Abstract
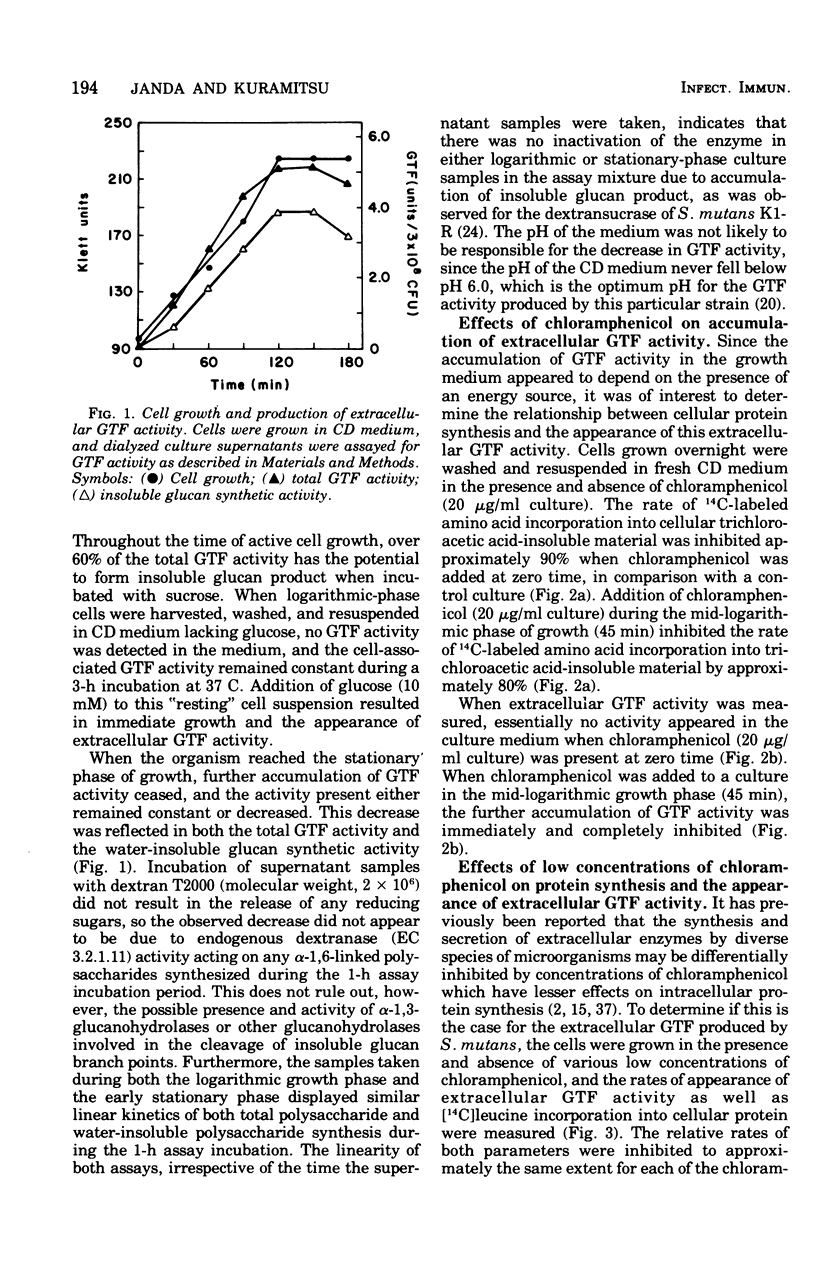
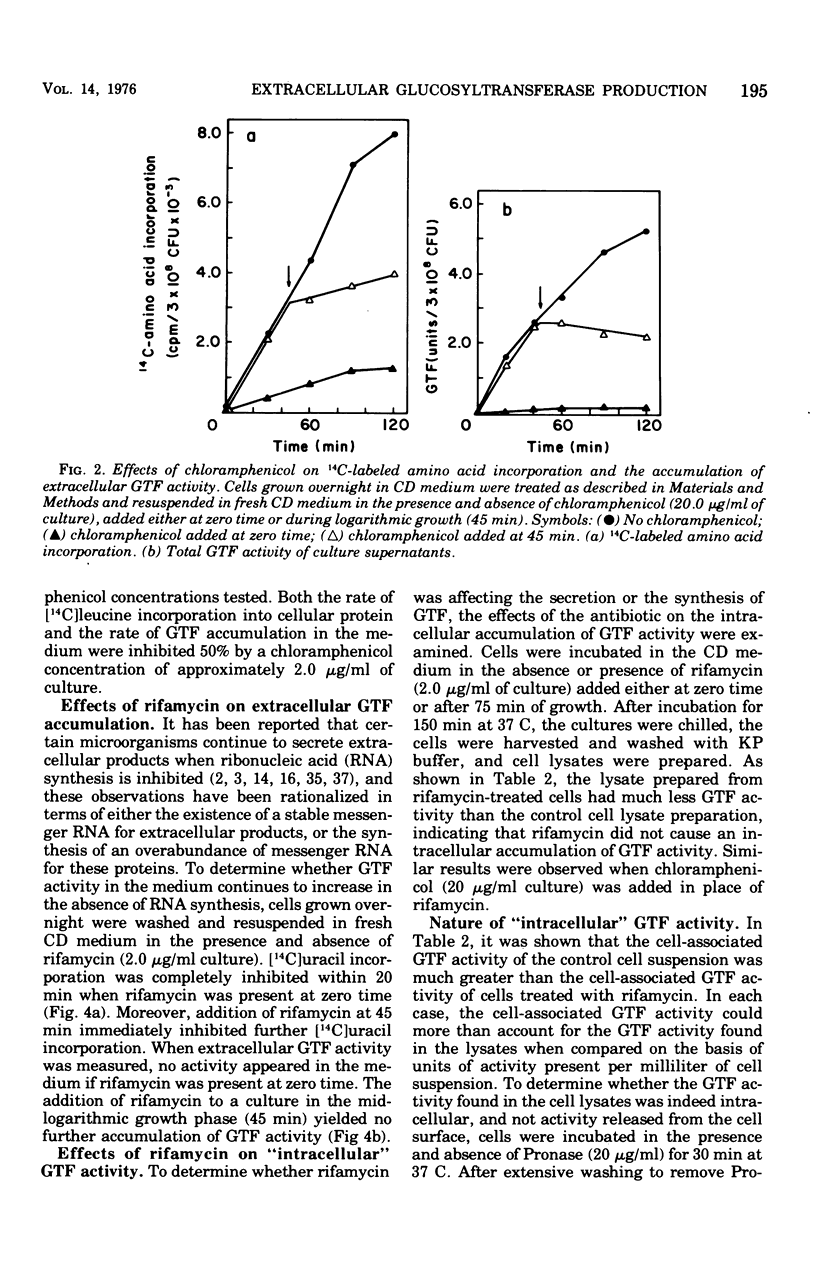
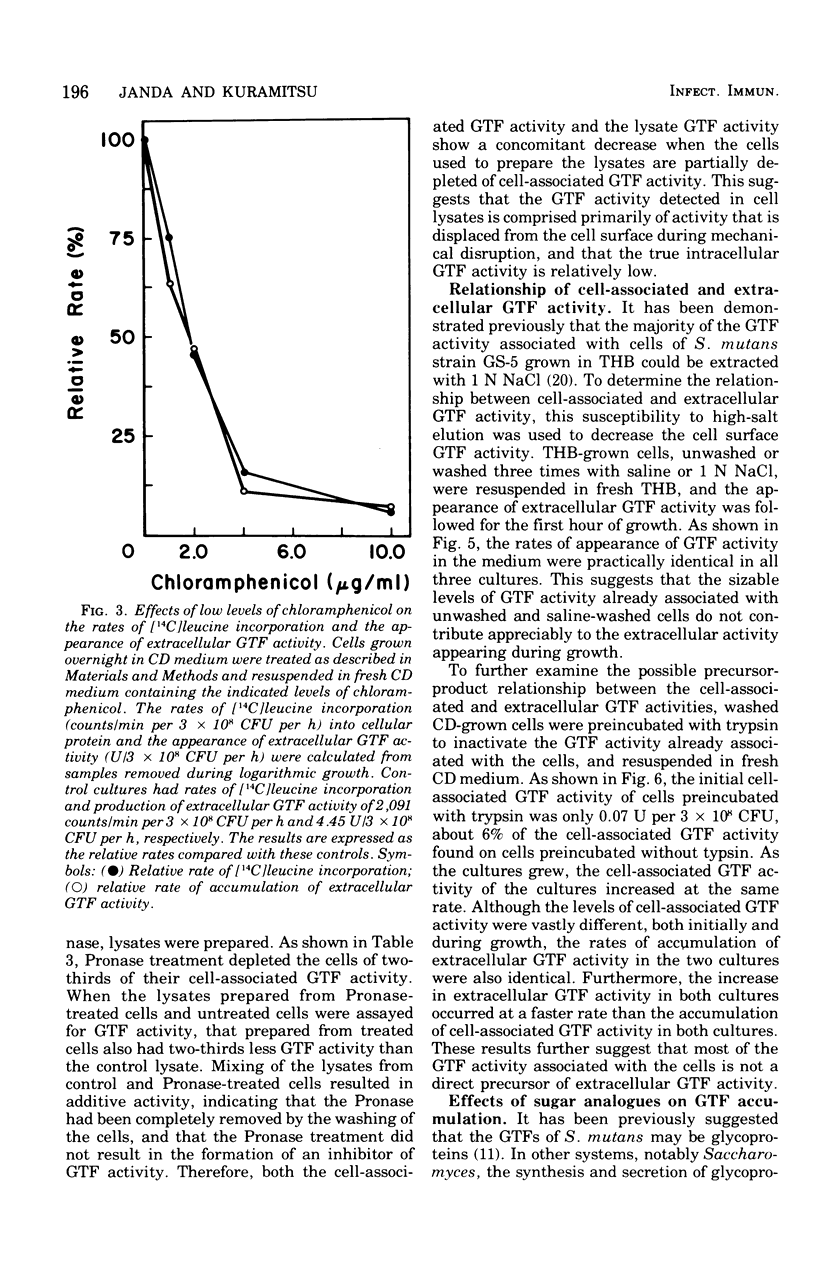
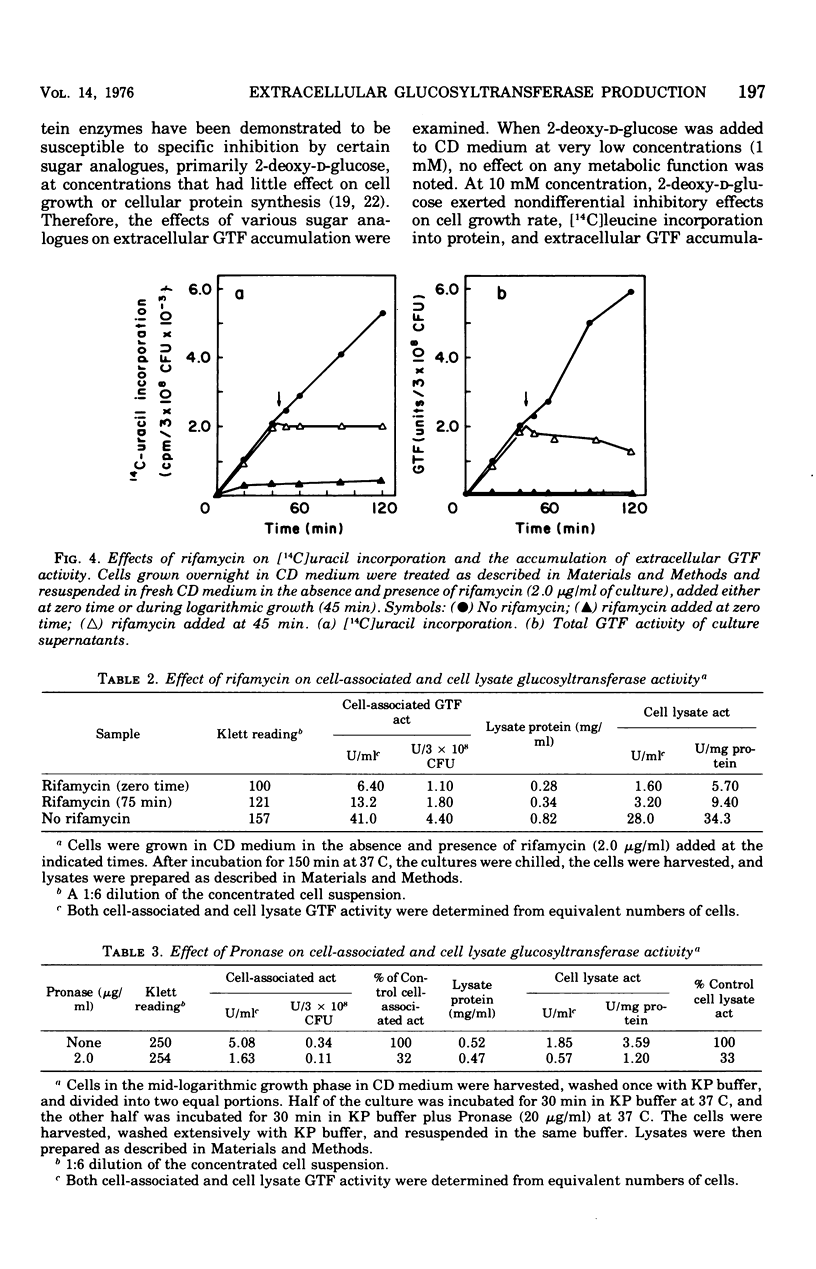
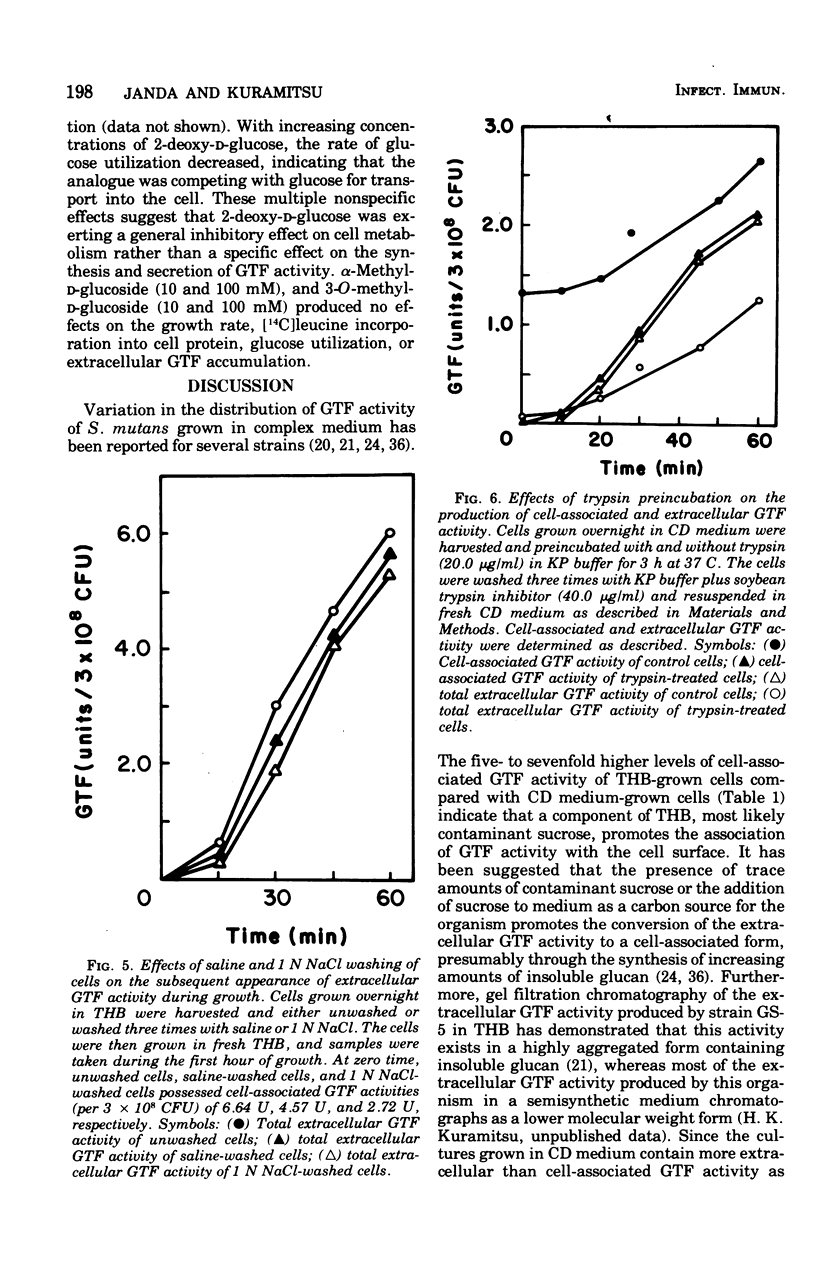
The regulation of extracellular glucosyltransferase production in Streptococcus mutans GS-5 has been studied using a chemically defined medium. Most of the glucosyltransferase activity produced by cells grown in the chemically defined medium was extracellular, in contrast with the distribution between cell-associated and extracellular glucosyltransferase activity when cells were grown in complex medium. The production of extracellular glucosyltransferase activity coincided with the logarithmic growth phase, and further accumulation ceased when glucose was exhausted from the medium. Accumulation of extracellular glucosyltransferase activity was inhibited immediately by chloramphenicol and rifamycin, added either at the beginning of growth or during mid-logarithmic growth. Low concentrations of chloramphenicol inhibited both cellular protein synthesis and the accumulation of extracellular glucosyltransferase activity to the same extent, indicating a close coupling between glucosyltransferase synthesis and secretion. Experiments using cell lysates showed that no intracellular accumulation of glucosyltransferase activity occurred in the presence of the inhibitors and that the intracellular activity is very low relative to the cell-surface activity. The utilization of cells depleted of cell-associated glucosyltransferase activity indicated that most of the cell-associated glucosyltransferase activity does not act as a precursor for the extracellular enzyme. Sugar analogue inhibitors of glycoprotein synthesis did not have any specific effects on the synthesis or secretion of extracellular glucosyltransferase activity.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Boethling R. S. Regulation of extracellular protease secretion in Pseudomonas maltophilia. J Bacteriol. 1975 Sep;123(3):954–961. doi: 10.1128/jb.123.3.954-961.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Both G. W., McInnes J. L., Hanlon J. E., May B. K., Elliott W. H. Evidence for an accumulation of messenger RNA specific for extracellular protease and its relevance to the mechanism of enzyme secretion in bacteria. J Mol Biol. 1972 Jun 20;67(2):199–217. doi: 10.1016/0022-2836(72)90236-7. [DOI] [PubMed] [Google Scholar]
- Braatz J. A., Heath E. C. The role of polysaccharide in the secretion of protein by Micrococcus sodonensis. J Biol Chem. 1974 Apr 25;249(8):2536–2547. [PubMed] [Google Scholar]
- Bratthall D. Demonstration of five serological groups of streptococcal strains resembling Streptococcus mutans. Odontol Revy. 1970;21(2):143–152. [PubMed] [Google Scholar]
- Chludzinski A. M., Germaine G. R., Schachtele C. F. Purification and properties of dextransucrase from Streptococcus mutans. J Bacteriol. 1974 Apr;118(1):1–7. doi: 10.1128/jb.118.1.1-7.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman C., Brown S. Relationship between exoprotease secretion and the synthesis of ribonucleic acid and protein in Bacillus amyloliquefaciens. Antimicrob Agents Chemother. 1975 Jun;7(6):840–844. doi: 10.1128/aac.7.6.840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwardsson S. Characteristics of caries-inducing human streptococci resembling Streptococcus mutans. Arch Oral Biol. 1968 Jun;13(6):637–646. doi: 10.1016/0003-9969(68)90142-8. [DOI] [PubMed] [Google Scholar]
- Fukui K., Fukui Y., Moriyama T. Purification and properties of dextransucrase and invertase from Streptococcus mutans. J Bacteriol. 1974 Jun;118(3):796–804. doi: 10.1128/jb.118.3.796-804.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Chludzinski A. M., Schachtele C. F. Streptococcus mutans dextransucrase: requirement for primer dextran. J Bacteriol. 1974 Oct;120(1):287–294. doi: 10.1128/jb.120.1.287-294.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons R. J., Banghart S. B. Synthesis of extracellular dextran by cariogenic bacteria and its presence in human dental plaque. Arch Oral Biol. 1967 Jan;12(1):11–23. doi: 10.1016/0003-9969(67)90137-9. [DOI] [PubMed] [Google Scholar]
- Gibbons R. J., Nygaard M. Synthesis of insoluble dextran and its significance in the formation of gelatinous deposits by plaque-forming streptococci. Arch Oral Biol. 1968 Oct;13(10):1249–1262. doi: 10.1016/0003-9969(68)90081-2. [DOI] [PubMed] [Google Scholar]
- Glenn A. R., Both G. W., McInnes J. L., May B. K., Elliott W. H. Dynamic state of the messenger RNA pool specific for extracellular protease in Bacillus amyloliquefaciens: its relevance to the mechanism of enzyme secretion. J Mol Biol. 1973 Jan 10;73(2):221–230. doi: 10.1016/0022-2836(73)90325-2. [DOI] [PubMed] [Google Scholar]
- Glew R. H., Heath E. C. Studies on the extracellular alkaline phosphatase of Micrococcus sodonensis. II. Factors affecting secretion. J Biol Chem. 1971 Mar 25;246(6):1566–1574. [PubMed] [Google Scholar]
- Gould A. R., May B. K., Elliott W. H. Accumulation of messenger RNA for extracellular enzymes as a general phenomenon in Bacillus amyloiquefaciens. J Mol Biol. 1973 Jan 10;73(2):213–219. doi: 10.1016/0022-2836(73)90324-0. [DOI] [PubMed] [Google Scholar]
- Gould A. R., May B. K., Elliott W. H. Release of extracellular enzymes from Bacillus amyloliquefaciens. J Bacteriol. 1975 Apr;122(1):34–40. doi: 10.1128/jb.122.1.34-40.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guggenheim B., Newbrun E. Extracellular glucosyltransferase activity of an HS strain of Streptococcus mutans. Helv Odontol Acta. 1969 Oct;13(2):84–97. [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Inhibition by 2-deoxy-D-glucose of synthesis of glycoprotein enzymes by protoplasts of Saccharomyces: relation to inhibition of sugar uptake and metabolism. J Bacteriol. 1972 Aug;111(2):419–429. doi: 10.1128/jb.111.2.419-429.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of cell-associated dextransucrase activity from glucose-grown cells of Streptococcus mutans. Infect Immun. 1974 Jul;10(1):227–235. doi: 10.1128/iai.10.1.227-235.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K. Characterization of extracellular glucosyltransferase activity of Steptococcus mutans. Infect Immun. 1975 Oct;12(4):738–749. doi: 10.1128/iai.12.4.738-749.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Liras P., Gascón S. Biosynthesis and secretion of yeast invertase. Effect of cycloheximide and 2-deoxy-D-glucose. Eur J Biochem. 1971 Nov 11;23(1):160–165. doi: 10.1111/j.1432-1033.1971.tb01603.x. [DOI] [PubMed] [Google Scholar]
- McCabe M. M., Smith E. E. Origin of the cell-associated dextransucrase of Streptococcus mutans. Infect Immun. 1973 Jun;7(6):829–838. doi: 10.1128/iai.7.6.829-838.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. I. Roles of insoluble dextran-levan synthetase enzymes and cell wall polysaccharide antigen in plaque formation. Infect Immun. 1973 Oct;8(4):555–562. doi: 10.1128/iai.8.4.555-562.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of adherence of Streptococcus mutans to smooth surfaces. II. Nature of the binding site and the adsorption of dextran-levan synthetase enzymes on the cell-wall surface of the streptococcus. Infect Immun. 1974 Feb;9(2):419–429. doi: 10.1128/iai.9.2.419-429.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukasa H., Slade H. D. Mechanism of the Adherence of Streptococcus mutans to Smooth Surfaces III. Purification and Properties of the Enzyme Complex Responsible for Adherence. Infect Immun. 1974 Nov;10(5):1135–1145. doi: 10.1128/iai.10.5.1135-1145.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson G. A., Guggenheim B., Small P. A., Jr Antibody-mediated inhibition of dextran-sucrose-induced agglutination of Streptococcus mutans. Infect Immun. 1974 Feb;9(2):273–278. doi: 10.1128/iai.9.2.273-278.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robrish S. A., Reid W., Krichevsky M. I. Distribution of enzymes forming polysaccharide from sucrose and the composition of extracellular polysaccharide synthesized by Streptococcus mutans. Appl Microbiol. 1972 Aug;24(2):184–190. doi: 10.1128/am.24.2.184-190.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Ghosh B. K., Lampen J. O. Characteristics of penicillinase secretion by growing cells and protoplasts of Bacillus licheniformis. J Bacteriol. 1969 Feb;97(2):820–826. doi: 10.1128/jb.97.2.820-826.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schachtele C. F. Glucose transport in Streptococcus mutans: preparation of cytoplasmic membranes and characteristics of phosphotransferase activity. J Dent Res. 1975 Mar-Apr;54(2):330–338. [PubMed] [Google Scholar]
- Schachtele D. F., Leung W. L. Effect of sugar analogues on growth, sugar utilization, and acid production by Streptococcus mutans. J Dent Res. 1975 May-Jun;54(3):433–440. doi: 10.1177/00220345750540030301. [DOI] [PubMed] [Google Scholar]
- Scherp H. W. Dental caries: prospects for prevention. Science. 1971 Sep 24;173(4003):1199–1205. doi: 10.1126/science.173.4003.1199. [DOI] [PubMed] [Google Scholar]
- Semets E. V., Glenn A. R., May B. K., Elliott W. H. Accumulation of messenger ribonucleic acid specific for extracellular protease in Bacillus subtilis 168. J Bacteriol. 1973 Nov;116(2):531–534. doi: 10.1128/jb.116.2.531-534.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinell D. M., Gibbons R. J. Influence of culture medium on the glucosyl transferase- and dextran-binding capacity of Streptococcus mutans 6715 cells. Infect Immun. 1974 Dec;10(6):1448–1451. doi: 10.1128/iai.10.6.1448-1451.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinson M. W., Merrick J. M. Extracellular enzyme secretion by Pseudomonas lemoignei. J Bacteriol. 1974 Jul;119(1):152–161. doi: 10.1128/jb.119.1.152-161.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanzer J. M., Chassy B. M., Krichevsky M. I. Sucrose metabolism by Streptococcus mutans, SL-I. Biochim Biophys Acta. 1971 Feb 28;261(2):379–387. doi: 10.1016/0304-4165(72)90062-1. [DOI] [PubMed] [Google Scholar]
- Terleckyj B., Willett N. P., Shockman G. D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975 Apr;11(4):649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]


