Abstract
In traditional immuno-PCR a single antibody recognition event is associated with one to three DNA tags, which are subsequently amplified by PCR. Here, we describe a nanoparticle amplified immuno-PCR (NPA-IPCR) assay that combines antibody recognition of ELISA with a 50-fold nanoparticle valence amplification step prior to tag amplification by PCR. The assay detects a respiratory syncytial virus (RSV) surface protein using an antibody bound to a 15 nm gold nanoparticle co-functionalized with thiolated DNA complementary to a hybridized to 76-base tag DNA with a tag DNA to antibody ratio of 50 to 1. The presence of virus particles triggers the formation of a “sandwich” complex comprised of the gold nanoparticle construct, virus and an antibody functionalized magnetic particle used for extraction. After extraction, DNA tags are released by heating to 95°C and detected via real-time PCR. The limit of detection of the assay was compared to ELISA and RT-PCR using RSV infected HEp-2 cell extracts. NPA-IPCR showed a ~4000-fold improvement in the limit of detection compared to ELISA and a 4-fold improvement compared to viral RNA extraction followed by traditional RTPCR. NPA-IPCR offers a viable platform for the development of an early-stage diagnostics requiring an exceptionally low limit of detection.
Keywords: Immuno-PCR, PCR, Viral Detection, Respiratory Syncytial Virus, Gold Nanoparticle, Magnetic Extraction
Introduction
Severe respiratory illness in young children and infants is often associated with respiratory syncytial virus (RSV). RSV is the major cause of infantile bronchiolitis and the most frequent cause of hospitalization of infants and young children in industrialized countries [1]. In the United States, RSV is estimated to account for over 125,000 infant hospitalizations for bronchiolitis or pneumonia per year [2]. Although not fatal to the majority of patients, RSV can be deadly in immuno-compromised populations, and in the U.S., RSV is responsible for 10,000 deaths annually in patients over the age of 65 [3] and [4]. Additionally, naturally acquired immunity to RSV is not complete, and recurrent infection can be frequent [5], [6] and [7].
Early attempts at developing a formalin-inactivated RSV vaccine were hindered by an increase in disease severity upon subsequent infection of vaccinated patients [8], [9] and [10]. Because of such problems, there is no approved vaccine for RSV [11]. Without a vaccine, treatment is typically limited to prophylactic passive immunization with neutralizing humanized monoclonal antibodies (palivizumab) or antivirals (ribavirin) [5] and [12]. The cost associated with monthly prophylactic passive immunization is expensive, so it typically is only utilized in premature or other high-risk infants [12] and [13]. Additionally, these prophylactic treatments have been associated with adverse outcomes in infants with cyanotic cardiac disease [5]. Another option, antivirals, is only effective when used early in the course of infection, making early detection crucial [14].
Although a median infective dose (ID50) for RSV has not been established in humans, one clinical study showed that as few as 120 to 440 plaque forming units (PFU) in two milliliters of Hank's balance salt solution caused an infection in 83% of healthy adult volunteers [15] and [16]. Another study using a temperature sensitive mutant RSV vaccine infected children with a dose as low as 27 PFU [17]. These are likely overestimates since as the RSV strain is passaged in laboratory culture, it is thought to become attenuated and that the dose of wild RSV actually required to infect an infant is likely even less than 20 PFU [18].
Given that treatment is most effective early in the course of an infection, and that even relatively few PFUs can result in infection, it is clear that sensitive diagnostics for RSV are essential for effective treatment. To date, one of the most sensitive diagnostics available for the detection of RSV has been the use of Reverse Transcription Polymerase Chain Reaction (RTPCR) to amplify and detect RSV genetic material [19] and [20]. However, detection of the negative-sense RNA genome or mRNA of the virus comes with a few drawbacks. First, RNA is very sensitive to degradation by RNases and typically has a shorter half-life than proteins [21] and [22]. This, in turn, places increased importance on preparation, pre-assay handling and storage of samples for RT-PCR diagnostics [23]. Secondly, studies have shown that RSV mRNA expressed during infection is cyclical and can limit the amount of mRNA present to detect at any given time; however, the surface fusion protein (F-protein) increases monotonically over time [24]. Further, the link between RT-PCR viral load and disease severity has yet to be established [20]. In contrast, an Immuno-PCR (I-PCR) based diagnostic for the detection of RSV comes with two inherent strengths. First, empty viral nucleocapsids can be detected because only the presence of a specific protein is required for a positive result [25]. Even fragments of cell wall that have the protein of interest expressed can be detected. Secondly, each virion may contain only a single copy of RSV genomic RNA, but the protein targets are often expressed at a higher copy number, each one of which can act as a potential target [26].
I-PCR, developed by Sano et al., offers processing similar to ELISA while coupling the signal amplification of PCR [27]. The original I-PCR design has been modified in a number of ways to strengthen various aspects of the assay. Different antibody-DNA linking strategies have been used; from streptavidin-protein A coupling, to strictly streptavidin-biotin coupling, and even phage display mediated I-PCR (PD-IPCR), where a single chain variable fragments are displayed on the phage and phage DNA itself acts as a DNA tag [27], [28], [26] and [29]. Capture antibodies have been bound to biogenic magnetosome nanoparticles (M-IPCR) instead of protein binding plates to enhance signal generation [30]. Biotinylated DNA-streptavidin nanostructures have been used to increase the valency of the antibody binding, and in turn increase the sensitivity of I-PCR [31]. However, these studies utilized an average DNA/complex ratio of only 5, as increased DNA ratios resulted in instability of the streptavidin conjugates [31].
Here, we report the development of a Nanoparticle Amplified Immuno-PCR (NPAIPCR) assay for the detection of RSV (Figure 1). The diagnostic assay offers a unique dual-amplification approach to achieve an exceptionally low limit of detection of 4.1 PFU/mL. The first step of signal amplification is achieved by using multivalent gold nanoparticles (AuNPs) to release multiple strands of reporter DNA per antibody binding event. After reporter DNA is released, real-time PCR is used to provide a second level of amplification. Additionally, the assay utilizes magnetic microparticles (MMPs) to capture antigen for improved washing to enable better target extraction. Our results suggest that the NPA-IPCR assay not only offers >1000-fold improvement in limit of detection over ELISA, but also a 4-fold improvement over detection via real-time RT-PCR.
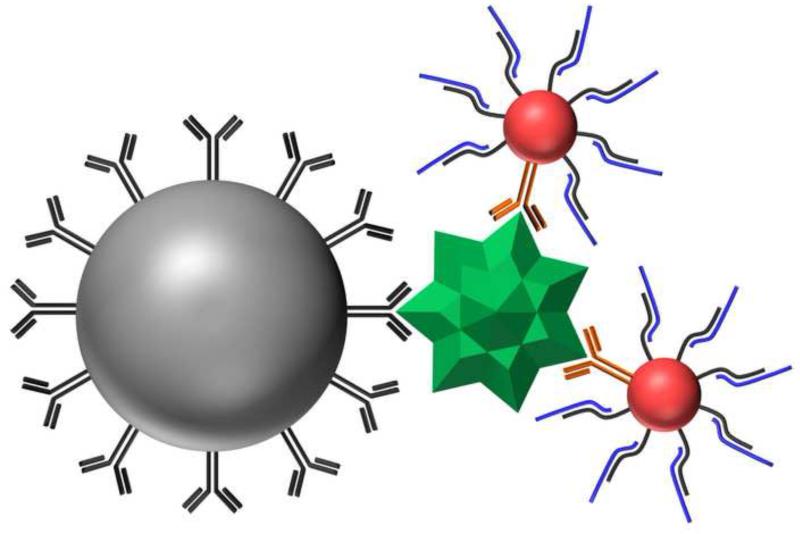
Figure 1.
Illustration of the imunno-nanoparticle-PCR sandwich. RSV antigen (green) is captured and separated using antibody functionalized 1 μm magnetic microparticles (gray). The magnetic bead- antigen complex is then reacted with antibody-DNA functionalized 15 nm AuNPs (red). Hybridized DNA (blue) is released from the AuNPs by heating and quantified via real-time PCR.
Methods and Materials
Coupling of Antibodies to Magnetic Microparticles
MagnaBind™ amine derivatized 1 μm magnetic microparticles (product #21352, Pierce Biotechnology) were activated with sulfosuccinimidyl-4-[N-maleimidomethyl]cyclohexane-1-carboxylate (Sulfo-SMCC). In a typical reaction, 200 μL of MMPs were washed three times with coupling buffer (CB) consisting of 50 mM phosphate buffer (pH 7.0), 0.15 M NaCl and 5 mM EDTA. The MMPs were then cleared from the suspension using a magnet. After 1 min, the solution became clear and MMPs were pulled to the side of the tube. When the clear supernatant and the external magnetic field were removed, the MMPs were resuspended in CB. After washing, the MMPS were activated with 20 μL of 1 mM Sulfo-SMCC. The solution was mixed and allowed to sit at room temperature for 1 hr. The MMPs were then washed 3 times to remove excess Sulfo-SMCC.
Antibody reduction was coordinated so that once the MMPs were activated, they could be immediately used. Dithiothreitol (DTT) was used to reduce anti-RSV fusion protein antibodies (clones 1269 and 1214). 15 μL of 1.2 mM DTT was added to 15 μL of purified antibodies at 30 mg/mL in phosphate buffered saline (PBS). The solution was mixed and allowed to sit at room temperature for 0.5 hr. After that time, the antibodies were separated from DTT using a NAP-5 column (product #17-0853-01, GE Healthcare Bio-Sciences Corp.). The activated MMPs were combined with the column-purified reduced antibodies and allowed to react for 1 h at room temperature. The conjugation was quenched by the addition of β-mercaptoethanol to a final concentration of 100 μM. After 1 h quenching, the MMPs were washed three times with PBS and then resuspended in a final volume of 300 μL.
Coupling of Antibodies and DNA to Gold Nanoparticles
Thiolated DNA sequences (Biosearch Technologies) were received as disulfides and were activated by cleaving the disulfide bond. Cleavage was performed in 100 mM DTT, 0.1 M phosphate buffer, pH 8.3. After 0.5 hr, thiolated DNA was desalted using Microcon YM-3 centrifugal filters (product #4410, Millipore). The purified DNA was resuspended in TE buffer and stored in small aliquots at −80°C.
In a typical reaction, 35 μL of 0.2 mg/mL Synagis® antibody (humanized monoclonal antibody known to target the A antigenic site of RSV's fusion protein, product #NDC 60574-4114-1, MedImmune, Inc.) were added to 10 mL of 2.3 nM, 15 nm diameter AuNPs (product #15704-1, Ted Pella) at pH 9.3 and placed on a rotator for 0.5 hr.
Then, 50.8 μL of 107 μM activated DNA (Comp_55, Table 1) was added; the AuNPs were then mixed on a rotator for another 0.5 hr. Sodium chloride, phosphate buffer and tween20 were added to the solution until it was brought to 0.1 M NaCl, 10 mM phosphate buffer, and 0.02% Tween20 and then incubated at room temperature for 1 hr. After this time, the concentration of NaCl was brought to 0.2 M and to 0.3 M after a third hour. Excess DNA was removed by centrifuging the solution for 20 minutes at 21,100 × gravity. The clear supernatant was then removed, and the red oily pellet was resuspended in a stock solution of 0.3 M NaCl, 10 mM phosphate buffer and 0.02% Tween20 (PB). This washing process was repeated three times. After washing, particles were resuspended, and tag DNA (Tag_76, Table 1) was added to bring the molar ratio of tag DNA:AuNPs to 200:1. The solution was allowed to sit at room temperature overnight. Finally, excess tag DNA was removed using the washing method described above.
Table 1.
Glossary of oligonucleotide sequences.
| Name | Sequence 5′ → 3′ |
|---|---|
| Comp_55 | [C6Thiol] TTTTT TTTTT TTTTT GCTTG TCTCG TAAGT TGAGA TTTCG CTATG CACGG TCCTT |
| Tag_76 | CTGCG ACGAT CTACC ATCGA CGTAC CAGGT CGGTT GAAGG ACCGT GCATA GCGAA ATCTC AACTT ACGAG ACAAG C |
| Tag Primer 1 | CTGCG ACGAT CTACC AT |
| Tag Primer 2 | GCTTG TCTCG TAAGT TGA |
| RSV Primer 1 | GCTCT TAGCA AAGTC AAGTT GAATG A |
| RSV Primer 2 | TGCTC CGTTG GATGG TGTAT T |
Preparation of RSV Infected Cell Lysate
HEp-2 cells (product #CCL-23, American Type Culture Collection) were cultured in OPTI-MEM media supplemented with 2% fetal bovine serum, 250 μg/mL of amphotericin-B, 10 mg/mL gentamicin and 200 mM L-glutamine and incubated at 37°C with 5% CO2. RSV stock was prepared by infecting a confluent T-150 flask of HEp-2 cells with RSV strain A2. Infection was performed with rapidly thawed virus diluted to 5 mL in cell media. Following the initial infections, cells were incubated for 1 h after which 35 mL of cell media was added to the flask. The infection was allowed to proceed for 4 days, after which cells were scraped from the surface of the T-150 flask. The supernatant containing the cells was collected in a 50 mL centrifuge tube and centrifuged for 5 min at 500 × gravity. Following removal of the supernatant, the cell pellet was resuspended to 5 mL in cell media. The cells were then frozen using a slurry of ethanol and dry ice in order to lyse cells and release virus particles. After freezing, the cells were thawed in a 37°C water bath. The freezing/thawing cycle was repeated three times to ensure the release of virus particles from the cell wall. After the third cycle, the cells were centrifuged at 100 × gravity for 5 min to pellet large cellular debris. The supernatant was then separated into aliquots of 0.5 mL and stored at −80°C.
Antibody-Magnetic Microparticle Characterization Experiment
Pulldown experiments were performed to validate the attachment of antibodies to magnetic microparticles (MMPs). 10 μL of antibody-conjugated MMPs, 100 μL of a stock solution of RSV (1.675×106 PFU/mL) and 100 μL 4% bovine serum albumin (BSA, product #A3059-100G, Sigma) were mixed and placed on a rotator for 2 hr. Unbound virus was then removed from the MMPs by cleaning as previously described in the antibody-MMP coupling reaction; this was done three times. Next, the MMPs were mixed with 100 μL of 2% BSA containing 12 μg/mL of F-mix antibody with a 655 nm quantum dot (QD) attached to the antibody. The solution was stirred and placed on a rotator for 2 hr; unbound quantum dot-coupled antibodies were removed by cleaning the beads three times. After cleaning, the MMPRSV-QD complexes were placed in a 96-well plate on a BioMag® 96-well Plate Side Pull Magnetic Separator (product #85072, Polysciences, Inc.) and imaged on a Zeiss Axiovert 200 inverted fluorescence microscope.
Validation of DNA-Gold Nanoparticle Functionalization
Antibodies and activated DNA (Comp_55, Table 1) were attached to AuNPs following the procedure stated above. After the removal of excess activated DNA, tag DNA (Tag_76, Table 1) was added to the AuNPs. As a control, tag DNA was also added to PB without AuNPs. The amount of complementary DNA added to 100 μL of AuNPs or PB was varied to determine the optimal molar ratio of tag DNA:AuNPs for hybridization. After incubation at room temperature for 24 hr, the solution was centrifuged for 0.5 h at 16,100 × gravity to pellet the AuNPs and tag DNA hybridized to them. 90 μL of supernatant was removed from both AuNP and PB samples. The absorbance at 260 nm of the supernatant was then obtained on an Agilent 8453 UV-Vis spectrophotometer to determine the difference between samples incubated with and without AuNPs.
To verify the release of tag DNA upon heating, antibody-CompDNA-AuNPs prior to tag DNA hybridization and post tag DNA hybridization were compared. Samples were heated to 95°C for 10 min then centrifuged at 21,100 × gravity for 15 min. The supernatant was then examined by measuring the absorbance at 260 and 280 nm.
Quartz Crystal Microbalance Validation of Antibody-Gold Nanoparticle Attachment
All quartz crystal microbalance (QCM) experiments were performed on a Maxtek, Inc. Research Quartz Crystal Microbalance with a flow rate of 30 μL/min. The crystals used were 5 MHz Ti/Au quartz crystals. After equilibrating for 0.5 h in PBS, a 5 minute PBS baseline was obtained. A RSV stock solution containing 4.0×105 PFU/mL was then flowed over the crystal for 10 min, followed by an additional five minutes of PBS. After RSV binding, non-specific binding was blocked using a 1% BSA solution flow over the crystal for 10 min followed by 10 min of PBS. At this point, the flow was switched to either AuNPs functionalized with anti-RSV antibodies and DNA or, as a control, AuNPs functionalized with DNA alone.
Nanoparticle Amplified Immuno-PCR (NPA-IPCR)
Pulldown experiments used 5 μL of antibody-conjugated MMPs, 100 μL of infected cell lysate at the desired dilution and 200 μL of 5% BSA. A stock solution of RSV infected HEp-2 cell lysate (8.33×106 PFU/mL) was serially diluted 10-fold to determine the lower limit of detection. The MMPs, virus, and BSA solution were mixed and placed on a rotator for 1 hr. Unbound virus was then removed from the MMPs by cleaning as previously described. After three washes, the MMPs were mixed with 5 μL of 5 nM antibody-DNA functionalized AuNPs and 300 μL of 5% BSA and placed back on the rotator for 1 hr. The MMPs were then washed two times in 5% BSA followed by three additional washes in PBS. After the final wash, MMPs were resuspended in 300 μL of nuclease-free water. Solutions were then held at 95°C for 10 min followed by placement in an external magnetic field and removal of 100 μL of supernatant. The supernatant was then analyzed via PCR.
Real-time PCR was performed using a Rotor-Gene Q 5-Plex thermal cycler system (Qiagen, Alameda, CA). Reactions were done in a 25 μL volume with 12.5 μL of 2X Rotor-Gene SYBR Green PCR Master Mix (product #204074, Qiagen), 200 nM left and right primers (Tag primers, Table 1), nuclease-free water and 5 μL of sample. DNA polymerase activation was performed 95°C for 3 min and 40 cycles of 95°C for 15 sec to denature, 60°C for 60 sec to anneal and extend, and 72°C for 15 sec to detect fluorescence. Following amplification, a melt curve from 50°C to 99°C was performed. Dynamic tube normalization was used to remove background fluorescence by monitoring the second derivative of each sample to determine the level of background fluorescence prior to amplification. The normalized fluorescence data was then graphed for each sample to determine the Ct using a threshold of 0.03. Serially diluted samples were used to determine the assay's linear range and uninfected control samples were used to determine the background of the assay. The limit of detection was then determined using the assay's linear range to calculate 3 standard deviations above background.
ELISA
To allow capture antibodies to bind to the Costar UV microtiter plate (product #3635, Corning), 100 μL of 10 μg/mL F-mix antibodies were added the 96-well plate and allowed to incubate at room temperature for 1 hr. After 1 hr, the wells were rinsed 3 times with PBS and blocked with 5% BSA in PBS for 1 hr. A stock solution of RSV infected HEp-2 cell lysate (8.33×106 PFU/mL) was serially diluted 3-fold to determine the lower limit of detection. Following blocking, BSA was removed from the wells and 100 μL of infected cell lysate at the desired dilution was added. The plate was then incubated at room temperature for 1 hr. Following antigen binding, the wells were rinsed 3 times with PBS and 100 μL of 10 μg/mL of Synagis antibodies diluted in 5% BSA were added to each well. Once again, the plate was incubated at room temperature for 1 hr. The wells were then rinsed 3 times with PBS and 100 μL of a 1:1000 dilution in 5% BSA of goat anti-human HRP conjugated secondary antibodies (product #2010-05, Southern Biotech) were added to each well. Following 1 h incubation at room temperature, the wells were rinsed 5 times with PBS. Next, 100 μL of TMB One Solution (product #G7431, Promega), was added to each well and the enzymatic reaction was allowed to proceed for 10 minutes, after which it was quenched with 100 μL of 2 M H2SO4. Finally, absorbance at 450 nm was measured using a Bio-Tek Synergy HT microplate reader. Serially diluted samples were used to determine the assay's linear range and uninfected control samples were used to determine the background of the assay. The limit of detection was then determined using the assay's linear range to calculate 3 standard deviations above background.
RNA Isolation and Real-Time Reverse Transcription PCR (Real-Time RT-PCR)
An RNeasy Mini Kit (product #74104, Qiagen) was used for RNA isolation. 300 μL of cell lysate was mixed with 400 μL lysis buffer (RLT) and homogenized by passing the sample through a 20 gauge needle 8 times. 700 μL of 70% was then added to the sample, and it was bound to an RNeasy spin column. On the column, DNA was digested using an RNase-Free DNase Set (product #79254, Qiagen). The remaining RNA was then washed with the appropriate buffers (RW1 and RPE). After washing, the RNA was eluted in 50 μL of nuclease-free water, and analyzed via real-time RT-PCR.
Real-time RT-PCR was performed using a Rotor-Gene Q 5-Plex thermal cycler system (Qiagen, Alameda, CA). Reactions were done in a 25 μL volume with 12.5 μL of 2X One-Step qRT-PCR Buffer plus SYBR (product #639518, Clontech), 0.5 μL of 50X QTaq DNA Polymerase Mix (product #639518, Clontech), 0.4 μL of 60X qRT Mix (product #639518, Clontech), 200 nM left and right primers (RSV primers, Table 1), nuclease-free water and 5 μL of sample. Reverse transcription was performed at 48°C for 20 min followed by an initial QTaq DNA polymerase activation step of 95°C for 3 min and 40 cycles of 95°C for 15 sec to denature, 60°C for 60 sec to anneal and extend, and 72°C for 15 sec to detect fluorescence. Following amplification, a melt curve from 50°C to 99°C was performed. Dynamic tube normalization was used to remove background fluorescence by monitoring the second derivative of each sample to determine the level of background fluorescence prior to amplification. The normalized fluorescence data was then graphed for each sample to determine the Ct using a threshold of 0.03. Serially diluted samples were used to determine the assay's linear range and uninfected control samples were used to determine the background of the assay. The limit of detection was then determined using the assay's linear range to calculate 3 standard deviations above background.
Results and Discussion
Assay Design Rationale
Nanoparticle constructs were designed utilizing AuNPs to facilitate the attachment of both tag DNA and antibodies on a single RSV probe. The AuNP acts as a scaffold to associate antibody recognition with multiple copies of a DNA tag of a specific sequence. Gold nanoparticles were chosen as they are biologically compatible, provide a large surface area on which to couple multiple probes and allow the use of facile gold-thiol chemistry [32], [33], [34] and [35]. Recently, AuNPs have been co-functionalized with antibodies and DNA for a number of applications [36], [37] and [38]. The 15 nm AuNPs used in this study were functionalized with Synagis® antibodies (humanized monoclonal antibodies targeted to the RSV fusion protein) to bind to antigen. The AuNPs were also functionalized with thiolated strands of DNA (Comp_55, Table 1) to provide a covalently attached DNA binding site for hybridization of reporter DNA tags (Tag_76, Table 1). The resulting AuNP contains both a recognition component (Synagis®) and a signal generating component (Tag_76) which is released upon heating. In the presence of antigen, the MMPs and AuNPs form a “sandwich” which can be separated from unbound AuNPs and cell debris using a magnetic field (Figure 1). After washing, the DNA tags bound to extracted AuNPs are released upon heating and quantified via PCR.
The F-protein of RSV, a surface protein often targeted in direct antigen detection diagnostics because it is highly conserved between different strains of RSV, is the primary target in this version of the assay. Published studies have shown that the F-protein of RSV has over 80% homology between different serotypes [39]. Initially, virus in the analyte solution is captured using anti-F protein antibodies functionalized to MMPs. MMPs were chosen as the capture antibody scaffold for three reasons. First, attaching the antibodies to a microparticle as opposed to a microtiter plate allow for more thorough sampling of the analyte solution. Secondly, MMPs allow for more thorough washing of captured antigen and AuNPs to decrease non-specific binding and reduce the background signal, which tends be a major drawback of IPCR [40]. Finally, the use of MMPs allows for ease of downstream automation in future applications.
Magnetic Microparticle Pulldown of RSV Cell Lysate
After attachment to MMPs, anti-RSV antibodies remain functional and effectively capture F-protein on RSV-infected cell lysates (Figure 2). To verify the functional attachment of anti-RSV antibodies to the MMPs, a pulldown experiment was performed. After conjugation of F-mix antibodies to the MMPs, the functionalized MMPs were added to RSV-infected cell lysate. An external magnetic field was used to extract captured virus. Captured RSV was labeled by the addition of F-mix antibodies coupled to fluorescent 655 nm quantum dots. Unbound quantum dots were removed by applying a magnetic field and washing. After washing, the MMPs were imaged with a fluorescence microscope (Figure 2). As a control, nonfunctionalized MMPs were exposed to RSV, followed by pulldown and addition of antibodies coupled to quantum dots. Figure 2A shows the fluorescence image from this experiment which contains negligible fluorescence. In Figure 2B, the fluorescence was generated by the presence of the fluorescent quantum dot bound to the MMP-RSV complexes. Formation of these complexes confirms that the antibodies remain functional after attachment to MMPs. Examining the average pixel intensity over the entire fluorescence images revealed that the functionalized MMPs (33.9 average pixel intensity) generated 10-fold greater fluorescence over the control MMPs (3.2 average pixel intensity). DIC images (not shown) both revealed striations thought to be due to the MMPs lining up when a magnetic field was applied in the bottom right corner of both images.
Figure 2.
Fluorescence images of MMP-virus-QD complexes. RSV was mixed with MMPs (A) unconjugated or (B) conjugated to F-mix antibodies and 655 nm quantum dots conjugated to F-mix antibodies. Magnetic particles and associated complexes were extracted and washed. Images are 20x magnification.
Tag DNA-Gold Nanoparticle Functionalization
Co-functionalization of tag DNA and anti-RSV antibodies on 15 nm AuNPs offer a valency of up to 70 to 1 (Figure 3). To determine the number of tag DNA molecules bound per AuNP, tag DNA was incubated with samples containing antibody-CompDNA-AuNPs or a PB control. Following incubation, both the sample and control were centrifuged to pellet AuNPs and any tag DNA hybridized to them. The absorbance at 260 nm was then used to determine the amount of unbound tag DNA in each supernatant. These studies revealed that saturation of the AuNPs with tag DNA, ~70 strands per particle, occurred when tag DNA was incubated at a molar ratio ≥ 400:1 of tag DNA:AuNP (Figure 3). However, a much lower concentration, 200:1, still allowed for the hybridization of ~50 strands per particle, which is the concentration that was used in subsequent experiments. To verify the release of tag DNA upon heating, antibody-CompDNA-AuNPs prior to tag DNA hybridization and post tag DNA hybridization were compared. The supernatant of pre-hybridized conjugates after heating revealed that a small amount of Comp DNA had been released from the AuNP, approximately 9 ± 1.3 strands per AuNP. The post-hybridized supernatant had a much higher concentration of DNA. After subtracting out the absorbance from the Comp DNA that was released, it was determined that approximately 38 ± 0.9 strands of tag DNA per AuNP was released upon heating. Additionally, the 260/280 absorbance ratio of the samples remained unchanged, assuring that the difference between samples was not due to the release of coupled antibodies.
Figure 3.
Number of strands of tag DNA bound per gold nanoparticle versus number of strands of tag DNA added per nanoparticle. Data shown as the mean ± s.d. (n = 3).
QCM Validation of Antibody-Gold Nanoparticle Attachment
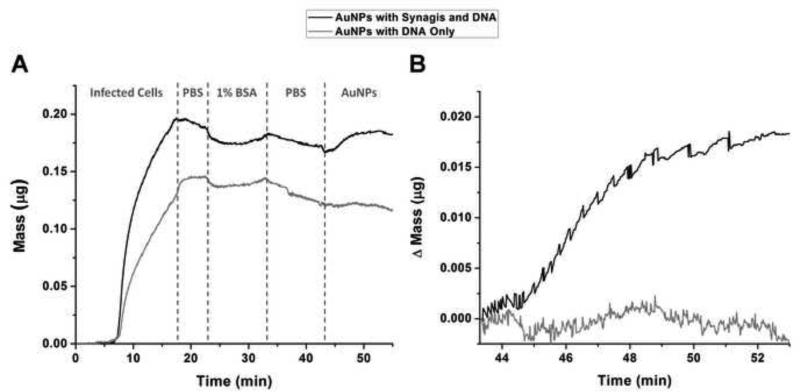
Upon functionalizing AuNPs with F-mix antibodies, their affinity for RSV greatly increases as compared to AuNPs conjugated to DNA alone (Figure 4). RSV was bound via hydrophobic interactions to the gold electrode of a quartz crystal microbalance (QCM). This was followed by washing and blocking steps. AuNPs functionalized with antibodies and DNA were then flowed over the crystal and into contact with any bound RSV. The binding of AuNPs to RSV resulted in a change of the crystal's resonance frequency. The degree of this change in frequency was used to determine the amount of mass bound to the crystal (Figure 4A). When antibody-DNA-AuNPs flowed along the crystal, binding occurred and can be observed by the 0.19 μg increase in mass detected by the microbalance (Figure 4B). However when DNAAuNPs which were not functionalized with antibodies were flowed along the crystal, no significant change in mass was observed (Figure 4B). Although the initial loading of cells on the quartz crystal would ideally be equal between experiments, it is not always possible to achieve when using two different crystals to allow the experiment to be run in parallel on the same QCM. In the QCM validation studies, the channel on which DNA-AuNPs were flowed had less binding of infected cells; however, this did not affect the outcome of the results. If the results are normalized to the mass of infected cells bound, the addition of antibody-DNA-AuNPs give an increase of 9.93% while DNA-AuNPs actually give a decrease of 0.26%. By comparing the addition of AuNPs with conjugated antibodies and DNA to the addition of AuNPs with DNA only, it is apparent that not only do antibodies coupled to the AuNP facilitate binding to RSV but also that AuNPs with DNA alone have very little non-specific binding to RSV, as evident by no change in mass under control conditions.
Figure 4.
Mass change of RSV exposed to AuNPs functionalized with either DNA alone (gray) or DNA and Synagis antibodies (black). (A) Complete time course of experiment showing total mass bound upon flowing RSV onto the quartz crystal, followed by a PBS wash, blocking with 1% BSA, a second PBS wash, and finally functionalized AuNPs. (B) Partial time course of the same experiment comparing the change in mass upon flowing co-functionalized AuNPs or tag DNA functionalized AuNPs over RSV infected cells bound to a QCM chip.
Nanoparticle amplified immuno-PCR Detection of RSV
Nanoparticle amplified immuno-PCR delivered >10,000 times more tag DNA to PCR analysis in RSV infected samples than uninfected control samples. Evaluation of the NPA-IPCR assay was performed on RSV infected HEp-2 cell lysates and compared to uninfected HEp-2 cell lysates and PBS (Figure 5). There is a distinct shift in cycle threshold (Ct) between infected cells and both control samples. The RSV samples, 8.3 × 106 PFU/mL, had an average Ct value of 8.49 while uninfected cells and PBS were much later, 21.9 and 22.1 respectively. Additionally, it is important to note that both the uninfected cells and PBS have amplification; however, they cross the cycle threshold later because there is a lower initial concentration of tag DNA present in the uninfected and PBS samples. Other have noted that this inherent background in I-PCR assays limits the sensitivity of this approach [40] and [41].
Figure 5.
RSV detection via nanoparticle amplified immuno-PCR. (A) Real-time PCR results of NPA-IPCR performed on RSV-infected HEp-2 cell lysates, uninfected HEp-2 cell lysates and PBS. (B) Comparison of the cycle thresholds of the same experiment. Data shown as the mean ± s.d. (n = 3).
To determine the lower limit of detection for NPA-IPCR, infected cells lysates were serially diluted and then assayed. The same cell lysate stock was serial diluted and assayed via RT-PCR and ELISA. The limit of detection was then determined using the assay's linear range to calculate 3 standard deviations above background, an uninfected cell lysate stock. ELISA was performed using the same antibodies as NPA-IPCR, to offer a direct comparison highlighting the inherent amplification potential of NPA-IPCR. RT-PCR was performed using primers that target the N-gene because it is preferentially transcribed due to its location near the promoter; resulting in higher copy numbers than other transcripts [20]. In order to compare all three methods, the signal generated from each was normalized (Figure 6). The normalized signal is obtained by subtracting out noise of the assay and dividing each value by the response obtained at the highest virus concentration. Both NPA-IPCR and RT-PCR have a substantially lower limit of detection than ELISA. The lower limit of detection for ELISA was 16,000 PFU/mL, while RT-PCR was 17.9 PFU/mL and NPA-IPCR was 4.1 PFU/mL. The limits of detection obtained for ELISA and RT-PCR in this study closely agree with previous studies and literature precedent (17,500 PFU/mL for ELISA and 14.13 PFU/mL for RT-PCR) [42] and [20].
Figure 6.
Detection of decreasing concentrations of RSV. (A) Comparison of RSV detection using ELISA, real-time RT-PCR and the developed NPA-IPCR assay. (B) The number of standard deviations above background using both RT-PCR and NPA-IPCR at low virus concentrations. The gray dashed line shows a 3 limit of detection. Data shown as the mean ± s.d. (n = 3). NPA-IPCR data has been shifted prevent overlapping with RT-PCR data.
Although the dilution curves for NPA-IPCR and RT-PCR appear similar, the lower dilutions, shown in Figure 6B, provide insight in determining why NPA-IPCR has a lower limit of detection than RT-PCR. At lower virus concentrations, ≤ 8.3 PFU/mL, RT-PCR has more variation between replicates. While both assays give an observable shift at 8.3 PFU/mL, only the NPA-IPCR is statistically above the limit of detection. The variability between replicates is due in part to the amount of amplification that is required to reach the cycle threshold, but may also be due to other properties of PCR [43]. At a concentration of 8.3 PFU/mL, RT-PCR requires 29.1 cycles to reach the threshold while NPA-IPCR requires only 19.4 cycles. NPA-IPCR effectively moved the low concentration dilutions up 10 cycles, in turn reducing the amount of PCR-induced variation between replicates.
Although the NPA-IPCR assay offered a lower limit of detection than RT-PCR, the 4-fold improvement was less than what was theoretically expected. Given the abundance of protein over RNA coupled with the tag DNA valency used in the NPA-IPCR assay, a theoretical improvement of 500-fold could be expected. However, in all I-PCR based assays, DNA tags are added to all samples, even negative ones. This, in turn, results in a background signal which can eclipse the signal of low concentration samples. Even though, after thorough washing, the amount of background DNA is low, PCR amplifies this signal. Steps to further reduce the background signal could lead to further improvement in the limit of detection of the NPA-IPCR assay.
From a practical standpoint, the NPA-IPCR-based assays were comparable in the amount of time it took to perform either RT-PCR or ELISA. RT-PCR required RNA isolation and reverse-transcription, which took approximately 1 hr to complete, prior to performing PCR which took approximately 2 hr to setup and perform. NPA-IPCR required viral capture and labeling, which took approximately 2 hr to complete, prior to performing PCR which took approximately 2 hr to setup and perform. For all NPA-IPCR assays, a capture and labeling time of 1 hr was used to keep the assay comparable to the equivalent ELISA steps. However, it is possible that these times could be reduced in the NPA-IPCR assays because a solution-based capture and labeling strategy is being used as opposed to the surface-based approach used in ELISA.
Conclusion
We report the development of a nanoparticle amplified immuno-PCR assay which offers highly sensitive detection of respiratory syncytial virus in HEp-2 cell lysates. The assay has a limit of detection better than RT-PCR detection of the RSV N-gene. The NPA-IPCR assay also has the potential to be improved to further lower the limit of detection. Larger AuNPs, such as 30 nm AuNPs, can be utilized to increase the surface area available for attaching tag DNA. More strands of tag DNA per AuNP would increase the number of tags released per binding event, increasing the pre-PCR amplification of the NPA-IPCR assay. The use of functionalized microparticles for virus extraction not only provides better analyte sampling, but also more thorough washing to help address the inherent background associated with I-PCR assays. Additionally, the dual-amplification approach reduces the effect of PCR-induced variation in sample replicates by increasing the concentration of tag DNA which provides a more robust PCR reaction [43]. In turn, the dual-amplification approach lowers the limit of detection of the assay by making low concentrations of antigen detectable with greater confidence. The NPA-IPCR assay holds promise as a highly sensitive diagnostic.
Acknowledgments
This work was financially supported by the NIH (EB009235) and the Vanderbilt University Discovery Grant.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Robert CW. Review of epidemiology and clinical risk factors for severe respiratory syncytial virus (RSV) infection. J. Pediatr. 2003;143:112–117. doi: 10.1067/s0022-3476(03)00508-0. [DOI] [PubMed] [Google Scholar]
- 2.Shay DK, Holman RC, Newman RD, Liu LL, Stout JW, Anderson LJ. Bronchiolitis-Associated Hospitalizations Among US Children, 1980-1996. J. Am. Med. Assoc. 1999;282:1440–1446. doi: 10.1001/jama.282.15.1440. [DOI] [PubMed] [Google Scholar]
- 3.Falsey AR, Walsh EE. Respiratory Syncytial Virus Infection in Elderly Adults. Drug Aging. 2005;22:577–587. doi: 10.2165/00002512-200522070-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Falsey AR, Hennessey PA, Formica MA, Cox C, Walsh EE. Respiratory Syncytial Virus Infection in Elderly and High-Risk Adults. N. Engl. J. Med. 2005;352:1749–1759. doi: 10.1056/NEJMoa043951. [DOI] [PubMed] [Google Scholar]
- 5.Hall CB. Respiratory Syncytial Virus and Parainfluenza Virus. N. Engl. J. Med. 2001;344:1917–1928. doi: 10.1056/NEJM200106213442507. [DOI] [PubMed] [Google Scholar]
- 6.Moore E, Barber J, Tripp R. Respiratory syncytial virus (RSV) attachment and nonstructural proteins modify the type I interferon response associated with suppressor of cytokine signaling (SOCS) proteins and IFN-stimulated gene-15 (ISG15) Virol. J. 2008;5:116. doi: 10.1186/1743-422X-5-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tripp RA. Pathogenesis of Respiratory Syncytial Virus Infection. Viral Immunol. 2004;17:165–181. doi: 10.1089/0882824041310513. [DOI] [PubMed] [Google Scholar]
- 8.Chin J, Magoffin RL, Shearer LA, Schieble JH, Lennette EH. Field evaluation of a respiratory syncytial virus vaccine and a trivalent parainfluenza virus vaccine in a pediatric population. Am. J. Epidemiol. 1969;89:449–463. doi: 10.1093/oxfordjournals.aje.a120957. [DOI] [PubMed] [Google Scholar]
- 9.Kapikian AZ, Mitchell RH, Chanock RM, Shvedoff RA, Stewart CE. An epidemiologic study of altered clinical reactivity to respiratory syncytial (RS) virus infection in children previously vaccinated with an inactivated RS virus vaccine. Am. J. Epidemiol. 1969;89:405–421. doi: 10.1093/oxfordjournals.aje.a120954. [DOI] [PubMed] [Google Scholar]
- 10.Kim HW, Canchola JG, Brandt CD, Pyles G, Chanock RM, Jensen K, Parrott RH. Respiratory syncytial virus disease in infants despite prior administration of antigenic inactivated vaccine. Am. J. Epidemiol. 1969;89:422–434. doi: 10.1093/oxfordjournals.aje.a120955. [DOI] [PubMed] [Google Scholar]
- 11.Kaur J, Tang RS, Spaete RR, Schickli JH. Optimization of plasmid-only rescue of highly attenuated and temperature-sensitive respiratory syncytial virus (RSV) vaccine candidates for human trials. J. Virol. Methods. 2008;153:196–202. doi: 10.1016/j.jviromet.2008.07.012. [DOI] [PubMed] [Google Scholar]
- 12.Prevention of Respiratory Syncytial Virus Infections: Indications for the Use of Palivizumab and Update on the Use of RSV-IGIV. Pediatrics. 1998;102:1211–1216. doi: 10.1542/peds.102.5.1211. [DOI] [PubMed] [Google Scholar]
- 13.Meissner HC, Bocchini JA, Jr., Brady MT, Hall CB, Kimberlin DW, Pickering LK. The Role of Immunoprophylaxis in the Reduction of Disease Attributable to Respiratory Syncytial Virus. Pediatrics. 2009;124:1676–1679. doi: 10.1542/peds.2009-2346. [DOI] [PubMed] [Google Scholar]
- 14.Reassessment of the Indications for Ribavirin Therapy in Respiratory Syncytial Virus Infections. Pediatrics. 1996;97:137–140. [PubMed] [Google Scholar]
- 15.Sami IR, Piazza FM, Johnson SA, Darnell MER, Ottolini MG, Hemming VG, Prince GA. Systemic Immunoprophylaxis of Nasal Respiratory Syncytial Virus Infection in Cotton Rats. J. Infect. Dis. 1995;171:440–443. doi: 10.1093/infdis/171.2.440. [DOI] [PubMed] [Google Scholar]
- 16.Kravetz HM, Knight V, Chanock RM, Morris JA, Johnson KM, Rifkind D, Utz JP. Respiratory Syncytial Virus. J. Am. Med. Assoc. 1961;176:647–667. [PubMed] [Google Scholar]
- 17.Parrott RH, Kim HW, Brandt CD, Chanock RM. Potential of attenuated respiratory syncytial virus vaccine for infants and children. Dev. Biol. Stand. 1975;28:389–399. [PubMed] [Google Scholar]
- 18.Hall CB, Douglas RG, Jr., Schnabel KC, Geiman JM. Infectivity of respiratory syncytial virus by various routes of inoculation. Infect. Immun. 1981;33:779–783. doi: 10.1128/iai.33.3.779-783.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodrich JS, Miller MB. Comparison of Cepheid's Analyte-Specific Reagents with BD Directigen for Detection of Respiratory Syncytial Virus. J. Clin. Microbiol. 2007;45:604–606. doi: 10.1128/JCM.01325-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Perkins SM, Webb DL, Torrance SA, El Saleeby C, Harrison LM, Aitken JA, Patel A, DeVincenzo JP. Comparison of a Real-Time Reverse Transcriptase PCR Assay and a Culture Technique for Quantitative Assessment of Viral Load in Children Naturally Infected with Respiratory Syncytial Virus. J. Clin. Microbiol. 2005;43:2356–2362. doi: 10.1128/JCM.43.5.2356-2362.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chomczynski P. Solubilzation in formamide protects RNA from degradation. Nucleic Acids Res. 1992;20:3791–3792. doi: 10.1093/nar/20.14.3791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hargrove JL, Schmidt FH. The role of mRNA and protein stability in gene expression. FASEB J. 1989;3:2360–2370. doi: 10.1096/fasebj.3.12.2676679. [DOI] [PubMed] [Google Scholar]
- 23.Mahony JB. Detection of Respiratory Viruses by Molecular Methods. Clin. Microbiol. Rev. 2008;21:716–747. doi: 10.1128/CMR.00037-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bentzen EL, House F, Utley TJ, Crowe JE, Wright DW. Progression of Respiratory Syncytial Virus Infection Monitored by Fluorescent Quantum Dot Probes. Nano Lett. 2005;5:591–595. doi: 10.1021/nl048073u. [DOI] [PubMed] [Google Scholar]
- 25.Adler M, Schulz S, Fischer R, Niemeyer CM. Detection of Rotavirus from stool samples using a standardized immuno-PCR (“Imperacer”) method with end-point and real-time detection. Biochem. Biophys. Res. Commun. 2005;333:1289–1294. doi: 10.1016/j.bbrc.2005.06.027. [DOI] [PubMed] [Google Scholar]
- 26.Barletta J, Bartolome A. Immuno-polymerase chain reaction as a unique molecular tool for detection of infectious agents. Expert Opin. Med. Diagn. 2007;1:267–288. doi: 10.1517/17530059.1.2.267. [DOI] [PubMed] [Google Scholar]
- 27.Sano T, Smith CL, Cantor CR. Immuno-PCR: very sensitive antigen detection by means of specific antibody-DNA conjugates. Science. 1992;258:120–122. doi: 10.1126/science.1439758. [DOI] [PubMed] [Google Scholar]
- 28.Guo YC, Zhou YF, Zhang XE, Zhang ZP, Qiao YM, Bi LJ, Wen JK, Liang MF, Zhang JB. Phage display mediated immuno-PCR. Nucleic Acids Res. 2006;34:e62. doi: 10.1093/nar/gkl260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhou H, Fisher RJ, Papas TS. Universal immuno-PCR for ultra-sensitive target proteindetection. Nucleic Acids Res. 1993;21:6038–6039. doi: 10.1093/nar/21.25.6038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wacker R, Ceyhan B, Alhorn P, Schueler D, Lang C, Niemeyer CM. Magneto Immuno-PCR: A novel immunoassay based on biogenic magnetosome nanoparticles. Biochem. Biophys. Res. Commun. 2007;357:391–396. doi: 10.1016/j.bbrc.2007.03.156. [DOI] [PubMed] [Google Scholar]
- 31.Niemeyer CM, Adler M, Pignataro B, Lenhert S, Gao S, Chi L, Fuchs H, Blohm D. Self-assembly of DNA-streptavidin nanostructures and their use as reagents in immuno-PCR. Nucleic Acids Res. 1999;27:4553–4561. doi: 10.1093/nar/27.23.4553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Braun PV. Nanoparticles: Spontaneous ligand organization. Nat. Mater. 2004;3:281–282. doi: 10.1038/nmat1125. [DOI] [PubMed] [Google Scholar]
- 33.Daniel MC, Astruc D. Gold Nanoparticles: Assembly, Supramolecular Chemistry, Quantum-Size-Related Properties, and Applications toward Biology, Catalysis, and Nanotechnology. Chem. Rev. 2003;104:293–346. doi: 10.1021/cr030698+. [DOI] [PubMed] [Google Scholar]
- 34.Hurst SJ, Lytton-Jean AK, Mirkin CA. Maximizing DNA Loading on a Range of Gold Nanoparticle Sizes. Anal. Chem. 2006;78:8313–8318. doi: 10.1021/ac0613582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Giulio FP, David GIK, Lawrence T. Colloidal gold nanoparticles: a novel nanoparticle platform for developing multifunctional tumor-targeted drug delivery vectors. Drug Dev. Res. 2006;67:47–54. [Google Scholar]
- 36.Nam JM, Thaxton CS, Mirkin CA. Nanoparticle-Based Bio-Bar Codes for the Ultrasensitive Detection of Proteins. Science. 2003;301:1884–1886. doi: 10.1126/science.1088755. [DOI] [PubMed] [Google Scholar]
- 37.Qiao FY, Liu J, Li FR, Kong XL, Zhang HL, Zhou HX. Antibody and DNA dual-labeled gold nanoparticles: Stability and reactivity. Appl. Surf. Sci. 2008;254:2941–2946. [Google Scholar]
- 38.Pompi H, Buelent C. M.N. Christof Sensitive Detection of Proteins Using Difunctional DNA-Gold Nanoparticles. Small. 2005;1:844–848. doi: 10.1002/smll.200500063. [DOI] [PubMed] [Google Scholar]
- 39.Zimmer G, Budz L, Herrler G. Proteolytic Activation of Respiratory Syncytial Virus Fusion Protein. J. Biol. Chem. 2001;276:31642–31650. doi: 10.1074/jbc.M102633200. [DOI] [PubMed] [Google Scholar]
- 40.Adler M, Wacker R, Niemeyer CM. Sensitivity by combination: immuno-PCR and related technologies. Analyst. 2008;133:702–718. doi: 10.1039/b718587c. [DOI] [PubMed] [Google Scholar]
- 41.Adler M, Gregory SM. Advances in Clinical Chemistry. Elsevier. 2005;39:239–292. [PubMed] [Google Scholar]
- 42.Perez JW, Haselton FR, Wright DW. Viral detection using DNA functionalized gold filaments. Analyst. 2009;134:1548–1553. doi: 10.1039/b904191e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stowers CC, Haselton FR, Boczko EM. An analysis of quantitative PCR reliability through replicates using the Ct method. J. Biomed. Sci. Engin. 2010;3:459–469. doi: 10.4236/jbise.2010.35064. [DOI] [PMC free article] [PubMed] [Google Scholar]