Abstract
Purpose
Present study assessed the influence of gallate esterification on the anti-cancer activity of procyanidin B2 (B2) in androgen-dependent human prostate carcinoma LNCaP cells employing B2-3,3'-di-O-gallate (B2-G2), two mono-gallate esters B2-3-O-gallate (B2-3G) and B2-3’-O-gallate (B2-3’G) and the parent compound B2, all isolated from grape seed extract (GSE).
Materials and Methods
Study compounds were isolated from GSE by several chromatographic steps and structures determined by a combination of enzymatic hydrolysis, mass spectrometry and comparisons with standards. Cells, treated with these compounds, were assessed for viability and apoptosis and examined by western blotting.
Results
Gallate esters B2-G2, B2-3G and B2-3’G significantly decreased LNCaP cell viability; however, B2 and gallic acid were ineffective. Furthermore, only B2-G2 also significantly decreased cell growth. Decreases in cell viability were largely due to apoptosis induction with B2-G2 and B2-3’G exhibiting comparable effects, whereas B2-3G was less effective. In mechanistic studies, B2-G2 and B2-3’G treatments caused caspases-9 and –3 and PARP cleavage, and down-regulated Bcl-2, Bcl-Xl and androgen receptor levels.
Conclusion
Together, our findings demonstrate anti-PCA efficacy of B2-G2 and suggest that a gallate ester moiety at 3’ position of procyanidin B2 contributes more extensively toward the biological activity of the di-gallate ester than esterification of position 3.
Keywords: Prostate cancer, chemoprevention, grape seed extract, procyanidins, apoptosis
INTRODUCTION
Prostate cancer (PCA) is the most common cancer in elderly men with one out of six men afflicted with this malignancy in the United States (1). According to estimates of the American Cancer Society for 2009, 192,280 new cases of PCA are expected to occur in the United States alone with 27,360 associated-deaths (2). Androgen receptor (AR) mediated signaling cascade is central to normal prostate physiology/homeostasis (3). Under pathological condition such as PCA, this signaling cascade functions as the contributory factor (3). The initial line of treatment for PCA involves limiting systemic testosterone levels which is effective initially; however, the malignancy resurfaces as an androgen-independent/hormone refractory stage of PCA (4). It has been observed, however, that despite the hormone-refractory stage, circulating levels of PSA, a downstream target of AR signaling, increase in most PCA cases suggesting that AR-dependent signaling remains functional even at this stage (5) and is critical to the progression of PCA. In spite of improvements in diagnostic and treatment strategies for PCA, prognosis remains poor at advanced stages (6) suggesting that newer strategies are still required to control PCA. Since PCA develops as a result of accumulation of various genetic and epigenetic changes over a period of years (7, 8), it could be effectively controlled by using various preventive/interventive measures. Variations in dietary patterns throughout the world are associated with higher/lower incidences of various cancers including PCA, and consumption of fruits and vegetables is particularly correlated with lower cancer incidences (9, 10). Plant-based therapies (herbal extracts) have also been used since ancient times to treat various diseases and are popular as complementary and alternative medicine for various health conditions including cancer (11–14).
Grape seed extract (GSE) is a commonly used health supplement in the United States and is rich in procyanidins (also referred to as proanthocyanidins) (15). GSE exhibits strong anti-oxidant capacity and is considered to be beneficial for treating various human health conditions (15–18). Several studies by us and others in recent years have shown the anti-cancer effects of GSE in various in vitro and in vivo models of lung, prostate, colon, skin and breast cancer (19–29); however, only recently, attempts have been made to isolate individual compounds and identify active constituents responsible for biological effects (30–32). Regarding anti-cancer and cancer preventive efficacy of individual constituents of GSE, Eng et al (33) reported that procyanidin B dimers inhibit androgen-dependent growth of aromatase-transfected MCF-7 cells and exhibit aromatase inhibitory activity in-vitro. We have shown that gallic acid, also a GSE constituent, inhibits the growth of DU145 xenograft in nude mice and prostate tumors in TRAMP mice (34, 35). We have also identified procyanidin B2-3,3'-di-O-gallate (B2-G2, Fig. 1) as a major constituent exhibiting anti-cancer efficacy against human PCA DU145 cells (36).
Fig. 1.
Structures and abbreviations of the procyanidin B2 dimer and gallate esters discussed in the text.
The findings that gallic acid and B2-G2 are active anti-PCA agents but procyanidin B2 is not (37) indicated the need for further studies to probe the effects of number and location of gallate groups on the activity of procyanidin B2. GSE is known to contain mono-gallate esters as well (37), so the goal of the present work was to isolate B2, B2-G2 and the mono-gallates, B2-3G and B2-3’G (Fig. 1) and to compare their effects in a PCA cell line. Since AR is recognized as an important molecular target in early as well as advanced stages of PCA, we used human PCA LNCaP cells that harbor functional AR, to investigate the activity of these compounds. The data obtained from these studies provide a better understanding of the role of gallate esterification on the activity of procyanidin B2 gallates and, therefore, will facilitate further studies to define the usefulness of these compounds as anti-cancer or chemopreventive agents in prostate and possibly other cancers.
MATERIALS AND METHODS
Materials
Gallic acid and Trypan blue were from Sigma-Aldrich Chemical Co. (St. Louis, MO). Vybrant Apoptosis Assay Kit 2 was from Molecular Probes (Eugene, OR). GSE was from Kikkoman Corp. (Noda City, Japan). Z-VAD.fmk was from MP Biomedicals (Solon, OH). Antibodies to cleaved poly (ADP-ribose) polymerase (PARP), cleaved caspase-3, cleaved caspase-9, Bcl-2 and anti-rabbit IgG horseradish peroxidase conjugated secondary antibody were from Cell Signaling Technology (Beverly, MA), antibody to Bcl-Xl was from Upstate Biotechnology, and anti-β-actin antibody and anti-mouse IgG horseradish peroxidase conjugated secondary antibody were from Sigma-Aldrich Chemical Co (St. Louis, MO). Antibody to androgen receptor was procured from Santa-Cruz Biotechnology, Inc. (Santa Cruz, CA).
Chromatography
Multiple chromatographic steps were utilized to isolate pure samples of the test compounds. Initially, ethyl acetate extracts of GSE (1–2 g) were subjected to size-exclusion chromatography (SEC) on a 2.5 × 40 cm column containing Toyopearl HW-40S resin (Tosoh Biosep, Montgomeryville, PA) as described in detail previously (36, 37). This column was eluted with methanol at 1.2 ml/min and monitored with a UV detector set at 280 nm. Eight fractions were collected over a total of 16 h; fractions 3–5 (5.5–10.0 h) containing B2 and B2-3G were combined, fraction 6 (10.0–11.5 h) contained B2-3’G and fraction 7 (11.5–13.5 h) contained B2-G2. This procedure also was scaled up to separate 10 g of extract on a 5.0 × 60 cm Toyopearl column eluted with methanol at 5 ml/min. Fractions from SEC were then processed by preparative HPLC on a 21.2 × 250 mm C18 (5 µ) Luna column (Phenomenex, Torrance, CA) at a flow rate of 10 ml/min. In a typical separation, 305 mg of combined fractions 3–5 yielded 15 mg of impure B2 and 14 mg of impure B2-3G. Each sample was then chromatographed by semi-preparative HPLC on a 10 × 250 mm C18 (5 µ) Luna column (Phenomenex) at a flow rate of 3 ml/min to yield 6.6 mg of pure B2 and 1.5 mg of pure B2-3G. The eluent for both HPLC procedures consisted of 0.1% trifluoroacetic acid in water (solvent A) and 0.1% trifluoroacetic acid in acetonitrile (solvent B) with a gradient from 8 % B in A raised to 20% B over 20 min, then to 50% B from 20 to 40 min and finally to 70% B from 40 to 45 min. Fraction 6 (302 mg) from SEC yielded 19 mg of impure B2-3’G from preparative HPLC and 13 mg of pure B2-3’G after semi-preparative HPLC. Likewise, SEC fraction 7 (325 mg) yielded 19 mg of impure material from preparative HPLC and 12.5 mg of pure B2-G2 after semi-preparative HPLC. The above chromatographic sequence was repeated several times to obtain sufficient material for all experiments and the purity of each compound was established (>95%) by analytical HPLC on a 4.6 × 250 mm Prodigy ODS-2 column (Phenomenex) at 1.0 ml/min with the solvent gradient described above and the detector set at 280 nm. All compounds were stored in the dark under argon at −80 °C and purity reconfirmed by HPLC prior to use.
Hydrolysis and LC-MS
The structures of the isolated compounds were determined by a combination of enzymatic hydrolysis and analysis by liquid chromatography-mass spectrometry (LC-MS). Purified samples of B2, B2-3G, B2-3’G and B2-G2 were analyzed by LC-MS on an Agilent SL LC/MSD instrument equipped with an electrospray ionization source and operated in the negative ion mode. Samples were introduced into the MS with a 1.0 × 150 mm Prodigy ODS-3 (5 µ) column (Phenomenex). The mobile phase consisted of 10 mM ammonium acetate in water (solvent A) and 10 mM ammonium acetate in acetonitrile (solvent B) utilizing a linear gradient increasing from 8 to 40% B in A over 30 min at a flow rate of 50 µL/min. The nebulizer and collision gasses were nitrogen and helium, respectively and the instrument was scanned over the range 150–1000 Da. Authentic samples of gallic acid and procyanidin B2 were purchased from Aldrich (Milwaukee, WI) and Indofine Chemical Co. (Hillsborough, NJ), respectively, and a sample of B2-G2 was available from our earlier work (36). Partial hydrolysis of B2-G2 was carried out by incubating 1 mg of the compound with Tannase (Wako Pure Chemical Industries, Osaka, Japan) in 1 mL of 0.1 M acetate buffer (pH 5.0) as described (36), except the amount of enzyme was reduced to 1 µg and the incubation conducted at room temperature to limit the extent of hydrolysis.
Cell Growth and Viability Assay
Human prostate carcinoma LNCaP cells were obtained from American Type Culture Collection (Manassas, VA). Cells were cultured in RPMI 1640 with 10% fetal bovine serum (Hyclone, Logan, UT) under standard culture conditions (37°C, 95% humidified air and 5% CO2). Cells were plated at a density of 1 × 105 cells/60 mm plates under standard culture conditions. After 36 h, cells were treated with either DMSO alone (control), or gallic acid, B2, B2-3G, B2-3’G and B2-G2 (0–50 µM) individually dissolved in DMSO. At the end of treatment time, cells were collected after brief trypsinization, washed with PBS, and then stained with Trypan blue. The total cell number (colorless and blue stained) and dead cells (blue stained) were counted under light microscope using hemocytometer.
Quantitative Apoptotic Cell Death Assay
To quantify whether the treatment of cells with these agents induce apoptotic death, at the end of the treatment protocol described above, the cells were collected and stained with annexin V and propidium iodide using Vybrant Apoptosis Assay Kit2 following the instructions provided with the kit. The extent of apoptosis was quantified by flow cytometry analysis of the stained cells as described recently (38). The extent of apoptotic death occurring after treatment with test compounds was also estimated using Cell Death Detection ELISA plus kit from Roche Diagnostics (Mannheim, Germany) in another experiment, where following treatment with test compounds for 6 h (in certain cases 2 h pre-treatment was made with caspase inhibitor Z-VAD.fmk at 50 µM), cells were collected as described for annexin V-PI staining, washed twice with ice cold PBS and then the extent of apoptotic death was measured following the procedure essentially as described in the kit.
Western Blot Analysis
LNCaP cells were treated with either DMSO alone (control) or individual compounds (B2-G2 or B2-3’G) at 25 and 50 µM concentrations for 12 h. At the end of treatment time, cells were collected with brief trypsinization, followed by two washings in ice cold PBS. The cell pellets were then suspended in non-denaturing lysis buffer (10 mM Tris-HCl, ph 7.4, 150 mM NaCl, 1% Triton X-100, 1mM EDTA, 1mM EGTA, 0.3 mM phenyl methyl sulfonylfluoride, 0.2 mM sodium orthovanadate, 0.5% NP-40, 5 units/ml aprotinin) and incubated on ice for 10 minutes. After clearing the cell lysates by centrifugation at 4°C, protein concentration was estimated using Bio-Rad DC protein assay kit (Bio-Rad, Hercules, CA). For western blotting, 80 µg of protein lysate per sample was denatured in 2X SDS–PAGE sample buffer and subjected to SDS–PAGE on 8 or 16% tris-glycine gels. The separated proteins were transferred onto nitrocellulose membrane, followed by blocking with 5% nonfat milk powder (w/v) in TBS (10 mM Tris, 100 mM NaCl, 0.1% Tween 20) for 1 h at room temperature. Membranes were probed with specific primary antibodies overnight at 4°C followed by peroxidase-conjugated appropriate secondary antibody for 1h at room temperature, and visualized by ECL detection system (GE Healthcare Bioscience). Membranes were stripped and reprobed with anti-β-actin antibody to check the equal protein loading.
Statistical Analyses
All statistical analyses were carried out with Sigma Stat software version 2.03 (Jandel Scientific, San Rafael, CA). Statistical significance of difference between the control and treated groups was determined either by unpaired Student’s t-test or one-way ANOVA followed by Bonferroni t-test where P<0.05 was considered statistically significant.
RESULTS
Isolation and structural analyses of procyanidins from GSE
GSE was separated by SEC into eight fractions as described previously (37); the compounds of interest were isolated from the respective fractions by preparative HPLC and further purified by semi-preparative HPLC as detailed in Materials and Methods. Procyanidins B2 and B2-G2 obtained in this way were identified by comparisons of their chromatographic, UV and MS properties with authentic samples, and the identities of mono-gallate esters of B2 were determined as follows.
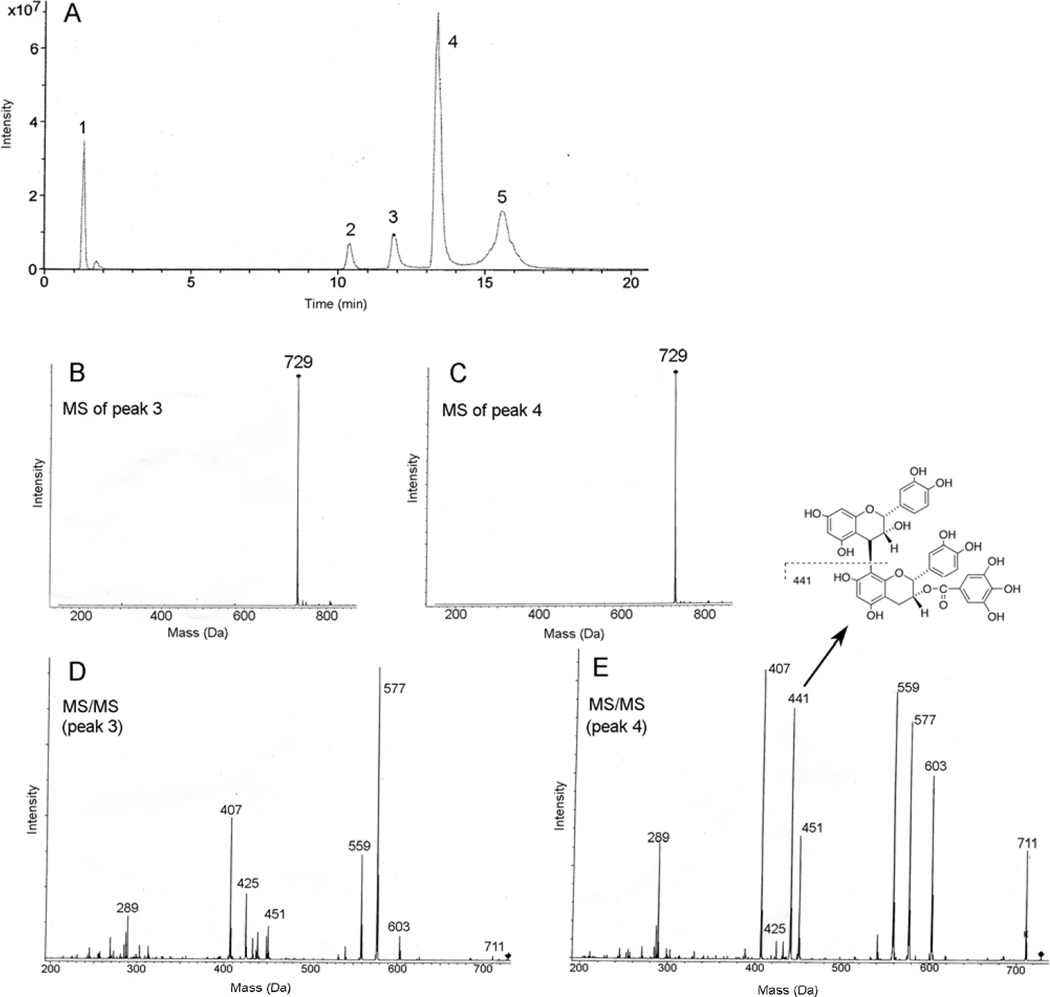
Partial enzymatic hydrolysis of B2-G2 yielded gallic acid, B2 and two mono-gallate esters shown in Fig. 2A. The mass spectra of peaks 3 and 4 both exhibited a deprotonated molecular ion (M - H)− at 729 Da (Figs. 2B and 2C, respectively), consistent with mono-gallate esters of procyanidin dimers. Collision-induced dissociation (MS/MS) of the 729 ions from each peak produced many of the same fragment ions, albeit in different relative intensities (Figs. 2D and 2E), all of which were also detected in the spectrum of B2-G2 (36). The ion that clearly differentiates B2-3G from B2-3’G occurs at 441 Da in the MS/MS spectrum from peak 4 that is not observed in the spectrum of peak 3. The 441 ion is also present in the spectrum of B2-G2 but not in the spectrum of B2 (36, 39) and is due to the lower epicatechin unit produced by cleavage of the interflavin bond with charge retention on epicatechin gallate shown in Fig. 2E. Previous MS studies of procyanidin dimers demonstrated that interflavin bond cleavage occurs with preferential charge retention on the lower unit (39, 40). These results, therefore, demonstrate that peak 3 corresponds to B2-3G and peak 4 to B2-3’G; the compounds isolated from GSE exhibited identical HPLC retention times and mass spectra as those from B2-G2 hydrolysis.
Fig. 2.
Negative ion LC-MS studies of hydrolysis products formed from B2-G2. A) Total ion current chromatogram of products from the partial enzymatic hydrolysis of B2-G2. Peaks 1, 2 and 5 correspond to gallic acid, B2 and B2G2, respectively, by comparison with authentic standards. B) MS spectrum of peak 3 and C) MS spectrum of peak 4, both demonstrating a deprotonated molecular ion (M – H)− at 729 Da. D) MS/MS spectrum of the 729 ion from peak 3. E) MS/MS spectrum of the 729 ion from peak 4. The unique ion at 441 Da corresponds to interflavin bond cleavage and charge retention the lower unit as shown.
Procyanidin B2 gallate esters reduce the viability of prostate carcinoma LNCaP cells
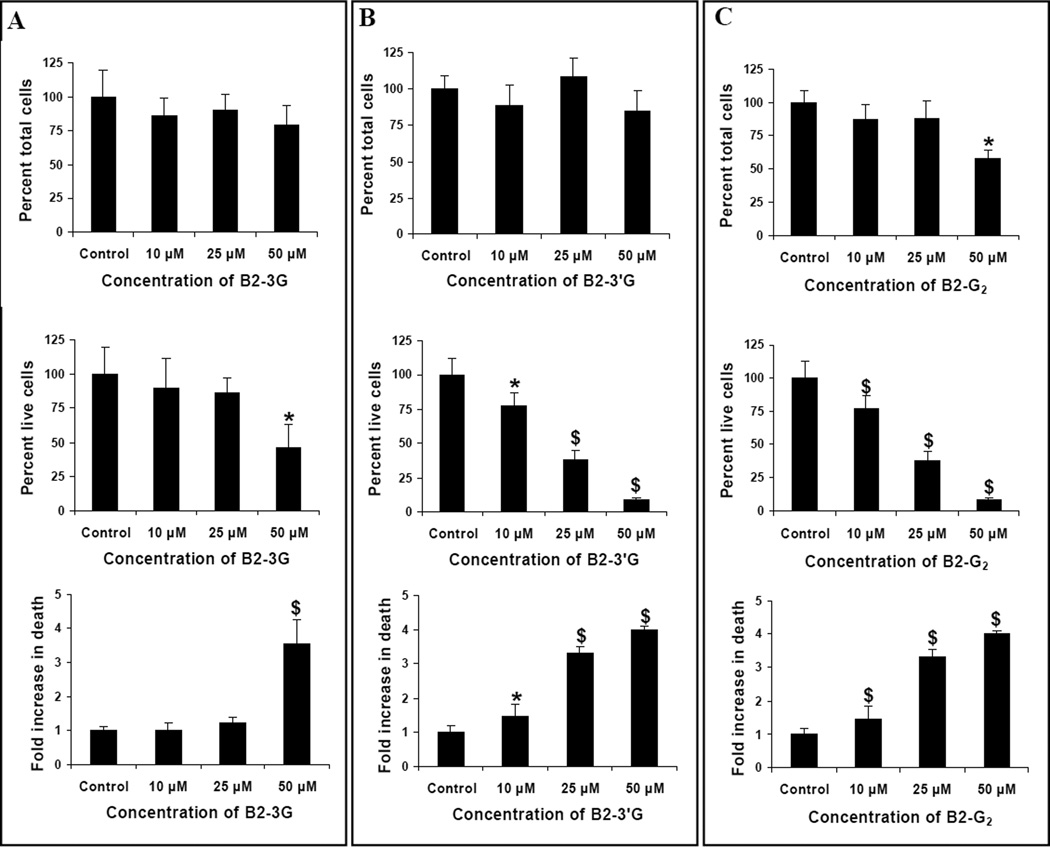
To investigate structure-activity relationships for procyanidin B2 and its gallate esters, we initially studied their effects on the viability of human prostate carcinoma LNCaP cells. LNCaP cells were treated with 10, 25 and 50 µM concentrations of B2, B2-3G, B2-3’G, B2-G2 or gallic acid for 12 h. We observed that neither B2 nor gallic acid (10–50 µM) affected the viability of these cells (data not shown). Treatment with B2-3G, B2-3’G or B2-G2 however, resulted in a decrease in cell viability to varying extents. With B2-3G, a 54% (P<0.05) decrease in the viability of LNCaP cells was observed only at a concentration of 50 µM and was accompanied by a 3.5 fold (P<0.001) increase in the percentage of dead cells relative to DMSO only treated controls. The lower concentrations of 10 and 25 µM B2-3G did not affect the viability of these cells. However, the percentage of total cell number (population) was not affected at any of the concentrations tested (Fig. 3A). With B2-3’G, the viability of LNCaP cells decreased by 23, 62 and 91% (P<0.05–0.001) at the concentrations of 10, 25 and 50 µM, respectively, after 12 h of treatment. The percentage of dead cells under similar treatment conditions increased by 1.45 fold (P<0.05) at 10 µM, 3.32 fold (P<0.001) at 25 µM and ~ 4 fold (P<0.001) at 50 µM of B2-3’G (Fig. 3B). The percentage of total cell number remained unaffected at all the concentrations tested. Treatment with B2-G2 resulted in significant decreases in the numbers of live cells with concomitant increases in the percentage of dead cells at all the test concentrations; however, the percentage of total cell number (population) decreased significantly only at 50 µM B2-G2 (P<0.01; Fig. 3C). The live cell number decreased by 40% (P<0.01) at 10 µM B2-G2; a further decrease of 68 and 92% (P<0.001) was observed at 25 and 50 µM B2-G2, respectively. A concomitant increase in the percentage of dead cells was observed at all concentrations of B2-G2 tested. At the lowest concentration of B2-G2 (10 µM), the dead cell population increased by 2.4 fold (P<0.001) over the DMSO treated controls, which further increased by ~4 fold (P<0.001) at 25 µM B2-G2 and 4.8 fold (P<0.001) at 50 µM B2-G2 after 12 h of treatment. From these results, it is clear that B2-G2 is most effective in reducing the viability of human prostate carcinoma LNCaP cells, followed by B2-3’G and lastly by B2-3G.
Fig. 3.
Comparative effect of procyanidins B2-3G, B2-3’G and B2-G2 on the growth and viability of human prostate carcinoma LNCaP cells in vitro. LNCaP cells were cultured in 60 mm culture plates for 36 h and were then treated with 0–50 µM concentrations of B2-3G (A), B2-3’G (B) and B2-G2 (C) in DMSO or DMSO alone for 12 h. At the end of treatment times, cell viability was measured by Trypan blue dye exclusion assay as described under Materials and Methods section. Data indicate mean ± SD, n=3. *, P<0.05; $, P<0.001 compared with control.
Procyanidin B2 gallates induce caspase-dependent apoptotic death in prostate carcinoma LNCaP cells
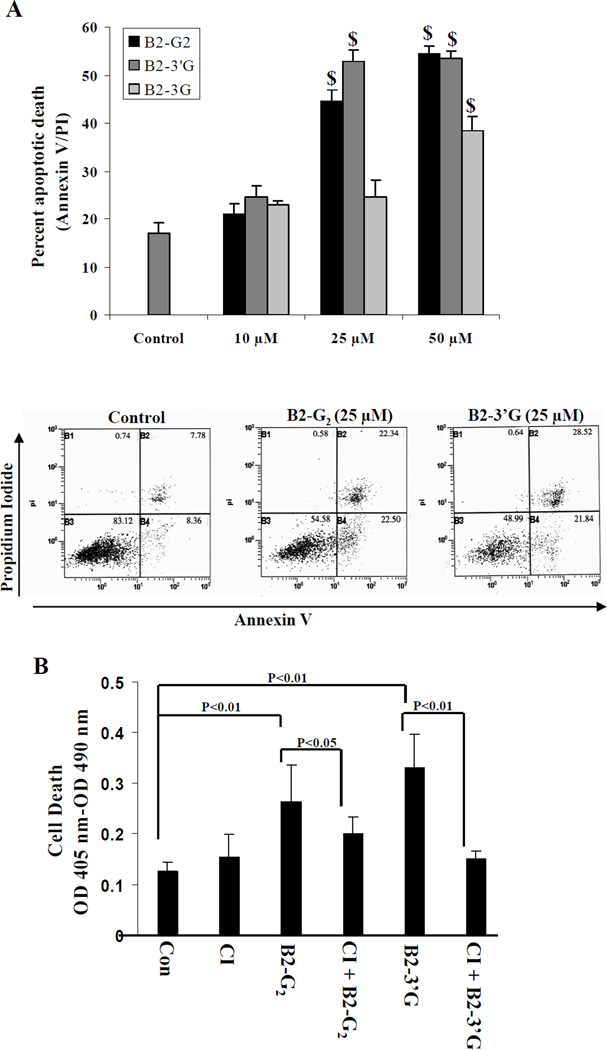
Since we observed that all the procyanidins except B2 caused significant death of LNCaP cells, we next determined the nature of death induced in these cells. In a cell viability assay, we observed that treatment of LNCaP cells with either B2-3G, B2-3’G or B2-G2 induced significant rounding up of cells and detachment from the tissue culture dishes which is similar to that observed with GSE treatment of these cells reported previously by us (36). On performing annexin V/PI staining followed by FACS analysis, we observed that these compounds caused significant apoptotic cell death (Fig. 4A). Treatment of LNCaP cells with B2-3G (0–50 µM concentrations for 12 h) resulted in a significant apoptotic death (38%, P<0.001) at 50 µM concentration only; the lower concentrations of this agent were ineffective in inducing significant apoptotic death of LNCaP cells. Treatment with B2-3’G at similar concentrations resulted in a significant apoptotic death (53%, P<0.001) at 25 µM with no further increase at 50 µM. However, treatment with B2-G2 at similar concentrations caused a significant and dose-dependent apoptotic death at 25 and 50 µM concentrations. At 25 µM concentration, 45% (P<0.001) apoptotic death was observed, which increased to 54% (P<0.001) at higher concentration of 50 µM. Collectively, we observed that B2-G2 and B2-3’G cause significant and comparable apoptosis induction, and therefore we focused further studies only on these two compounds.
Fig. 4.
Apoptosis induction in human prostate carcinoma LNCaP cells by procyanidins B2-3G, B2-3’G and B2-G2 in vitro. LNCaP cells were plated for 36 h at a density of 5000 cells per cm2 in 60 mm culture dishes. A) Cells were treated with 10, 25 and 50 µM concentrations of B2-3G, B2-3’G or B2-G2 for 12 h. At the end of treatment time, cells were collected and stained with annexin V-PI followed by flow cytometric analysis. Bars indicate mean ± SD, n=3. $, P<0.001 represents statistical significance of differences between control and test compound treated groups. Representative flow cytometric profiles are also shown below the bars for control and lower-dose agent treated cells. B) Cells were pretreated with pan caspase inhibitor (CI) Z-VAD.fmk (50 µM) for 2 h followed by treatment with 25 µM of either B2-G2 or B2-3’G for 6 h. Cells were also treated with DMSO, Z-VAD.fmk, B2-G2 or B2-3’G individually for 6 h. At the end of treatment time, the extent of apoptotic cell death was measured by using Cell Death ELISA kit as described in methods. Bars indicate mean ± SD, n=3.
In order to determine if the apoptosis induced by B2-G2 and B2-3’G occurs through caspase-dependent or -independent pathway, we pretreated the LNCaP cells with pan-caspase inhibitor Z-VAD.fmk (50 µM) for 2 h followed by treatment with either B2-G2 or B2-3’G (25 µM) for 6 h. We observed that pretreatment with Z-VAD.fmk resulted in a 24% (P<0.05) reversal of apoptotic cell death from treatment with B2-G2 and a 50% (P<0.01) reversal of cell death from B2-3’G, thereby indicating that caspases are involved in the induction of apoptosis by these compounds (Fig. 4B).
Apoptosis induction by procyanidins B2-G2 and B2-3’G involves caspases-9 and -3 and PARP cleavage and modulation of Bcl-2 family members
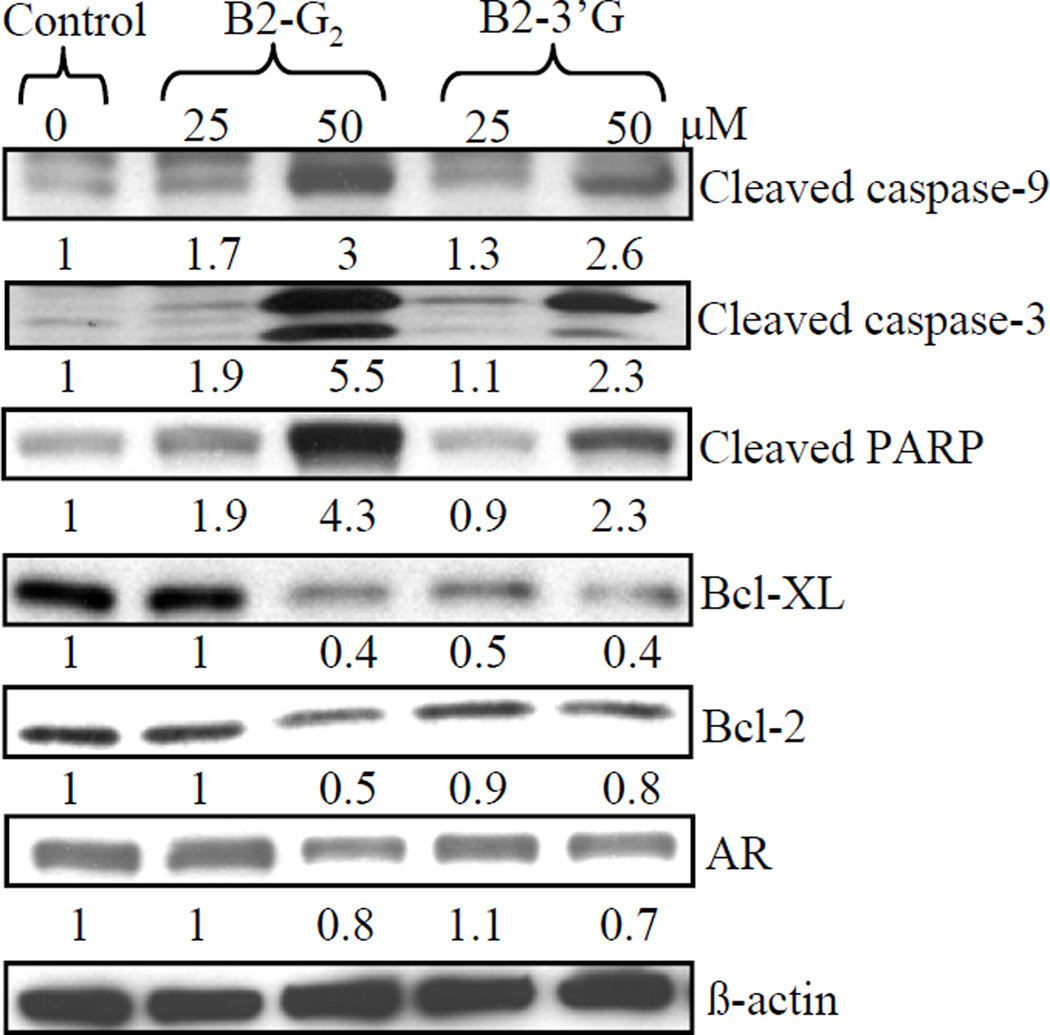
Since we observed that procyanidins B2-G2 and B2-3’G cause apoptotic death of LNCaP cells that is reversed by pan-caspases inhibitors, we further confirmed the role of caspases activation by these agents in their apoptotic activity by conducting western blot analysis for cleaved forms of caspases-9 and -3. Treatment of cells with these agents resulted in the cleavage of both caspases-9 and -3 in a concentration-dependent manner, together with the cleavage of PARP which is a downstream substrate for activated caspase-3 (Fig. 5). Next, we also studied the involvement of Bcl-2 family members in the induction of apoptosis by procyanidins B2-G2 and B2-3’G. Treatment of LNCaP cells with either procyanidin B2-G2 or B2-3’G resulted in a decrease in the levels of Bcl-2 and Bcl-Xl in a concentration-dependent manner (Fig. 5); however, no changes were observed in the levels of pro-apoptotic members such as Bax and Bad (data not shown). Further, we also observed that treatment of these cells with either procyanidin B2-G2 or B2-3’G results in a decrease in the levels of AR at higher concentration of 50 µM.
Fig. 5.
Apoptosis induction by procyanidins B2-G2 and B2-3’G is associated with caspase-9 and -3 and PARP cleavage and modulation in the levels of Bcl-2 family members. LNCaP cells were plated, left overnight and then treated with 0–50 µM of either B2-G2 or B2-3’G for 12 h. Total cell lysates were prepared and analyzed by western immunoblotting for cleaved caspase-9, -3, cleaved PARP, Bcl-xl, Bcl-2 and AR. Membranes were stripped and reprobed with β-actin as a loading control. Densitometric value shown below each band represents fold-change as compared to control after normalization with respective loading controls.
DISCUSSION
GSE has been shown to possess anti-cancer/cancer preventive efficacy against cancers of different anatomical sites in various studies (19–29). The chemical constituents of this extract (mostly procyanidins) have also been identified in numerous studies but few have reported the bioavailability of these procyanidins in the plasma of the rats and humans (30, 41). There is a lack of studies, however, to evaluate the biological activity of individual chemical constituents of GSE responsible for its various beneficial health effects. Toward this end, after demonstrating the anti-cancer/chemopreventive efficacy of GSE in animal models of prostate and colon cancer, we have also focused our efforts to identify the individual constituents responsible for its anti-cancer efficacy. Such studies are expected to contribute to the understanding of the mechanism of anti-cancer efficacy as well as to develop potential mechanism-based anti-cancer drugs. In our previous studies, we fractionated GSE into gallic acid and several constituent procyanidins by chromatographic techniques and employed reverse phase HPLC and electrospray ionization LC-MS to provide structural information. We found that gallic acid and several procyanidins had significant biological activity against human PCA DU145 cells; B2-G2 was the most effective compound (34–37). DU145 cells represent the androgen-independent stage of PCA; however, as described earlier, even at the androgen-independent stage, AR plays a significant role in the growth and progression of PCA. Therefore, in the present study we focused on the anti-cancer potential of procyanidin B2-G2 against human PCA LNCaP cells which express AR and are androgen-dependent/responsive for their growth. We also compared the efficacy of the mono-gallate esters, namely B2-3G and B2-3’G as well as gallic acid against human PCA LNCaP cells. Such studies with B2-G2 and mono-gallate esters were designed to identify the contributions of gallate ester groups to the anti-cancer efficacy of procyanidin B2-G2. Our findings clearly show that procyanidin B2 and gallic acid alone do not exhibit a growth inhibitory effect on LNCaP cells, even though gallic acid exhibited strong cytotoxic effects against human PCA DU145 cells as we reported recently, suggesting that the cytotoxic effects of gallic acid are cell specific in nature. We also found that procyanidins B2-G2, B2-3G and B2-3’G were effective in decreasing the viability of LNCaP cells, although to varying degrees. Procyanidins B2-G2 and B2-3’G exhibited similar anti-cancer efficacy. We observed that induction of apoptosis is the common phenomenon responsible for decreases in the viability of LNCaP cells by these compounds.
GSE as well as the polyphenolic constituents present therein have been shown to possess both anti and pro-oxidant activities dependent on the cellular context and the concentration. For example, B2-G2 and B2-3’G have been shown to possess anti-oxidant capacity which is higher than procyanidin B2 itself; they have been shown to exhibit potent DNA polymerase α inhibitory activity (42). However, in our previous study, we observed that reactive oxygen species production by GSE, rather than its anti-oxidant capacity, is responsible for its anti-cancer efficacy against LNCaP cells (38). These results suggest that more studies are needed in future to fully define the relative contribution of both anti and pro-oxidant activities toward the anti-cancer efficacy of these compounds. It is also important to emphasize here that we observed the biological response of these compounds at 25 µM concentration; however, whether such a level could be biologically achieved and is physiologically/pharmacologically relevant remained to be established due to a lack of sufficient evidence in literature about their bioavailability.
The activation of caspases is associated with the process of apoptosis (43) and activation of caspase-3 is a downstream event in apoptosis cascade (43). PARP is cleaved by activated caspase-3 and is studied as a marker of apoptosis (44). In addition to caspase-dependent apoptosis, the process of apoptosis also occurs through caspase-independent pathways (45). In our study, we found that B2-G2 and B2-3’G induced apoptosis involves the activation of caspase-3 with upstream activation of caspase-9 and downstream cleavage of PARP; however, more extensive studies are required in future to elucidate the involvement of intrinsic pathway of apoptosis. We also observed that apoptosis induced by these two compounds was partially reversed by pre-treatment with pan-caspase inhibitor after 6 h of treatment time, thereby implying that caspase-independent mechanisms might also be involved. Pre-treatment with pan-caspase inhibitor, however, failed to reverse significantly the apoptosis induced by both these compounds. In our study it seems that Z-VAD.fmk pre-treatment is only able to delay the process of apoptotic death rather than completely preventing it. This phenomenon has been reported previously in various systems where caspase inhibitors are not able to completely prevent apoptosis; rather they promote delayed apoptotic response to various apoptotic stimuli (46, 47). Further mechanistic studies are required in future to fully elucidate this aspect. Apoptosis is a highly regulated process and Bcl-2 family members serve as important regulators of the pathway (48). This family consists of both pro- and anti-apoptotic members where pro-apoptotic members such as Bax, Bak, Bad etc control the permeability of outer mitochondrial membrane and cause release of pro-apoptotic factors whereas anti-apoptotic members preserve the integrity of mitochondrial membranes and thus prevent apoptosis (49). In our study, we found that both B2-G2, and B2-3’G down regulate the expression of Bcl-2 and Bcl-xl without any effect on the pro-apoptotic members Bax and Bad, suggesting that anti-apoptotic members are the potential targets for apoptosis inducing efficacy by these compounds. The protein levels of AR also decreased moderately in response to treatment with these two procyanidins only at higher concentration of 50 µM. More studies are required in future to determine whether the decrease in AR levels is due to induction of apoptosis or vice versa.
Collectively, the present findings clearly show that both B2-G2 and B2-3’G have strong anti-cancer efficacy against human PCA LNCaP cells, and that the presence of a gallate moiety at the 3’ rather than the 3 position is responsible for most of the biological activity of procyanidin B2-G2. Based on these findings, further preclinical studies are warranted to fully elucidate the anti-cancer efficacy of these procyanidin gallate esters.
ACKNOWLEDGENMENTS
This study was supported by the National Cancer Institute, NIH RO1 grant CA091883.
ABBREVIATIONS
- AR
androgen receptor
- B2
procyanidin B2
- B2-G2
procyanidin B2-3,3'-di-O-gallate
- B2-3’G
procyanidin B2-3’-O-gallate
- B2-3G
procyanidin B2-3-O-gallate
- GSE
grape seed extract
- PCA
prostate cancer
- PARP
poly (ADP-ribose) polymerase
- SEC
size-exclusion chromatography
REFERENCES
- 1.Brawley OW, Ankerst DP, Thompson IM. Screening for prostate cancer. CA Cancer J Clin. 2009;59:264–273. doi: 10.3322/caac.20026. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Siegel R, Ward E, Hao Y, Xu J, Thun MJ. Cancer statistics, 2009. CA Cancer J Clin. 2009;59:225–249. doi: 10.3322/caac.20006. [DOI] [PubMed] [Google Scholar]
- 3.Nguyen MM, Wang Z. Manipulation of androgens and alterations in the androgen receptor axis in prostate cancer. Minerva Urol Nefrol. 2008;60:15–29. [PubMed] [Google Scholar]
- 4.Kawakami J, Cowan JE, Elkin EP, Latini DM, DuChane J, Carroll PR and CaPSURE Investigators. Androgen-deprivation therapy as primary treatment for localized prostate cancer:data from Cancer of the Prostate Strategic Urologic Research Endeavor (CaPSURE) Cancer. 2006;106:1708–1714. doi: 10.1002/cncr.21799. [DOI] [PubMed] [Google Scholar]
- 5.Yuan X, Balk SP. Mechanisms mediating androgen receptor reactivation after castration. Urol Oncol. 2009;27:36–41. doi: 10.1016/j.urolonc.2008.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stratton KL, Chang SS. Locally advanced prostate cancer: the role of surgical management. BJU Int. 2009;104:449–454. doi: 10.1111/j.1464-410X.2009.08741.x. [DOI] [PubMed] [Google Scholar]
- 7.Reynolds MA. Molecular alterations in prostate cancer. Cancer Lett. 2008;271(1):13–24. doi: 10.1016/j.canlet.2008.04.047. [DOI] [PubMed] [Google Scholar]
- 8.Schulz WA, Hoffmann MJ. Epigenetic mechanisms in the biology of prostate cancer. Semin Cancer Biol. 2009;19:172–180. doi: 10.1016/j.semcancer.2009.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Trichopoulou A, Lagiou P, Kuper H, Trichopoulos D. Cancer and Mediterranean dietary traditions. Cancer Epidemiol Biomarkers Prev. 2000;9:869–873. [PubMed] [Google Scholar]
- 10.Grant WB. A multicountry ecologic study of risk and risk reduction factors for prostate cancer mortality. Eur Urol. 2004;45:271–279. doi: 10.1016/j.eururo.2003.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Raskin I, Ribnicky DM, Komarnytsky S, Ilic N, Poulev A, Borisjuk N, Brinker A, Moreno DA, Ripoll C, Yakoby N, O'Neal JM, Cornwell T, Pastor I, Fridlender B. Plants and human health in the twenty-first century. Trends Biotechnol. 2002;20:522–531. doi: 10.1016/s0167-7799(02)02080-2. [DOI] [PubMed] [Google Scholar]
- 12.Dhiman RK, Chawla YK. Herbal medicines for liver diseases. Dig Dis Sci. 2005;50:1807–1812. doi: 10.1007/s10620-005-2942-9. [DOI] [PubMed] [Google Scholar]
- 13.Surh YJ. Cancer chemoprevention with dietary phytochemicals. Nat Rev Cancer. 2003;3:768–780. doi: 10.1038/nrc1189. [DOI] [PubMed] [Google Scholar]
- 14.Sagar SM, Yance D, Wong RK. Natural health products that inhibit angiogenesis: a potential source for investigational new agents to treat cancer-Part 1. Curr Oncol. 2006;13:14–26. doi: 10.3747/co.v13i1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bagchi D, Bagchi M, Stohs S, Ray SD, Sen CK, Preuss HG. Cellular protection with proanthocyanidins derived from grape seeds. Ann N Y Acad Sci. 2002;957:260–270. doi: 10.1111/j.1749-6632.2002.tb02922.x. [DOI] [PubMed] [Google Scholar]
- 16.Yoshimura Y, Nakazawa H, Yamaguchi F. Evaluation of the NO scavenging activity of procyanidin in grape seed by use of the TMA-PTIO/NOC 7 ESR system. J Agric Food Chem. 2003;51(22):6409–6412. doi: 10.1021/jf034129e. [DOI] [PubMed] [Google Scholar]
- 17.Yamaguchi F, Yoshimura Y, Nakazawa H, Ariga T. Free radical scavenging activity of grape seed extract and antioxidants by electron spin resonance spectrometry in an H(2)O(2)/NaOH/DMSO system. J Agric Food Chem. 1999;47:2544–2548. doi: 10.1021/jf9806762. [DOI] [PubMed] [Google Scholar]
- 18.Bagchi D, Garg A, Krohn RL, Bagchi M, Tran MX, Stohs SJ. Oxygen free radical scavenging abilities of vitamins C and E, and a grape seed proanthocyanidins extract in vitro. Res Commun Mol Pathol Pharmacol. 1997;95:179–189. [PubMed] [Google Scholar]
- 19.Tyagi A, Agarwal R, Agarwal C. Grape seed extract inhibits EGF-induced and constitutively active mitogenic signaling but activates JNK in human prostate carcinoma DU145 cells: possible role in antiproliferation and apoptosis. Oncogene. 2003;22:1302–1316. doi: 10.1038/sj.onc.1206265. [DOI] [PubMed] [Google Scholar]
- 20.Singh RP, Tyagi AK, Dhanalakshmi S, Agarwal R, Agarwal C. Grape seed extract inhibits advanced human prostate tumor growth and angiogenesis and upregulates insulin-like growth factor binding protein-3. Int J Cancer. 2004;108:733–740. doi: 10.1002/ijc.11620. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal C, Singh RP, Dhanalakshmi S, Agarwal R. Anti-angiogenic efficacy of grape seed extract in endothelial cells. Oncol Rep. 2004;11:681–685. [PubMed] [Google Scholar]
- 22.Kaur M, Singh RP, Gu M, Agarwal R, Agarwal C. Grape seed extract inhibits in vitro and in vivo growth of human colorectal carcinoma cells. Clin Cancer Res. 2006;12:6194–6202. doi: 10.1158/1078-0432.CCR-06-1465. [DOI] [PubMed] [Google Scholar]
- 23.Raina K, Singh RP, Agarwal R R, Agarwal C. Oral grape seed extract inhibits prostate tumor growth and progression in TRAMP mice. Cancer Res. 2007;67:5976–5982. doi: 10.1158/0008-5472.CAN-07-0295. [DOI] [PubMed] [Google Scholar]
- 24.Kijima I, Phung S, Hur G, Kwok SL, Chen S. Grape seed extract is an aromatase inhibitor and a suppressor of aromatase expression. Cancer Res. 2006;66:5960–5967. doi: 10.1158/0008-5472.CAN-06-0053. [DOI] [PubMed] [Google Scholar]
- 25.Kowalczyk MC, Walaszek Z, Kowalczyk P, Kinjo T, Hanausek M, Slaga TJ. Differential effects of several phytochemicals and their derivatives on murine keratinocytes in vitro and in vivo: implications for skin cancer prevention. Carcinogenesis. 2009;30:1008–1015. doi: 10.1093/carcin/bgp069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Singletary KW, Meline B. Effect of grape seed proanthocyanidins on colon aberrant crypts and breast tumors in a rat dual-organ tumor model. Nutr Cancer. 2001;39:252–258. doi: 10.1207/S15327914nc392_15. [DOI] [PubMed] [Google Scholar]
- 27.Nomoto H, Iigo M, Hamada H, Kojima S, Tsuda H. Chemoprevention of colorectal cancer by grape seed proanthocyanidin is accompanied by a decrease in proliferation and increase in apoptosis. Nutr Cancer. 2004;49:81–88. doi: 10.1207/s15327914nc4901_11. [DOI] [PubMed] [Google Scholar]
- 28.Ye X, Krohn RL, Liu W, Joshi SS, Kuszynski CA, McGinn TR, Bagchi M, Preuss HG, Stohs SJ, Bagchi D. The cytotoxic effects of a novel IH636 grape seed roanthocyanidin extract on cultured human cancer cells. Mol Cell Biochem. 1999;196:99–108. [PubMed] [Google Scholar]
- 29.Zhao J J, Wang J, Chen Y, Agarwal R. Anti-tumor-promoting activity of a polyphenolic fraction isolated from grape seeds in the mouse skin two-stage initiation-promotion protocol and identification of procyanidin B5-3'-gallate as the most effective antioxidant constituent. Carcinogenesis. 1999;20:1737–1745. doi: 10.1093/carcin/20.9.1737. [DOI] [PubMed] [Google Scholar]
- 30.Prasain JK, Peng N, Dai Y, Moore R, Arabshahi A, Wilson L, Barnes S, Wyss J. Michael, Kim H, Watts RL. Liquid chromatography tandem mass spectrometry identification of proanthocyanidins in rat plasma after oral administration of grape seed extract. Phytomedicine. 2009;16:233–243. doi: 10.1016/j.phymed.2008.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Monagas M, Gómez-Cordovés C, Bartolomé B, Laureano O, Ricardo da Silva JM. Monomeric, oligomeric, and polymeric flavan-3-ol composition of wines and grapes from Vitis vinifera L. Cv. Graciano, Tempranillo, and Cabernet Sauvignon. J Agric Food Chem. 2003;51:6475–6481. doi: 10.1021/jf030325+. [DOI] [PubMed] [Google Scholar]
- 32.Auger C, Gérain P, Laurent-Bichon F, Portet K, Bornet A, Caporiccio B, Cros G, Teissédre PL, Rouanet JM. Phenolics from commercialized grape extracts prevent early atherosclerotic lesions in hamsters by mechanisms other than antioxidant effect. J Agric Food Chem. 2004;52:5297–5302. doi: 10.1021/jf040125d. [DOI] [PubMed] [Google Scholar]
- 33.Eng ET, Ye J, Williams JD, Phung S, Moore RE, Young MK, Gruntmanis U, Braunstein G, Chen S. Suppression of estrogen biosynthesis by procyanidin dimmers in red wine and grape seeds. Cancer Res. 2003;63:8516–8522. [PubMed] [Google Scholar]
- 34.Kaur M, Velmurugan B, Rajamanickam S, Agarwal R, Agarwal C. Gallic acid, an active constituent of grape seed extract, exhibits anti-proliferative, pro-apoptotic and anti-tumorigenic effects against prostate carcinoma xenografts growth in nude mice. Pharm Res. 2009;26:2133–2140. doi: 10.1007/s11095-009-9926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raina K, Rajamanickam S, Deep G, Singh M, Agarwal R R, Agarwal C. Chemopreventive effects of oral gallic acid feeding on tumor growth and progression in TRAMP mice. Mol Cancer Ther. 2008;7:1258–1267. doi: 10.1158/1535-7163.MCT-07-2220. [DOI] [PubMed] [Google Scholar]
- 36.Agarwal C, Veluri R, Kaur M, Chou SC, Thompson JA, Agarwal R. Fractionation of high molecular weight tannins in grape seed extract and identification of procyanidin B2-3,3'-di-O-gallate as a major active constituent causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2007;28:1478–1484. doi: 10.1093/carcin/bgm045. [DOI] [PubMed] [Google Scholar]
- 37.Veluri R, Singh RP, Liu Z, Thompson JA, Agarwal R, Agarwal C. Fractionation of grape seed extract and identification of gallic acid as one of the major active constituents causing growth inhibition and apoptotic death of DU145 human prostate carcinoma cells. Carcinogenesis. 2006;27:1445–1453. doi: 10.1093/carcin/bgi347. [DOI] [PubMed] [Google Scholar]
- 38.Kaur M, Agarwal R, Agarwal C. Grape seed extract induces anoikis and caspase-mediated apoptosis in human prostate carcinoma LNCaP cells: possible role of ataxia telangiectasia mutated-p53 activation. Mol Cancer Ther. 2006;5:1265–1274. doi: 10.1158/1535-7163.MCT-06-0014. [DOI] [PubMed] [Google Scholar]
- 39.Gu L, Kelm MA, Hammerstone JF, Beecher G, Holden J, Haytowitz D, Prior RL. Screening of foods containing proanthocyanidins and their structural characterization using LC-MS/MS and thiolytic degradation. J. Agric. Food Chem. 2003;51:7513–7521. doi: 10.1021/jf034815d. [DOI] [PubMed] [Google Scholar]
- 40.Friedrich W, Eberhardt A, Galensa R. Investigation of proanthocyanidins by HPLC with electrospray ionization mass spectrometry. Eur. Food Res. Technol. 2000;211:56–64. (2000) [Google Scholar]
- 41.Sano A, Yamakoshi J, Tokutake S, Tobe K, Kubota Y, Kikuchi M. Procyanidin B1 is detected in human serum after intake of proanthocyanidin-rich grape seed extract. Biosci Biotechnol Biochem. 2003;67:1140–1143. doi: 10.1271/bbb.67.1140. [DOI] [PubMed] [Google Scholar]
- 42.Saito A, Mizushina Y, Ikawa H, Yoshida H, Doi Y, Tanaka A, Nakajima N. Systematic synthesis of galloyl-substituted procyanidin B1 and B2, and their ability of DPPH radical scavenging activity and inhibitory activity of DNA polymerases. Bioorg Med Chem. 2005;13:2759–2771. doi: 10.1016/j.bmc.2005.02.023. [DOI] [PubMed] [Google Scholar]
- 43.Pop C, Salvesen GS. Human caspases: activation, specificity, and regulation. J Biol Chem. 2009;284:21777–21781. doi: 10.1074/jbc.R800084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Duriez PJ, Shah GM. Cleavage of poly(ADP-ribose) polymerase: a sensitive parameter to study cell death. Biochem Cell Biol. 1997;75:337–349. [PubMed] [Google Scholar]
- 45.Tait SW, Green DR. Caspase-independent cell death: leaving the set without the final cut. Oncogene. 2008;27:6452–6461. doi: 10.1038/onc.2008.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McCarthy NJ, Whyte MK, Gilbert CS, Evan GI. Inhibition of Ced3/ICE related proteases does not prevent cell death induced by oncogenes, DNA damage, or the Bcl-2 homologue Bak. J Cell Biol. 1997;136:215–227. doi: 10.1083/jcb.136.1.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Borner C, Monney L. Apoptosis without caspases: an inefficient molecular guillotine? Cell Death Differ. 1999;6:497–507. doi: 10.1038/sj.cdd.4400525. [DOI] [PubMed] [Google Scholar]
- 48.Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. doi: 10.1038/onc.2008.307. [DOI] [PubMed] [Google Scholar]
- 49.Lomonosova E, Chinnadurai G G. BH3-only proteins in apoptosis and beyond: an overview. Oncogene. 2008;27(Suppl 1):S2–S19. doi: 10.1038/onc.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]