protein. Int J Parasitol. 2009;39:119–32. doi: 10.1016/j.ijpara.2008.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Franco A, Barnaba V, Natali P, Balsano C, Musca A, Balsano F. Expression of class I and class II major histocompatibility complex antigens on human hepatocytes. Hepatology. 1988;8:449–54. doi: 10.1002/hep.1840080302. [DOI] [PubMed] [Google Scholar]
- 39.Senaldi G, Lobo-Yeo A, Mowat AP, Mieli-Vergani G, Vergani D. Class I and class II major histocompatibility complex antigens on hepatocytes: importance of the method of detection and expression in histologically normal and diseased livers. J Clin Pathol. 1991;44:107–14. doi: 10.1136/jcp.44.2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lobo-Yeo A, Senaldi G, Portmann B, Mowat AP, Mieli-Vergani G, Vergani D. Class I and class II major histocompatibility complex antigen expression on hepatocytes: a study in children with liver disease. Hepatology. 1990;12:224–32. doi: 10.1002/hep.1840120208. [DOI] [PubMed] [Google Scholar]
- 41.Herkel J, Jagemann B, Wiegard C, et al. MHC class II-expressing hepatocytes function as antigen-presenting cells and activate specific CD4 T lymphocytes. Hepatology. 2003;37:1079–85. doi: 10.1053/jhep.2003.50191. [DOI] [PubMed] [Google Scholar]
- 42.Renia L, Grillot D, Marussig M, et al. Effector functions of circumsporozoite peptide-primed CD4+ T cell clones against Plasmodium yoelii liver stages. J Immunol. 1993;150:1471–8. [PubMed] [Google Scholar]
- 43.McCall MB, Sauerwein RW. Interferon-gamma--central mediator of protective immune responses against the pre-erythrocytic and blood stage of malaria. J Leukoc Biol. 2010;88:1131–43. doi: 10.1189/jlb.0310137. [DOI] [PubMed] [Google Scholar]
- 44.Vreden SG, van den Broek MF, Oettinger MC, Verhave JP, Meuwissen JH, Sauerwein RW. Cytokines inhibit the development of liver schizonts of the malaria parasite Plasmodium berghei in vivo. Eur J Immunol. 1992;22:2271–5. doi: 10.1002/eji.1830220914. [DOI] [PubMed] [Google Scholar]
- 45.Tarun AS, Peng X, Dumpit RF, et al. A combined transcriptome and proteome survey of malaria parasite liver stages. Proc Natl Acad Sci U S A. 2008;105:305–10. doi: 10.1073/pnas.0710780104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Doolan DL, Southwood S, Freilich DA, et al. Identification of Plasmodium falciparum antigens by antigenic analysis of genomic and proteomic data. Proc Natl Acad Sci U S A. 2003;100:9952–7. doi: 10.1073/pnas.1633254100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Adegnika AA, Verweij JJ, Agnandji ST, et al. Microscopic and sub-microscopic Plasmodium falciparum infection, but not inflammation caused by infection, is associated with low birth weight. Am J Trop Med Hyg. 2006;75:798–803. [PubMed] [Google Scholar]
- 48.Lejeune D, Souletie I, Houze S, et al. Simultaneous determination of monodesethylchloroquine, chloroquine, cycloguanil and proguanil on dried blood spots by reverse-phase liquid chromatography. J Pharm Biomed Anal. 2007;43:1106–15. doi: 10.1016/j.jpba.2006.09.036. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.