Abstract
Aims
The aim of this study was to compare long-term safety and efficacy of the basal insulin analogue degludec with glargine in insulin-naive subjects with Type 2 diabetes.
Methods
This open-label trial included a 52-week core period followed by a 52-week extension. Participants were randomized 3:1 to once-daily degludec or glargine, administered with metformin ± dipeptidyl peptidase-4 inhibitors. Basal insulin was titrated to target pre-breakfast plasma glucose 3.9–4.9 mmol/l.
Results
At end of treatment (104 weeks), mean HbA1c reductions were similar for degludec and glargine; estimated treatment difference between degludec and glargine was 1 mmol/mol (95% CI −1 to 3) [0.07% (95% CI −0.07 to 0.22)], P = 0.339 in the extension trial set (degludec 551, glargine 174), comprising subjects who completed core trial and continued into the extension trial. Overall confirmed hypoglycaemia rates (1.72 vs. 2.05 episodes/patient-year), rates of adverse events possibly or probably related to trial product (0.19 events/patient-year), weight gain (2.7 vs. 2.4 kg) and mean daily insulin doses (0.63 U/kg) were similar between treatments in the safety analysis set (degludec 766, glargine 257) comprising all treated subjects. Rates of nocturnal confirmed hypoglycaemia (0.27 vs. 0.46 episodes/patient-year; P = 0.002) and severe hypoglycaemia (0.006 vs. 0.021 episodes/patient-year, P = 0.023) were significantly lower with degludec for the safety analysis set (analysis based on intention-to-treat full analysis set comprising all randomized subjects).
Conclusions
In Type 2 diabetes, insulin degludec in combination with oral anti-diabetic drugs, safely and effectively improves long-term glycaemic control, with a significantly lower risk of nocturnal hypoglycaemia as compared with glargine.
Introduction
Insulin degludec, a new basal insulin analogue with an ultra-long duration of action 1, was compared with glargine in a 52-week, randomized, treat-to-target study (BEGIN Once Long) in insulin-naive participants with Type 2 diabetes, inadequately controlled with oral anti-diabetic drugs. Administration of degludec or glargine once daily in combination with oral anti-diabetic drugs [with all participants using metformin and < 2% using dipeptidyl peptidase-4 (DPP-4) inhibitors at end of treatment] provided similar improvements in glycaemic control, with a lower rate of nocturnal hypoglycaemia with degludec 2. The objective of the extension study reported here was to compare the long-term safety and tolerability of degludec with glargine for 104 weeks of treatment.
Patients and methods
The BEGIN Once Long study design has been previously described 2. The core trial was a 52-week randomized, controlled, parallel-group, open-label, multinational, treat-to-target, non-inferiority trial in which 1030 insulin-naive patients with Type 2 diabetes, inadequately controlled with oral anti-diabetic drugs, were randomly assigned in a 3:1 ratio to treatment with once-daily insulin degludec or once-daily insulin glargine, respectively. The insulin was administered subcutaneously in combination with metformin and a DPP-4 inhibitor (use of the latter was dependent on country-specific approved labelling that allowed combining the DPP-4 inhibitor with insulin). Subjects who completed the 52-week core trial and provided informed consent entered the 52-week extension trial maintaining prior randomization. The extension trial was conducted between 9 September 2010 and 20 December 2011 in accordance with the Declaration of Helsinki 3 and Good Clinical Practice 4. Protocols were approved by independent ethics committees/institutional review boards prior to the trials.
What’s new?
Insulin degludec, a basal insulin analogue, uses a novel protraction mechanism, resulting in a flat, stable profile and a duration of action greater than 42 h.
Consistent with its pharmacokinetic and pharmacodynamic profile, insulin degludec in combination with oral anti-diabetic drugs provided long-term glycaemic control similar to insulin glargine with a lower risk for nocturnal hypoglycaemia in insulin-naive patients with Type 2 diabetes, in a 1-year, randomized study.
This extension study reports 2-year data, confirming that insulin degludec in combination with oral anti-diabetic drugs maintains stable glycaemic control with a sustained benefit in reducing hypoglycaemic risk in Type 2 diabetes.
Insulin degludec was administered once daily with the main evening meal and glargine was administered once daily at the same time every day, as chosen by patient and investigator, as per approved labelling. In the core trial, the starting dose for both insulins was 10 Units. Basal insulin was titrated to target pre-breakfast plasma glucose of 3.9–4.9 mmol/l based on the mean of pre-breakfast self-monitored blood glucose values of the preceding two or three consecutive days. The initial insulin dose in the extension trial was to be the same as the dose at end of treatment of the core trial, but could be adjusted at investigator discretion.
To assess the immunogenicity of degludec and to minimize interference with antibody measurements, a 1-week basal insulin washout period was scheduled at end of treatment of both the 52-week core trial and the extension period, during which participants in both arms were switched to twice-daily neutral protamine Hagedorn (NPH) insulin (with a 20% reduction in total basal dose).
Safety and efficacy assessments in the extension trial were as described previously for the core trial 2. As the primary objective of the extension trial was to investigate the long-term safety and tolerability of insulin degludec, the following safety variables were the primary endpoints in the extension trial: adverse events (including injection-site reactions), hypoglycaemia episodes, insulin dose, body weight, clinical evaluations (including physical examination, vital signs, fundoscopy, electrocardiogram) and central laboratory tests (including insulin antibodies). Safety endpoints were summarized and analysed using the safety analysis set (comprising all subjects exposed to treatment). The statistical analysis of hypoglycaemia episodes and weight change observed in the safety analysis set was based on the full analysis set (comprising all randomized subjects). Hypoglycaemia was also analysed in the extension trial set (comprising completers of the core trial that continued into the extension trial) for the entire trial period and (post hoc) in the maintenance period (when the average insulin dose had stabilized, i.e. from week 16 to the end of 104 weeks of treatment). The number of hypoglycaemic episodes per patient-year were analysed by use of a negative binomial regression model that included treatment category, antecedent anti-diabetic therapy at screening, sex and region as fixed factors and age as covariate. However, severe hypoglycaemia was analysed using a Poisson regression model, as the negative binomial regression model could not be fitted to the sparse data for severe hypoglycaemia.
Efficacy variables assessed in the core and extension trial included HbA1c, central laboratory-measured fasting plasma glucose, 9-point self-monitored blood glucose profile and a questionnaire based on patient-reported outcomes. Treatment differences in the efficacy variables and in body weight were analysed using analysis of variance (ANOVA) with treatment, anti-diabetic therapy at screening, sex and region as fixed factors, and age and baseline value as covariates. In addition to analysing glycaemic efficacy in the full analysis set, the extension trial set was analysed for HbA1c and post hoc for fasting plasma glucose and self-monitored blood glucose. Baseline was defined as the time of randomization in the core trial. Post-baseline missing values were imputed using the last-observation-carried-forward method. Statistical analysis results include estimated mean treatment differences (or ratios) with their two-sided 95% confidence intervals and P-values (post hoc) for two-sided testing with an α (type I error probability) of 0.05.
Results
Of 773 participants randomized to degludec, 607 (79%) completed the core (52-week) trial; of these, 551 (71%) continued into the extension period and 505 (65%) completed the second year of study. Of 257 participants initially randomized to glargine, 197 (77%) completed the core trial; 174 (68%) of these continued into the extension period and 154 (60%) completed the 104-week trial. Participants were withdrawn in the extension period from the degludec and glargine groups because of adverse events [12 (1.6%) and five (1.9%)], ineffective therapy [three and one (0.4% each)], non-compliance [two (0.3%) and four (1.6%)], for meeting withdrawal criteria [six (0.8%) and three (1.2%), see also Supporting Information, Table S1] and ‘other’ reasons [23 (3.0%) and seven (2.7%)], with loss to follow-up being the most common ‘other’ reason. Baseline characteristics of the two treatment groups in the extension trial set were well matched and similar to those of the full analysis set (see also Supporting Information, Table S2).
Safety
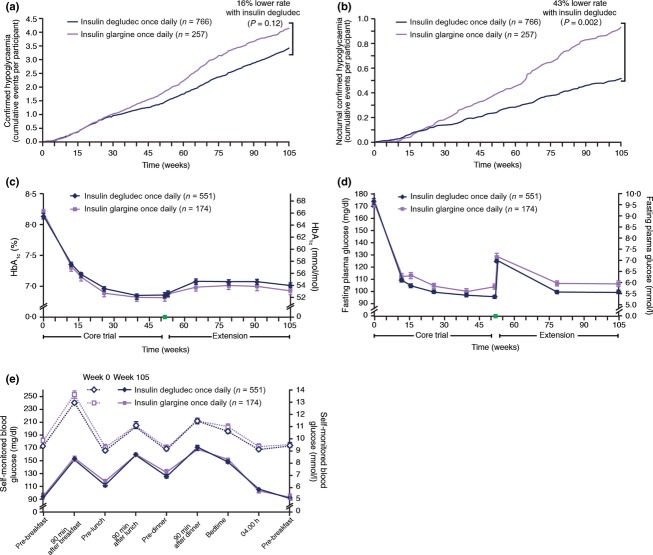
In the safety analysis set, comprising subjects receiving treatment (degludec 766, glargine 257), the mean basal insulin dose at end of treatment was identical: 0.63 (± 0.39) U/kg for degludec and 0.63 (± 0.36) U/kg for glargine. Confirmed hypoglycaemia (see Fig. 1 legend for definition) was reported by 58% and 55% of subjects treated with degludec and glargine, respectively. Overall confirmed hypoglycaemia rates were similar between degludec and glargine when considering the entire trial period [1.72 and 2.05 episodes/patient-year; estimated rate ratio of 0.84 (95% CI 0.68–1.04), P = 0.115] and maintenance period [1.80 and 2.21 episodes/patient-year; estimated rate ratio of 0.80 (95% CI 0.63–1.01), P = 0.063] (Fig. 1a and Table 1). Nocturnal confirmed hypoglycaemia was significantly lower by 43% with degludec at end of trial [0.27 vs. 0.46 episodes/patient-year; estimated rate ratio of 0.57 (95% CI 0.40–0.81), P = 0.002] and significantly lower by 53% in the maintenance period [0.28 vs. 0.53 episodes/patient-year; estimated rate ratio of 0.47 (95% CI 0.32–0.69), P < 0.001] (Fig. 1b and Table 1). The rate of severe hypoglycaemia was significantly lower with degludec than glargine when considering the entire trial period for the safety analysis set [0.006 vs. 0.021 episodes/patient-year; estimated rate ratio of 0.31 (95% CI 0.11–0.85), P = 0.023] (Table 1). The results for overall confirmed, nocturnal confirmed and severe hypoglycaemia in the extension trial set (Table 1) were consistent with those observed for the safety analysis set.
Figure 1.
Confirmed hypoglycaemia and glycaemic efficacy in the insulin degludec and glargine groups. (a) Overall confirmed hypoglycaemic episodes. (b) Nocturnal confirmed hypoglycaemic episodes. (c) HbA1c vs. time. (d) Fasting plasma glucose vs. time. The green box in (c) and (d) on the horizontal axes between weeks 52 and 53 denotes the 1-week basal insulin washout period during which participants switched to NPH and total insulin dose was reduced by 20%. (e) Nine-point profiles of self-monitored blood glucose calibrated to plasma glucose, at baseline (week 0) and after 104 weeks of treatment. Hypoglycaemia data correspond to observed data for the safety analysis set comprising all subjects exposed to treatment. Confirmed hypoglycaemic episodes included either episodes confirmed by self-monitored blood glucose corresponding to plasma glucose value < 3.1 mmol/l or severe episodes requiring assistance. Episodes occurring between 00:01 and 05:59 h (both inclusive) were classified as nocturnal. Glycaemic efficacy data are reported as the mean ± standard error of the mean (sem) for the extension trial set, comprising participants who completed the core trial and entered the extension trial. Missing post-baseline data were imputed using the last-observation-carried-forward approach. Baseline was defined as the time of randomization in the core trial.
Table 1.
Hypoglycaemic episodes in the insulin degludec and insulin glargine groups
| Safety analysis set, entire trial period | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Insulin degludec once daily (N = 766) | Insulin glargine once daily (N = 257) | Estimated rate ratio; insulin degludec/insulin glargine (95% CI)* | P-value | |||||||
| Participants | Episodes | Rate | Participants | Episodes | Rate | |||||
| n | % | n | % | |||||||
| Severe | 6 | 0.8 | 7 | 0.006 | 7 | 2.7 | 8 | 0.021 | 0.31 (0.11–0.85) | 0.023 |
| Overall confirmed | 444 | 58.0 | 2081 | 1.72 | 141 | 54.9 | 789 | 2.05 | 0.84 (0.68–1.04) | 0.115 |
| Nocturnal confirmed | 158 | 20.6 | 325 | 0.27 | 61 | 23.7 | 176 | 0.46 | 0.57 (0.40–0.81) | 0.002 |
| Subjects in the safety analysis set with at least 16 weeks of exposure, maintenance period (16 weeks to end of treatment) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Insulin degludec once daily (N = 685) | Insulin glargine once daily (N = 226) | Estimated rate ratio; insulin degludec/insulin glargine (95% CI)* | P-value | |||||||
| Participants | Episodes | Rate | Participants | Episodes | Rate | |||||
| n | % | n | % | |||||||
| Severe | 6 | 0.9 | 7 | 0.007 | 5 | 2.2 | 6 | 0.019 | 0.42 (0.14–1.26) | 0.122 |
| Overall confirmed | 389 | 56.8 | 1777 | 1.80 | 125 | 55.3 | 687 | 2.21 | 0.80 (0.63–1.01) | 0.063 |
| Nocturnal confirmed | 138 | 20.1 | 274 | 0.28 | 55 | 24.3 | 164 | 0.53 | 0.47 (0.32–0.69) | < 0.001 |
| Extension trial set, entire trial period | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Insulin degludec once daily (N = 551) | Insulin glargine once daily (N = 174) | Estimated rate ratio; insulin degludec/insulin glargine (95% CI)† | P-value | |||||||
| Participants | Episodes | Rate | Participants | Episodes | Rate | |||||
| n | % | n | % | |||||||
| Severe | 6 | 1.1 | 7 | 0.006 | 6 | 3.4 | 7 | 0.021 | 0.33 (0.12–0.96) | 0.042 |
| Overall confirmed | 376 | 68.2 | 1903 | 1.74 | 120 | 69.0 | 701 | 2.06 | 0.83 (0.65–1.05) | 0.121 |
| Nocturnal confirmed | 137 | 24.9 | 290 | 0.27 | 53 | 30.5 | 144 | 0.42 | 0.58 (0.39–0.86) | 0.002 |
Hypoglycaemic episodes (severe, overall confirmed and nocturnal confirmed) occurring on or after the first day of exposure to treatment and no later than 7 days after the last day of treatment with insulin degludec or insulin glargine are included for all exposed subjects in the safety analysis set and for subjects completing the core trial that continued into the extension trial in the extension trial set. Hypoglycaemic episodes occurring in subjects with at least 16 weeks of exposure to treatment (i.e. from week 16 onwards to the end of 104 weeks of treatment) are included in the maintenance period.
Statistical analysis based on the full analysis set comprising all randomized subjects.
Statistical analysis based on the extension trial set.
N, subjects contributing to analysis; n, number of participants with hypoglycaemic episodes; Rate, episodes per patient-year of exposure;
%, proportion of participants with hypoglycaemic episodes.
At end of treatment, 81% of degludec-treated and 77% of glargine-treated subjects reported an adverse event; 96% of events were mild or moderate. The rate of adverse events possibly or probably related to insulin was similar for both degludec and glargine (0.19 events per patient-year). The most frequently reported adverse events in both treatment groups were nasopharyngitis, headache and diarrhoea. Serious adverse events were reported by 15.1 and 16.0% of subjects in the degludec and glargine treatment groups, respectively. The rate of serious adverse events was low; 0.15 (degludec) and 0.17 (glargine) events per patient-year. The most frequently reported serious adverse events were cardiac disorders (0.05 events per patient-year in each group). The rate of serious adverse events judged to be possibly or probably related to treatment by the investigator was the same in both treatment groups (0.01 events per patient-year). The rate of injection-site reactions was similar in the degludec and glargine groups (0.07 and 0.08 events per patient-year, respectively). Observed mean weight gain at end of treatment was similar with degludec (2.7 kg) and glargine (2.4 kg); estimated treatment difference of 0.37 kg (95% CI −0.35 to 1.10), P = 0.31.
One death in each group, both considered unrelated to treatment, was reported during the main trial 2. Seven additional deaths were reported in the extension trial: four in the degludec group (small-cell lung cancer, pseudomembranous colitis/large intestine perforation/multiple organ failure, rectal cancer and death as the result of an unknown cause) and three in the glargine group (motor vehicle accident, myocardial infarction and non-treatment-emergent cardiac arrest). Four of nine deaths were major adverse cardiovascular events: death by unknown cause and sudden cardiac death in the degludec group; cardiac arrest and myocardial infarction in the glargine group. The rate of major adverse cardiovascular events was 0.03 (degludec) and 0.01 (glargine) events per patient-year (not significant). No clinically relevant differences in other safety assessments were observed between the treatment groups.
Immunogenicity of insulin degludec, assayed by degludec-specific antibodies (median = 0% bound/total radioactivity) and antibodies cross-reacting between degludec and human insulin (median = 0% bound/total radioactivity), was low throughout treatment 2.
Efficacy
In the extension trial set, after 104 weeks of treatment, the observed mean (sd) HbA1c decreased from 65 ± 9 mmol/mol (8.1 ± 0.8%) at baseline to 53 ± 10 mmol/mol (7.0 ± 0.9%) with degludec and from 66 ± 9 mmol/mol (8.2 ± 0.8%) at baseline to 52 ± 9 mmol/mol (6.9 ± 0.8%) with glargine, and there was no statistical difference between treatments; estimated treatment difference of 1 mmol/mol (95% CI −1 to 3) [0.07% (95% CI −0.07 to 0.22), P = 0.339] (Fig. 1c). Similar results were obtained in analyses of the full analysis set: estimated treatment difference of 1 mmol/mol (95% CI 0–3) [0.12% (95% CI −0.01 to 0.25), P = 0.078]. In the extension trial set, laboratory-measured fasting plasma glucose decreased rapidly in the first 12 weeks and did not increase over the remainder of the 52-week core trial (Fig. 1d). The fasting plasma glucose increased abruptly during the 1-week basal insulin washout period (occurring between the core and extension trial), when participants switched to NPH and reduced their total daily insulin dose by 20%. Observed mean (sd) fasting plasma glucose decreased from 9.66 ± 2.37 mmol/l at baseline to 5.56 ± 1.82 mmol/l at end of treatment with degludec, and from 9.53 ± 2.36 mmol/l to 5.93 ± 1.69 mmol/l with glargine (Fig. 1d). These values remained above the target fasting plasma glucose to which insulin was titrated. The observed mean reduction in laboratory-measured fasting plasma glucose was significantly greater with degludec (4.17 mmol/l) than with glargine (3.56 mmol/l) [estimated treatment difference −0.36 mmol/l (95% CI −0.67 to −0.05), P = 0.021] (Fig. 1d). Similar results were seen for the full analysis set [estimated treatment difference −0.38 mmol/l (95% CI −0.70 to −0.06), P = 0.019]. The 9-point self-monitored blood glucose profiles were similar at baseline and at end of treatment for both treatments in the extension trial set (Fig. 1e) and the full analysis set (data not shown), with no significant difference in prandial increments.
Discussion
The 2-year exposure to insulin degludec or glargine in previously insulin-naive patients with Type 2 diabetes provided an opportunity to compare overall safety of degludec with that of glargine, while evaluating long-term glycaemic efficacy.
The principal findings of the 52-week, core trial 2, namely that degludec provides improved glycaemic control similar to glargine (similar HbA1c reduction with greater reduction in fasting plasma glucose), using similar insulin doses, with lower rates of nocturnal and severe hypoglycaemia and similar weight gain, were sustained for a full 104 weeks of treatment. Although rates of diurnal confirmed hypoglycaemia (confirmed hypoglycaemia occurring between 06:00 and 00:00 h) were similar between treatments, insulin degludec showed a consistently and significantly lower rate of nocturnal hypoglycaemia as compared with glargine, and this difference widened over the course of 2 years (P < 0.01). The lower rate of nocturnal hypoglycaemia can be attributed to the flat pharmacokinetic and pharmacodynamic profile of insulin degludec combined with the reduced variability within subjects between days and also between subjects 5,6.
The degludec phase 3 programme was a global clinical development programme, and the window of time for defining nocturnal hypoglycaemia was chosen, taking into consideration the variability of timing of meals around the world to ensure that the period chosen was truly nocturnal and not confounded by meal ingestion or use of bolus insulin (relevant for trials in the degludec development programme using basal–bolus insulin therapy). Thus, all analyses of nocturnal hypoglycaemia in the clinical development programme for insulin degludec were carried out according to the definition set a priori for nocturnal hypoglycaemia— hypoglycaemic episodes occurring between 00:01 and 05:59 h (both inclusive). The relevance of the (absolute) difference in hypoglycaemia rates between the two treatments in this study is apparent when considering the clinical impact. Only five patients with Type 2 diabetes need to be treated for a year with insulin degludec to observe the benefit of avoiding one nocturnal confirmed hypoglycaemic episode compared with glargine as the treatment alternative—that is, treating 100 patients for a year with insulin degludec would result in 19 fewer episodes of nocturnal confirmed hypoglycaemia compared with insulin glargine treatment.
The sustained treatment benefits of degludec are important to the management of diabetes, a chronic disease, and in potentially lowering the risk of diabetic complications. As hypoglycaemia and fear of hypoglycaemia are two of the major barriers to optimizing glycaemic control 7,8, insulin degludec appears to provide a significantly improved clinical response in patients with Type 2 diabetes.
Abstract presentation
An abstract including some of the study results was accepted for presentation in a poster (B. PS 074) at the 48th Annual Meeting of the European Association for Study of Diabetes held in Berlin, Germany, 1−5 October 2012: Rodbard et al. Reduced nocturnal hypoglycaemia with insulin degludec as compared to insulin glargine: results of a 2-year randomized trial in type 2 diabetes. Diabetologia 2012; 55: S378.
Funding sources
The study was sponsored by Novo Nordisk. Novo Nordisk contributed to study design and conduct, data collection, analysis and interpretation.
Competing interests
HWR has received grants/research support from Amylin, Eli Lilly, Merck and Sanofi-Aventis; served as a consultant for Biodel; served on advisory panels of Amylin Pharmaceuticals, Roche Diagnostics, AstraZeneca, Biodel, Novartis, Novo Nordisk, Janssen, Bristol-Myers Squibb and Sanofi-Aventis; served on the speaker’s bureau for Amylin Pharmaceuticals, AstraZeneca, Bristol-Myers Squibb, Boehringer Ingelheim, Eli Lilly, Merck and Co., and Novo Nordisk. BC has served on the advisory panel for Eli Lilly and Company, Genfit, Novo Nordisk and Sanofi-Aventis, and received grants/research support from Novo Nordisk and Sanofi-Aventis. BZ has served on the advisory panel for Eli Lilly and Company, Novo Nordisk and Sanofi-Aventis and received research support from Novo Nordisk. YH has received grants/research support from Boehringer Ingelheim, ConjuChem, Daiichi-Sankyo, GlaxoSmithKline, Lexicon, Novo Nordisk, Takeda, Sanofi-Aventis, Xoma and Tolerx; served as consultant for Amylin Pharmaceuticals, Daiichi-Sankyo, Gilead, Genentech, GlaxoSmithKline, Novo Nordisk, Merck, Xoma, Tolerx, Janssen, Halozyme, Amarin, Liposcience and Santarus; served on speakers’ bureaus for Amylin, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Daiichi-Sankyo, Eli Lilly, GlaxoSmithKline, Novo Nordisk, and Santarus; and is President of the American Association of Clinical Endocrinologists. AP-T has served on the advisory panel for Novo Nordisk, Merck and Co., Sanofi-Aventis, and Amylin Pharmaceuticals, and has received research support from Takeda Pharmaceutical, Merck and Co., Sankyo, Novo Nordisk, Sanofi-Aventis, Eli Lilly and Company, Amylin Pharmaceuticals, AstraZeneca LP and Pfizer. TVS is an employee of Novo Nordisk and owns stock in the company. AR is an employee of Novo Nordisk and owns stock in the company. CM has served on the advisory panel for Novo Nordisk, Sanofi-Aventis, Merck Sharp and Dohme Ltd., Eli Lilly and Company, Novartis, Bristol-Myers Squibb, AstraZeneca LP, Pfizer, Johnson and Johnson and Mannkind; has received research support from Novo Nordisk, Sanofi-Aventis, Merck Sharp and Dohme Ltd., Eli Lilly and Company, and Novartis; and has served on the speakers’ bureau for Novo Nordisk, Sanofi-Aventis, Merck Sharp and Dohme, Eli Lilly and Company and Novartis.
Acknowledgments
The authors would like to thank all the investigators, trial staff and participants. The authors also thank Pei-Ling Chu, an employee of Novo Nordisk Inc., Princeton, USA for statistical analyses, Abha Chandra, an employee of Novo Nordisk Inc., Princeton, USA, for providing medical writing assistance, and Watermeadow Medical (sponsored by Novo Nordisk) for preparation of figures and submission assistance.
Supporting Information
Additional Supporting Information may be found in the online version of this article:
Table S1. Participants meeting withdrawal criteria in extension study.
Table S2. Demographic and baseline characteristics.
Appendix S1. List of investigators in the BEGIN™ Once Long study.
References
- Jonassen I, Havelund S, Hoeg-Jensen T, Steensgaard DB, Wahlund PO, Ribel U. Design of the novel protraction mechanism of insulin degludec, an ultra-long-acting basal insulin. Pharm Res. 2012;29:2104–2114. doi: 10.1007/s11095-012-0739-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zinman B, Philis-Tsimikas A, Cariou B, Handelsman Y, Rodbard HW, Johansen T, et al. Insulin degludec versus insulin glargine in insulin-naive patients with type 2 diabetes: a 1-year, randomized, treat-to-target trial (BEGIN Once Long) Diabetes Care. 2012;35:2464–2471. doi: 10.2337/dc12-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Declaration of Helsinki. Ethical principles for medical research involving human subjects. J Indian Med Assoc. 2009;107:403–405. [PubMed] [Google Scholar]
- ICH Harmonised Tripartite Guideline. Guideline for good clinical practice. J Postgrad Med. 2001;47:199–203. [PubMed] [Google Scholar]
- Heise T, Nosek L, Bottcher SG, Hastrup H, Haahr H. Ultra-long-acting insulin degludec has a flat and stable glucose-lowering effect in type 2 diabetes. Diabetes Obes Metab. 2012;14:944–950. doi: 10.1111/j.1463-1326.2012.01638.x. [DOI] [PubMed] [Google Scholar]
- Heise T, Hermanski L, Nosek L, Feldman A, Rasmussen S, Haahr H. Insulin degludec: four times lower pharmacodynamic variability than insulin glargine under steady-state conditions in type 1 diabetes. Diabetes Obes Metab. 2012;14:859–864. doi: 10.1111/j.1463-1326.2012.01627.x. [DOI] [PubMed] [Google Scholar]
- Cryer PE. Hypoglycaemia: the limiting factor in the glycaemic management of Type I and Type II diabetes. Diabetologia. 2002;45:937–948. doi: 10.1007/s00125-002-0822-9. [DOI] [PubMed] [Google Scholar]
- Jabbour S. Primary care physicians and insulin initiation: multiple barriers, lack of knowledge or both? Int J Clin Pract. 2008;62:845–847. doi: 10.1111/j.1742-1241.2008.01757.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Participants meeting withdrawal criteria in extension study.
Table S2. Demographic and baseline characteristics.
Appendix S1. List of investigators in the BEGIN™ Once Long study.