Abstract
Background
Since the 2008 inception of universal childhood influenza vaccination, national rates have risen more dramatically among younger children than older children and reported rates across racial/ethnic groups are inconsistent. Interventions may be needed to address age and racial disparities to achieve the recommended childhood influenza vaccination target of 70%.
Purpose
To evaluate an intervention to increase childhood influenza vaccination across age and racial groups.
Methods
In 2011–2012, 20 primary care practices treating children were randomly assigned to Intervention and Control arms of a cluster randomized controlled trial to increase childhood influenza vaccination uptake using a toolkit and other strategies including early delivery of donated vaccine, in-service staff meetings, and publicity.
Results
The average vaccination differences from pre-intervention to the intervention year were significantly larger in the Intervention arm (n=10 practices) than the Control arm (n=10 practices), for children aged 2–8 years (10.2 percentage points (pct pts) Intervention vs 3.6 pct pts Control) and 9–18 years (11.1 pct pts Intervention vs 4.3 pct pts Control, p<0.05), for non-white children (16.7 pct pts Intervention vs 4.6 pct pts Control, p<0.001), and overall (9.9 pct pts Intervention vs 4.2 pct pts Control, p<0.01). In multi-level modeling that accounted for person- and practice-level variables and the interactions among age, race and intervention, the likelihood of vaccination increased with younger age group (6–23 months), white race, commercial insurance, the practice’s pre-intervention vaccination rate, and being in the Intervention arm. Estimates of the interaction terms indicated that the intervention increased the likelihood of vaccination for non-white children in all age groups and white children aged 9–18 years.
Conclusions
A multi-strategy intervention that includes a practice improvement toolkit can significantly improve influenza vaccination uptake across age and racial groups without targeting specific groups, especially in practices with large percentages of minority children.
Introduction
Since 2008, recommendations for annual influenza vaccination have included all children aged 6 months and older.1 Although vaccination rates among younger children have nearly reached or exceeded the national target of 70%,2 rates among older children are disappointingly low. For example, infants (aged 6–23 months) are most frequently vaccinated (77%), followed by preschoolers (aged 2–4-years, 66%), young children (aged 5–12 years, 59%) and older children (aged 13–18 years, 43%).3 Similar rates in the younger age groups and lower rates in the older age groups were reported in a study of urban children in a single city.4 Research examining age disparities is scant, but they may be explained by lower contact with the healthcare system among older children. In a study of children aged 6–23 months, those with more frequent contact with the practice during influenza season were more likely to be vaccinated.5 It is unknown whether intervention strategies designed to increase childhood influenza vaccination rates are effective for children of all ages because most of the intervention studies that have included several age groups predate universal vaccination and focus either on infants or high-risk older children. One recent RCT using text message reminders for influenza vaccination reported significant increases in rates among younger children but not among those aged 5–18 years.6
In addition to age disparities in influenza vaccination rates, there is some evidence of racial disparities. In adults, most non-white groups have reported lower influenza vaccination rates than non-Hispanic whites (34% for Hispanics, 36% for blacks, 45% for Asians, 41% for American Indians/Alaska Natives, and 45% for whites),3 whereas, national data for 2012–2013 among children aged 6 months–17 years indicated that Hispanic (61%), Asian (66%), and black (57%) children all had higher vaccination rates than non-Hispanic white (54%) and American Indian/Alaska Native (53%) children.3 Thus, national data reveal that overall rates in 2012–2013 were higher and racial differences were smaller among children than among adults.
Other research among children in specific locales has demonstrated varying differences in influenza vaccination rates across racial groups, with: (1) no differences reported between black and Latino low-income children7; (2) higher rates among white children than black children in inner-city practices8, 9; (3) higher rates among white children than Latino and non-Latino black children in practices in low-income urban communities4; and (4) higher rates among Asian and Hispanic children than among non-Hispanic white children in community health centers.10 Three of these studies were conducted before universal vaccination,7–9 and two4, 10 were based on data collected during the first year of universal vaccination recommendations. As with age disparities, the effectiveness of interventions to raise childhood influenza vaccination uptake across racial groups is unknown. The purpose of this study is to determine whether an intensive intervention based on a toolkit of strategies (the 4 Pillars Toolkit, pittvax.pitt.edu/childflu/papertoolkit), implemented in primary care practices in a cluster randomized controlled trial, was effective for increasing the proportion of children who received influenza vaccine across various age and racial groups in 2011–2012.
Methods
This randomized cluster trial was approved by the University of Pittsburgh IRB.
Sites
Twenty primary care pediatric and family medicine practices from two practice-based research networks and one clinical network in Southwestern Pennsylvania were solicited for participation. All sites were part of the UPMC Health System and used a common electronic medical record (EMR), EpicCare, with the exception of one practice with two offices that used a different EMR system (Allscripts Professional). Participating practices were stratified by location––inner city, urban, suburban, and rural.
Cluster Randomization
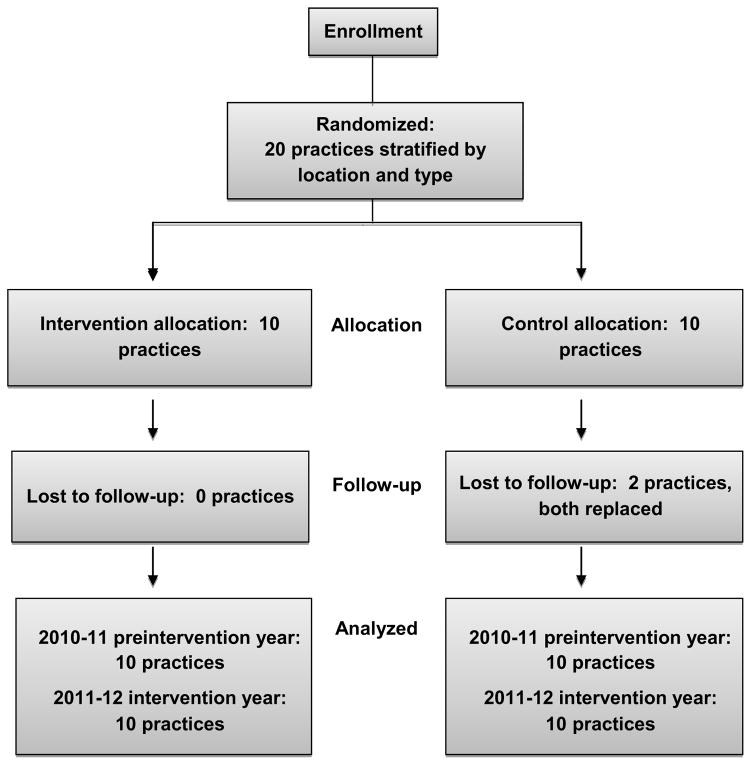
Cluster randomization allocates units or groups rather than individuals to the intervention arms11; hence, each practice or office was considered to be a cluster for randomization purposes. To be eligible, the office must have had a patient population of children aged 6 months–18 years, access to vaccination data via an EMR, and willingness to make office changes to increase influenza vaccination rates. Practices were stratified by location and within each stratum, were randomized into the Intervention or Control arms (Figure 1).
Figure 1.
Randomization scheme
Interventions
The intervention has been previously described,12 in which all CONSORT criteria for a cluster randomized controlled trial11 have been met. The intervention was based on the 4 Pillars Toolkit, a practice improvement toolkit that was developed for use in raising adult immunization rates13, 14 and recommends strategies around four key elements: Pillar 1, Convenient vaccination services; Pillar 2, Notification of patients about the importance of immunization and the availability of vaccines; Pillar 3, Enhanced office systems to facilitate immunization; and Pillar 4, Motivation through an office immunization champion who monitors progress and encourages adherence to vaccination-promoting office practices. Intervention sites were encouraged to increase the length of the influenza vaccination season by vaccinating as soon as vaccine supplies arrived. Intervention sites received donated vaccines for vaccinating disadvantaged children until practices received their Vaccines for Children (VFC) supplies. This allowed Intervention sites to capitalize on early season visits to vaccinate children who would normally need to return to the office later in the season when VFC vaccine became available. Appendix Table A-1 shows the strategies used by the practices.
Data Collection and Analysis
De-identified demographic and influenza vaccination data were derived from EMR data extractions performed by the UPMC Center for Assistance in Research using the eRecord and the EMR of the non-UPMC sites following the 2011–2012 influenza season.
Descriptive analyses were performed for selected patient demographic characteristics (age, sex, race, and health insurance). Chi-square tests were used to examine whether children’s characteristics differed between the Intervention and Control arms. Influenza vaccination was calculated for the pre-intervention and intervention years. The denominator was defined as the number of children who had been seen at least once during a 12-month period in each year (3/1/2010–2/28/2011 for the pre-intervention year and 3/1/2011–2/29/2012 for the intervention year) to capture only active patients in the practice. The numerator for each year was defined as the number of children who had received at least one dose of influenza vaccine during that influenza season (8/1/2010–2/28/2011 for the pre-intervention year and 8/1/2011–2/29/2012 for the intervention year). The proportion of children vaccinated against influenza in the pre-intervention and intervention years were compared within each intervention arm, overall and by age and race, using chi-square tests. Within years, proportions vaccinated across age and racial groups were also compared using chi-square tests.
Pre-intervention to intervention change in influenza vaccination uptake for each site were calculated. The average differences in influenza vaccination uptake were compared between intervention arms overall, by age, and by race. Within each arm, changes in vaccination uptake from pre-intervention to intervention were compared using paired t-tests. These within-arm differences were compared between Intervention and Control arms using t-tests.
To determine which patient and site characteristics were related to likelihood of childhood influenza vaccination while accounting for the clustered nature of the data, two-level generalized linear mixed modeling was conducted using influenza vaccination status (vaccinated versus not vaccinated) as a binary outcome variable. The Level 1 independent variables were age, race, and health insurance, which were divided into two categories, public/uninsured and private. The Level 2 independent variables were pre-intervention vaccination rate calculated to account for age, race and insurance status, and intervention arm (Intervention versus Control). A random intercept model with compound symmetric covariance structure was chosen as the final model based on the lowest value of the Akaike information criterion. Estimates and within-cluster independence were obtained and tested using residual pseudo-likelihood methods. Furthermore, in order to assess whether the intervention was effective across age and racial groups, we examined three interaction terms: (1) age by intervention; (2) race by intervention; and (3) age by race and intervention in separate regression models. The statistical analyses were conducted using SAS, version 9.3 (SAS Institute Inc., Cary NC). Statistical significance of two-sided tests was set at type I error (alpha)=0.05.
Results
Demographics
Twenty primary care practices were randomly assigned to either the Intervention or Control arm. Two Control sites dropped out of the study and were replaced with two other sites with similar characteristics and those data were used for all analyses. Table 1 summarizes the characteristics of sites during the pre-intervention year. The Intervention and Control arms did not differ significantly by percentage of non-white children, percentage of children publicly or self-insured, percentage of female patients, or age distribution.
Table 1.
Demographic characteristics of intervention and control arms
| Characteristic | Intervention sites | Control sites |
|---|---|---|
| Practices | ||
| Type (n) | ||
| Family medicine | 2 | 2 |
| Pediatrics | 8 | 8 |
| Total | 10 | 10 |
| Location (n) | ||
| Inner city | 2 | 1 |
| Rural | 1 | 1 |
| Suburban | 5 | 6 |
| Urban | 2 | 2 |
| Patients | ||
| Pre-intervention (n) | 43,292 | 38,306 |
| Non-white, mean % (SD) | 31.0 (32.8) | 22.3 (18.1) |
| Health insurance, M % (SD) | ||
| Self or publicly insured | 40.4 (25.4) | 37.9 (27.6) |
| Commercial | 59.6 (25.4) | 62.1 (27.6) |
| Age group, M % (SD) | ||
| 6–23 months | 12.1 (3.7) | 11.1 (2.4) |
| 2–8 years | 44.8 (8.4) | 39.8 (6.4) |
| 9–18 years | 43.2 (11.8) | 49.1 (8.1) |
| Female, M % (SD) | 49.7 (1.2) | 49.3 (2.3) |
Vaccination Uptake
Pre-intervention influenza vaccination uptake in both Intervention and Control arms was significantly higher among younger children than older children (p<0.001) and among whites compared to non-whites (p<0.001) (Table 2). During the intervention year, vaccination uptake improved significantly across all age and racial groups in both Intervention and Control arms as indicated by the combined levels shown in Table 2.
Table 2.
Influenza vaccination during pre-intervention (2010–2011) and intervention (2011–2012), overall and by age and race for each arm
| Group | Intervention arm | Control arm | ||||
|---|---|---|---|---|---|---|
|
| ||||||
| Pre-intervention year n=43,292 | Intervention year n=49,037 | p-value for difference between years | Pre-intervention year n=38,306 | Intervention year n=38,626 | p-value for difference between years | |
| Vaccinated n (%) | Vaccinated n (%) | Vaccinated n (%) | Vaccinated n (%) | |||
| Overall | 19,903 (46.0) | 26,447 (53.9) | <0.001 | 17,492 (45.7) | 19,335 (50.1) | <0.001 |
| Age group† | ||||||
| 6–23 months | 3,890 (70.2) | 4,680 (75.0) | <0.001 | 2,914 (66.3) | 3,088 (72.7) | <0.001 |
| 2–8 years | 9,945 (49.8) | 12,938 (57.9) | <0.001 | 9,450 (52.8) | 9,138 (56.6) | <0.001 |
| 9–18 years | 6,068 (34.1) | 8,829 (43.2) | <0.001 | 6,128 (34.2) | 7,109 (39.0) | <0.001 |
| Race† | ||||||
| Non-white | 3,334 (33.7) | 6,660 (50.9) | <0.001 | 3,046 (36.4) | 3,440 (43.1) | <0.001 |
| White | 16,569 (49.6) | 19,787 (55.0) | <0.001 | 14,446 (48.3) | 15,895 (51.9) | <0.001 |
Note: Boldface indicates statistical significance.
Differences across age groups and racial groups are significant (p<0.001) in both pre-intervention and intervention years.
Numbers represent number of children, not number of doses; vaccination rates were calculated for all sites combined for each intervention arm (Intervention and Control).
Because sites varied considerably in the racial distribution of their patient populations, each site was examined separately to compare influenza vaccination uptake by race. At pre-intervention, racial disparities in vaccination were statistically evident in eight Intervention sites and nine Control sites and the average proportion vaccinated was 15.9 percentage points (pct pts; 49.6 minus 33.7, p<0.001) higher for white compared with non-white children in the Intervention arm and 11.9 pct pts higher (48.3 minus 36.4, p<0.001) for white compared with non-white children in the Control arm (Table 2). During the intervention season, racial disparities remained in four of ten Intervention sites and six of ten Control sites, and the average uptake among Intervention sites was 4.1 pct pts (55 minus 50.9, p<0.001) higher among white children than non-white children compared with 14.8 pct pts (51.9 minus 43.1, p<0.001) higher vaccination uptake among white children than non-white children in Control sites (Table 2).
The average vaccination differences from pre-intervention to the intervention year are shown in Table 3. These differences were significantly larger in the Intervention arm than the Control arm overall (9.9 pct pts Intervention vs 4.2 pct pts Control, p<0.01), for children aged 9–18 years (11.1 pct pts Intervention vs 4.3 pct pts Control, p<0.05), and for non-white children (16.7 pct pts Intervention vs 4.6 pct pts Control, p<0.001). The average increase in uptake in the Intervention arm for non-white children was twice that of white children (16.7 pct pts vs 8.1 pct pts, p<0.05), whereas, in the Control arm, the average increase in uptake did not differ between non-white and white children (4.6 pct pts vs 3.7 pct pts). These findings indicate a differential effect of the intervention on non-white children.
Table 3.
Comparison of average differences in influenza vaccination across sites from pre-intervention to intervention
| Group | Intervention arm | Control arm | |||
|---|---|---|---|---|---|
|
| |||||
| Cluster vaccination increase, M (SD) | p-value* for difference within Intervention arm | Cluster vaccination increase, M (SD) | p-value* for difference within Control arm | p-value† for difference between Intervention and Control arms | |
| Overall | 9.9 (6.6) | 0.001 | 4.2 (4.2) | 0.012 | 0.007 |
| Age group | |||||
| 6–23 months | 7.2 (8.8) | 0.028 | 5.2 (7.5) | 0.057 | 0.586 |
| 2–8 years | 10.2 (7.6) | 0.002 | 3.6 (6.8) | 0.126 | 0.057 |
| 9–18 years | 11.1 (7.4) | 0.001 | 4.3 (4.4) | 0.012 | 0.024 |
| Race | |||||
| Non-white | 16.7 (7.2) | <0.001 | 4.6 (4.7) | 0.013 | <0.001 |
| White | 8.1 (5.8) | 0.002 | 3.7 (4.0) | 0.018 | 0.063 |
Note: Differences from pre-intervention (2010–2011) to intervention (2011–2012) years were calculated for each site (cluster), then averaged for each intervention arm (Intervention and Control). Boldface indicates statistical significance.
By Paired t-tests.
By t-test.
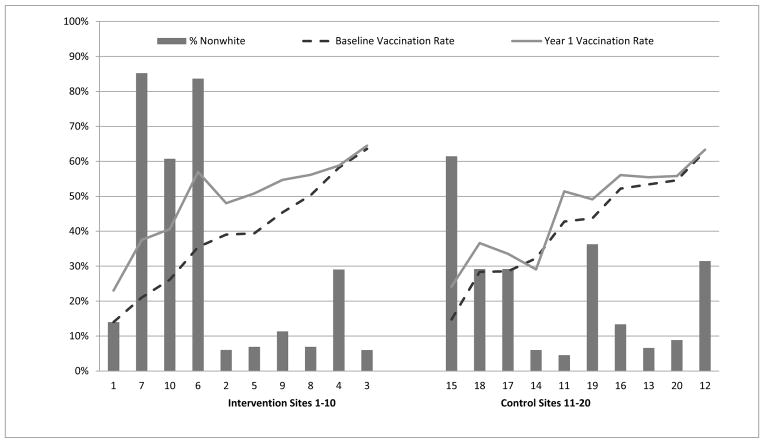
Figure 2 depicts pre-intervention and intervention vaccination levels and the percentage of non-white patients in each practice. The highest absolute increases (14.5–21.5 pct pts) in vaccination uptake from pre-intervention to intervention year occurred in practices in the Intervention arm with the highest percentages (61%–84%) of children of color.
Figure 2.
Pre-intervention (2010–2011) and intervention (2011–2012) influenza vaccination rates by proportion of non-white children aged 6 months–18 years
The number of doses given in the Intervention and Control arms was also examined (data not shown). In the Control arm, there was no significant change in the number or percentage of doses given to white and non-white children, with 18% of doses given to non-white and 82% given to white children both years. In the Intervention arm, 20% of 24,176 doses (4,866) were given to non-white children in the pre-intervention year, increasing to 25% of 29,074 doses (7,280) in the intervention year (p<0.001), suggesting that more of the additional doses given overall reached non-white children.
The results of regression analyses with a base model and models with significant interaction terms are shown in Appendix Table A-2. In regression analyses, patient-level variables of race, age group, and insurance type were significantly related to vaccination status, with white children, those aged 6–23 months, white, and privately insured children more likely to be vaccinated than those in comparison groups (p<0.01). Among practice-level variables, higher pre-intervention vaccination rate and being in the Intervention arm significantly increased the likelihood of vaccination (p<0.05).
Additional analyses were conducted to examine possible interactions among age, race, and intervention. The interaction terms were statistically significant (p<0.01) in all three models (Table A-2). The ORs and 95% CIs for interaction terms are summarized in Table 4. The model with the age and intervention interaction term indicates that the intervention was not significantly effective in raising influenza vaccination rates for the 2–8-year-old group compared with the same aged children in the Control arm, but was effective for children aged 6–23 months and 9–18 years. In the model including the race and intervention interaction term, the intervention was shown to be effective for both white and non-white children. The model including age, race, and intervention interaction term was found to significantly increase the likelihood of vaccination for non-white children in all age groups and for white children aged 9–18 years. Non-white children of all ages in the Intervention arm were 32%–45% more likely to be vaccinated than their counterparts in the Control arm while controlling for health insurance, percentage of the practice that was non-white, and the practice’s pre-intervention vaccination rate. White children aged 9–18 years in the Intervention arm were 18% less likely to be vaccinated than their counterparts in the Control arm.
Table 4.
OR and 95% CI estimates for interaction terms in a cluster RCT to increase childhood vaccination
| Age and racial groups | Model with age and intervention interaction | Model with race and intervention interaction | Model with age, race, and intervention interaction |
|---|---|---|---|
|
| |||
| OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Age | |||
| 6–23 months: Intervention versus Control | 1.26 (1.02, 1.57) | ||
| 2–8 years: Intervention versus Control | 1.18 (0.97, 1.44) | ||
| 9–18 years: Intervention versus Control | 1.34 (1.09, 1.64) | ||
| Race | |||
| White: Intervention versus Control | 1.21 (1.00, 1.48) | ||
| Non-white: Intervention versus Control | 1.39 (1.13, 1.71) | ||
| Age and race | |||
| 6–23 months, white: Intervention versus Control | 1.22 (0.98, 1.52) | ||
| 6–23 months, non-white: Intervention versus Control | 1.38 (1.06, 1.78) | ||
| 2–8 years, white: Intervention versus Control | 1.14 (0.94, 1.38) | ||
| 2–8 years, non-white: Intervention versus Control | 1.32 (1.07, 1.63) | ||
| 9–18 years, White: Intervention versus Control | 1.27 (1.05–1.54) | ||
| 9–18 years, Non-white: Intervention versus Control | 1.46 (1.18–1.80) | ||
Note: Boldface indicates statistical significance.
Discussion
Since the 2008 recommendations by the Advisory Committee on Immunization Practices for universal influenza vaccination of all children aged 6 months and older, little research has been published on efforts to increase uptake across the childhood age spectrum. Stockwell, et al.6 used text messages to increase influenza vaccine uptake 3.7% across all ages, with significant increases only among children <5 years old. The present study used a package of interventions including a practice improvement toolkit to raise influenza vaccination rates among all children aged >6 months in pediatric and family medicine practices. These intervention strategies included community-wide publicity and education, early delivery of donated vaccine for low-income children, in-service meetings to engage staff in the effort, and immunization rate tracking provided to an in-office immunization champion, among others. As previously reported, this intervention resulted in significant overall increases in influenza vaccination uptake in eight of ten intervention sites, a final Intervention arm vaccination rate of 54%, and significantly larger increases in mean uptake in the Intervention arm than the Control arm.12 However, those results did not examine rates by age and racial group, whereas the present study did.
National observational data indicate that influenza vaccine uptake in 2012–2013 had increased to 77% among 6–23-month-olds, 66% among 2–4-year-olds, 59% among 5–12-year-olds, and 43% among 13–18-year-olds.3 Studies of interventions to increase influenza vaccination among all children aged 6 months–18 years were difficult to identify, but one intervention study reported intervention group rates of 59% among 6–23-month-olds, 46% among 2–4-year-olds, and 27.8% among 5–18-year-olds.6 Significant increases in vaccination rates among children aged >5 years were not observed. In unadjusted analyses, the 4 Pillars Toolkit intervention was successful in raising the proportion of children vaccinated against influenza in 2011–2012 among all age groups (i.e., 75% for children aged 6–23 months, 58% for children aged 2–8 years, and 43% among children aged 9–18 years). Although the comparative age groupings differ somewhat, national rates did not reach these levels until 1 year later.3 Moreover, the practices participating in this study serve a sizable proportion of disadvantaged children, whose representation is unknown in the national rates.
Racial disparities in influenza vaccination rates among adults are well documented, whereas among children, the data do not consistently favor one racial or ethnic group, suggesting that national data may mask differences in uptake in specific geographic areas. Low vaccination rates and disparities in vaccine uptake among any racial or ethnic group require attention. Yet, few studies of interventions to increase childhood influenza vaccination have examined the effectiveness of an intervention across racial or ethnic groups.8, 9 In this study, children in practices with a greater proportion of non-white children were more likely to be vaccinated than in practices with fewer non-white children, when controlling for other factors. The Intervention practices with greater proportions of non-white children were the same practices with the largest percentage of non-commercially insured children, which itself was significantly related to lower likelihood of vaccination. However, analyses that accounted for interactions among age, race, and intervention, and controlled for insurance type, showed that non-white children in Intervention practices were 32%–45% more likely to be vaccinated than non-white children in Control practices. In fact, practices that had the largest proportions of non-white and publicly insured children generally achieved the largest increases in overall uptake. Some aspects of the intervention might explain these findings. One of the team members made numerous visits to social service and community agencies and places of worship frequented by the local black community to promote influenza vaccination both directly and indirectly through the organizations’ leadership. This type of promotion could affect both Intervention and Control sites. Secondly, Intervention sites were given donated influenza vaccine and preferential early delivery of VFC influenza vaccines to enable them to use early season visits to vaccinate low-income children. Finally, previous research has shown that increasing vaccination rates among all patients in a practice can eliminate racial differences in vaccination rates that were observed pre-intervention.15 This phenomenon may help to explain the larger increases in vaccine uptake among older children observed in this study. Additional research studies that compare intervention effectiveness across a broader racial/ethnic spectrum as well as all childhood age groups are warranted.
Strengths and Limitations
To date, this study is one of few trials to examine the effect of an evidence-based intervention on childhood influenza vaccination rates across age and racial groups. Only one previous non-randomized study was identified that looked at the relationship of a similar set of interventions in a toolkit on rates among high-risk children and adolescents.16 The present study’s limitations included the fact that two offices of one rural practice were each randomized into the Intervention and Control arms, which may have resulted in some carryover of the intervention to the Control site. Children aged <9 years who received at least one dose of vaccine were counted in the numerator as “vaccinated,” as we were unable to determine which children were first-time vaccinees and required two doses.
Conclusions
Implementation of a multi strategy intervention that includes a practice improvement toolkit can significantly improve vaccination rates across several age and racial groups and is especially effective in practices with large percentages of minority children. It may be possible to improve influenza vaccination rates among children of all ages and racial/ethnic groups without efforts targeted to specific subgroups.
Supplementary Material
Acknowledgments
The authors thank the University of Pittsburgh Clinical and Translational Science Institute Pediatric PittNet practice-based research network and the following site investigators: Tracey Conti, MD, Mark Diamond, MD, Harold Glick, MD, Phillip Iozzi, DO, Kenneth Keppel, MD, John J. Labella, MD, Sanjay Lambore, MD, Sheldon Levine, MD, Thomas G. Lynch, MD, Elaine McGhee, MD, Paul Rowland, MD, Robert Rutowski, MD, Pamela Schoemer, MD, Emeil Shenouda, MD, Aaron Smuckler, MD, Scott Tyson, MD, Donald Vigliotti, MD, David Wolfson, MD, and Rana Ziadeh, MD. The authors also thank Sanofi Pasteur for the donation of 2,000 doses of influenza vaccine used in the study.
This investigation was supported by a grant (No. U01 IP000321) from the CDC. The views expressed herein are those of those authors and not those of the funding agency. The project was also supported by the NIH through grant No. UL1 RR024153 and UL1TR000005.
Dr. Nowalk has received research funding from Merck and Co, Inc., Pfizer, Inc. and MedImmune, LLC and consults for MedImmune, LLC. Dr. Lin has received research funding from Merck and Co, Inc., Pfizer, Inc. and Sanofi Pasteur and consults for MedImmune, LLC. Dr. Huang has received research funding from Merck and Co, Inc. and Sanofi Pasteur. Dr. Zimmerman has received research funding from Merck and Co, Inc., Pfizer, Inc. and Sanofi Pasteur and MedImmune, LLC and consulted for MedImmune, LLC. Dr. Hannibal, Dr. Reis, Dr. Gallik, Dr. Allred, Dr. Wolfson have no financial relationships relevant to this article to disclose. Ms. Moehling has no financial relationships relevant to this article to disclose.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting galley proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Fiore AE, Uyeki TM, Broder K, et al. Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Morb Mortal Wkly Rep. 2010;59(RR-8):1–62. [PubMed] [Google Scholar]
- 2.USDHHS. Healthy People 2020: Immunization and Infectious Diseases Overview. 2013 healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=23.
- 3.CDC. Flu Vaccination Coverage, US, 2012–13 Influenza Season. 2013 cdc.gov/flu/fluvaxview/coverage-1213estimates.htm.
- 4.Hofstetter AM, Natarajan K, Rabinowitz D, et al. Timeliness of pediatric influenza vaccination compared with seasonal influenza activity in an urban community, 2004–2008. Am J Public Health. 2013;103(7):e50–8. doi: 10.2105/AJPH.2013.301351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poehling KA, Fairbrother G, Zhu YW, et al. Practice and child characteristics associated with influenza vaccine uptake in young children. Pediatrics. 2010;126(4):665–73. doi: 10.1542/peds.2009-2620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stockwell MS, Kharbanda EO, Martinez RA, Vargas CY, Vawdrey DK, Camargo S. Effect of a text messaging intervention on influenza vaccination in an urban, low-income pediatric and adolescent population: a randomized controlled trial. JAMA. 2012;307(16):1702–8. doi: 10.1001/jama.2012.502. [DOI] [PubMed] [Google Scholar]
- 7.Uwemedimo OT, Findley SE, Andres R, Irigoyen M, Stockwell MS. Determinants of influenza vaccination among young children in an inner-city community. J Community Health. 2012;37(3):663–72. doi: 10.1007/s10900-011-9497-9. [DOI] [PubMed] [Google Scholar]
- 8.Zimmerman RK, Hoberman A, Nowalk MP, et al. Improving influenza vaccination rates of high-risk inner-city children over 2 intervention years. Ann Fam Med. 2006;4(6):534–40. doi: 10.1370/afm.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zimmerman RK, Nowalk MP, Lin CJ, et al. Interventions over 2 years to increase influenza vaccination of children aged 6–23 months in inner-city family health centers. Vaccine. 2006;24(10):1523–9. doi: 10.1016/j.vaccine.2005.10.016. [DOI] [PubMed] [Google Scholar]
- 10.O’Connor ME, Everhart RM, Berg M, Federico SG, Hambidge SJ. Pediatric influenza immunization in an integrated safety net health care system. Vaccine. 2012;30(19):2951–5. doi: 10.1016/j.vaccine.2012.02.060. [DOI] [PubMed] [Google Scholar]
- 11.Campbell MK, Piaggio G, Elbourne DR, DGA Consort 2010 statement: extension to cluster randomised trials. BMJ. 2012;345:e5661. doi: 10.1136/bmj.e5661. [DOI] [PubMed] [Google Scholar]
- 12.Zimmerman RK, Norwalk MP, Hannibal K, et al. Cluster randomized trial of a toolkit and early vaccine delivery to improve childhood influenza vaccination rates in primary care. Vaccine. 2014;32(29):3656–63. doi: 10.1016/j.vaccine.2014.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nowalk MP, Nutini J, Raymund M, Ahmed F, Albert SM, Zimmerman RK. Evaluation of a toolkit to introduce standing orders for influenza and pneumococcal vaccination in adults: a multimodal pilot project. Vaccine. 2012;30(41):5978–82. doi: 10.1016/j.vaccine.2012.07.023. [DOI] [PubMed] [Google Scholar]
- 14.Nowalk MP, Nolan BAD, Nutini J, et al. Success of the 4 pillars toolkit for influenza and pneumococcal vaccination in adults. J Healthc Qual. 2013 doi: 10.1111/jhq.12020. in press. [DOI] [PubMed] [Google Scholar]
- 15.Zimmerman RK, Nowalk MP, Raymund M, et al. Tailored interventions to increase influenza vaccination in neighborhood health centers serving the disadvantaged. Am J Public Health. 2003;93(10):1699–705. doi: 10.2105/ajph.93.10.1699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Britto MT, Schoettker PJ, Pandzik GM, Weiland J, Mandel KE. Improving influenza immunisation for high-risk children and adolescents. Qual Saf Health Care. 2007;16(5):363–8. doi: 10.1136/qshc.2006.019380. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.