Abstract
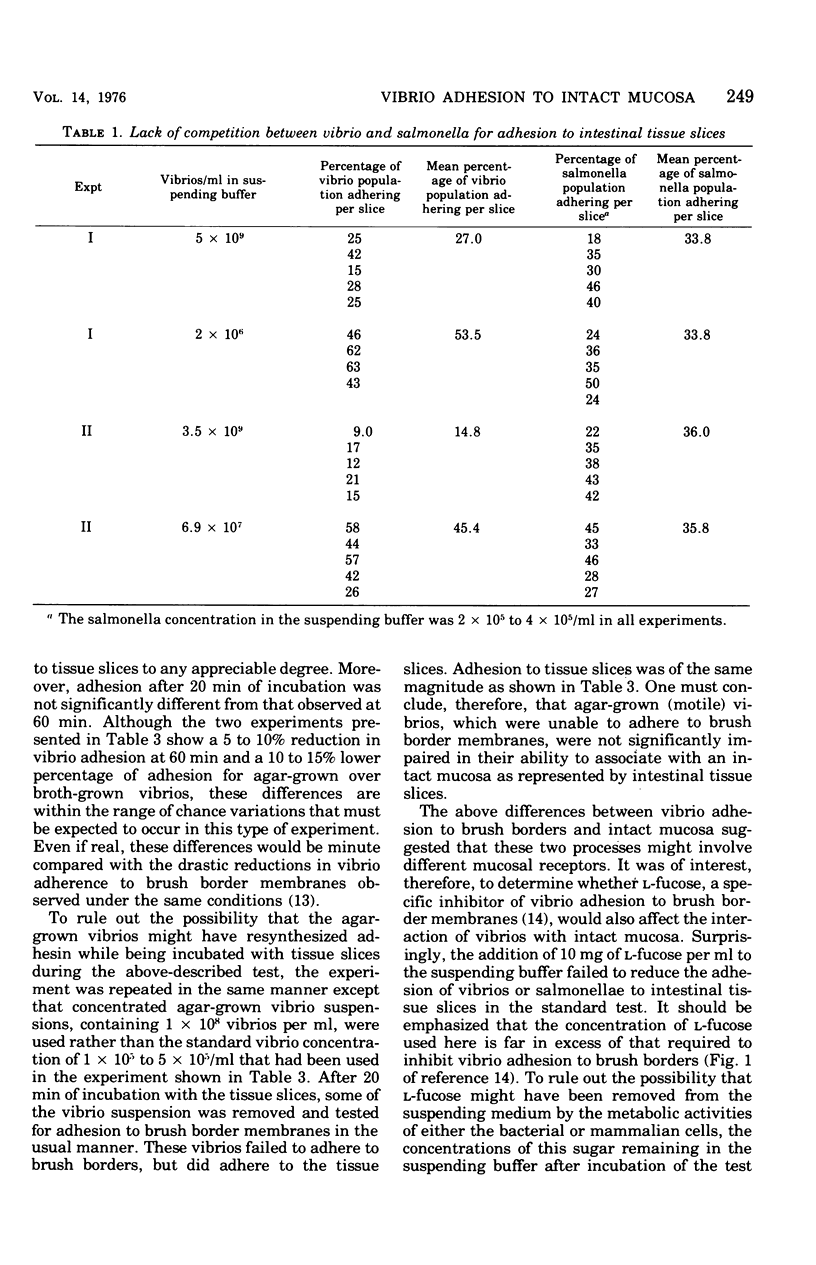
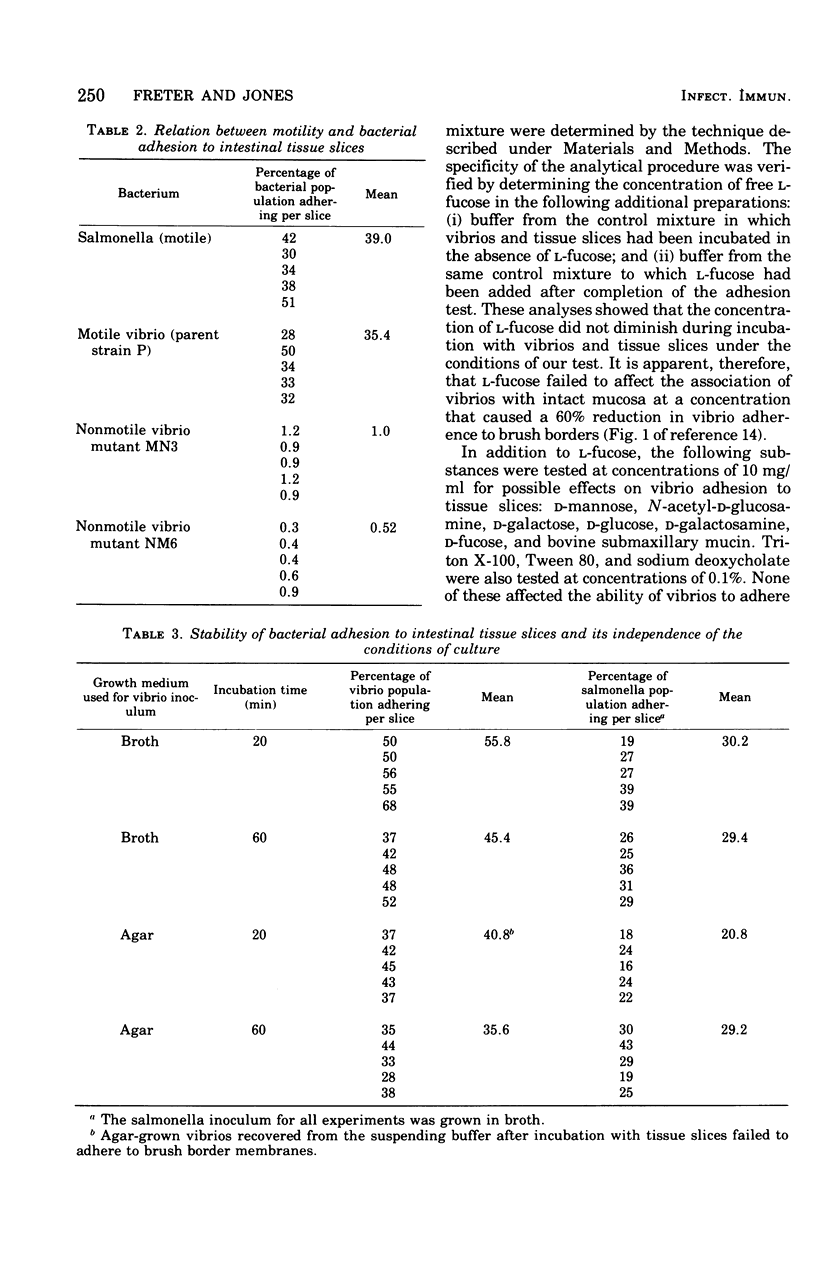
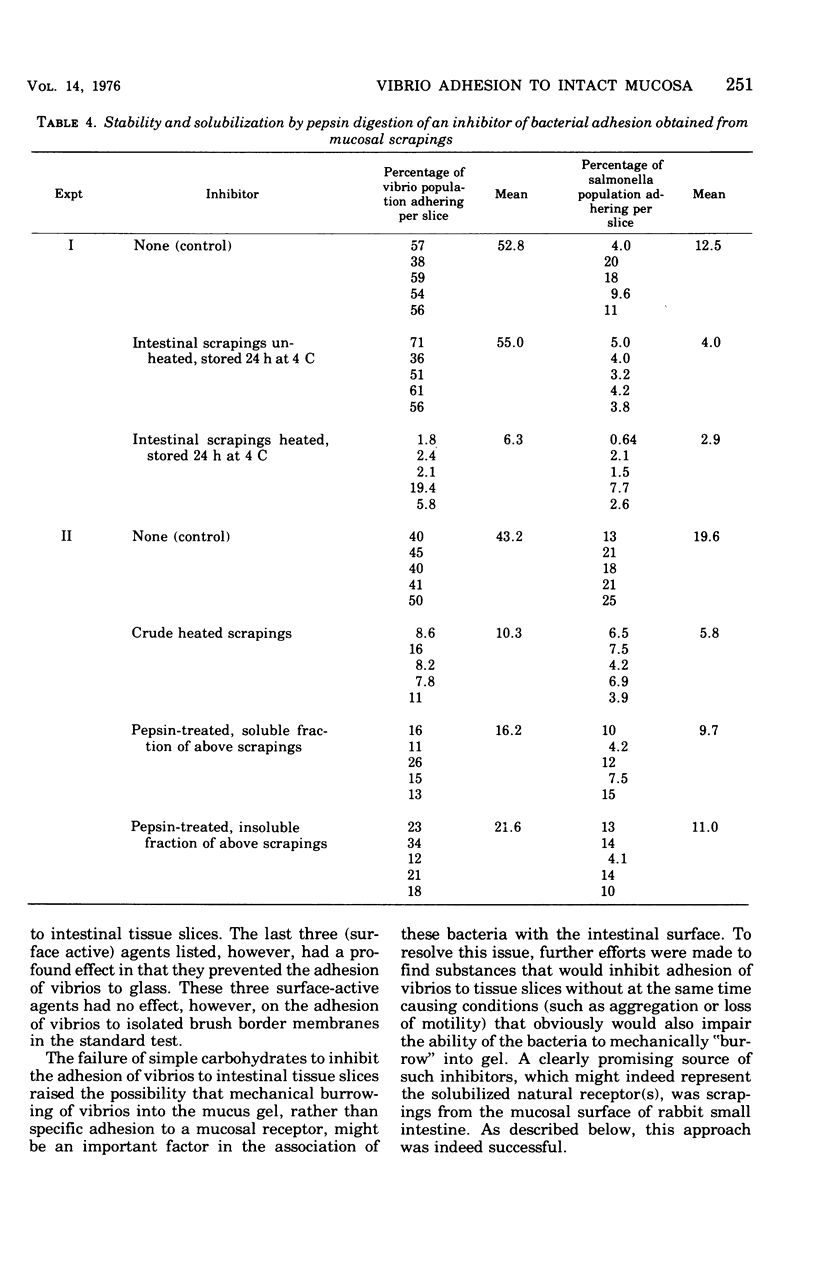
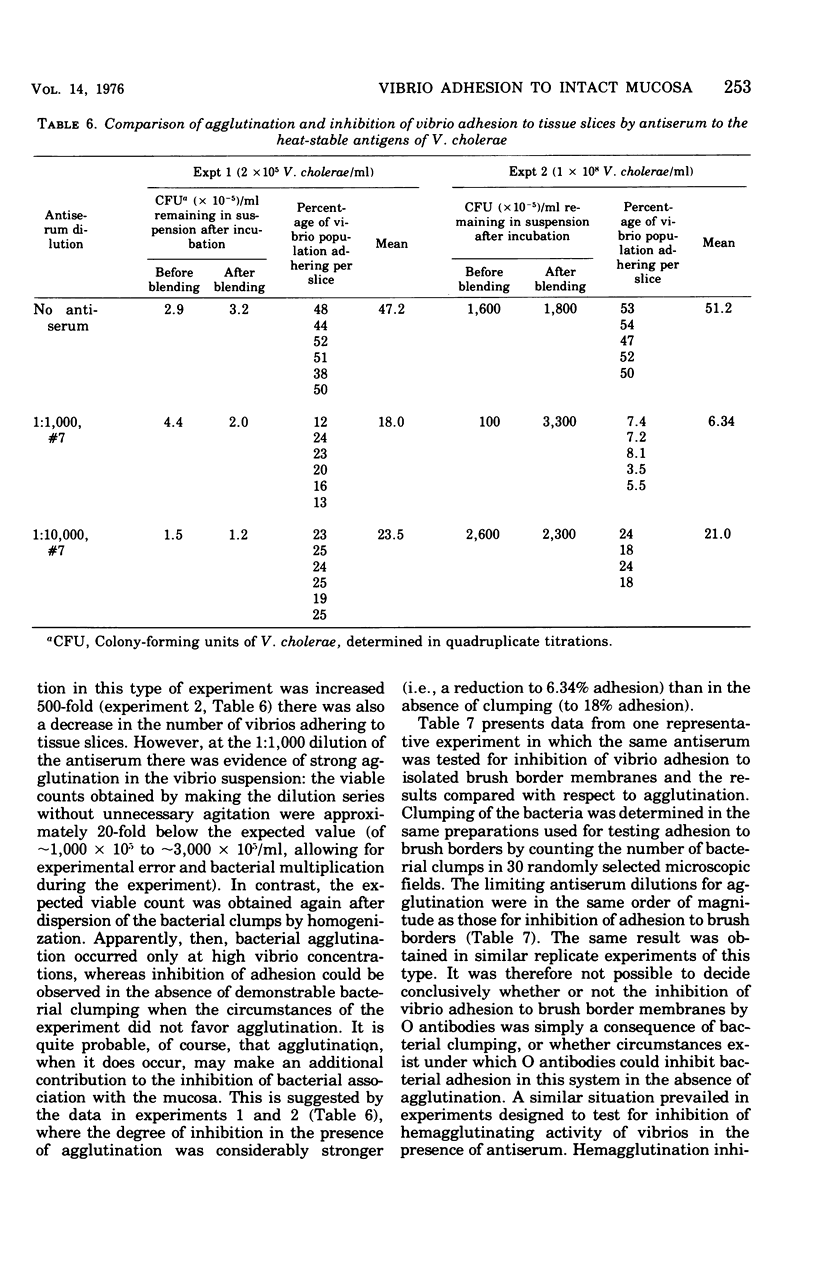
Two companion papers in this series have characterized the interaction between Vibrio cholerae and the surfaces of eukaryotic cells. The present paper reports studies of the association between vibrios or Salmonella enteritidis and intact slices of intestinal tissue. A significant number of differences were noted in the characteristics of bacterial adhesion in these systems. The results are interpreted to indicate the presence of at least two receptors for vibrio adhesion on the mucosal surface of the rabbit small intestine. The receptor mediating the adhesion of salmonella appeared to be distinct from these. A primary role for bacterial motility in the process of adhesion of vibrios to mucosal surfaces could not be demonstrated in the assay systems studied. Rather, loss of motility in mutant vibrios appeared to be correlated with the simultaneous loss of adhesive factors (adhesins) from the bacterial surface. The inhibition of vibrio adhesion to slices of intestinal tissues by antibody to the heat-stable antigens of V. cholerae occurred in the absence of bacterial agglutination. Agglutination in this assay system appeared to be an artifact in that it could be observed only in experiments where extremely high concentrations of vibrios were used. We speculate that such high vibrio concentrations are not likely to be present in humans at the time of infection and that agglutination in the lumen of the intestine might therefore play only a minor role in prophylactic immunity against natural cholera and other enteric infections of humans.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., FRENCH E. L., LIND P. E. Purification and properties of neuraminidase from Vibrio cholerae. J Gen Microbiol. 1961 Mar;24:409–425. doi: 10.1099/00221287-24-3-409. [DOI] [PubMed] [Google Scholar]
- Bellamy J. E., Knop J., Steele E. J., Chaicumpa W., Rowley D. Antibody cross-linking as a factor in immunity to cholera in infant mice. J Infect Dis. 1975 Aug;132(2):181–188. doi: 10.1093/infdis/132.2.181. [DOI] [PubMed] [Google Scholar]
- Chaicumpa W., Rowley D. Experimental cholera in infant mice: protective effects of antibody. J Infect Dis. 1972 May;125(5):480–485. doi: 10.1093/infdis/125.5.480. [DOI] [PubMed] [Google Scholar]
- Etzler M. E., Branstrator M. L. Differential localization of cell surface and secretory components in rat intestinal epithelium by use of lectins. J Cell Biol. 1974 Aug;62(2):329–343. doi: 10.1083/jcb.62.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRETER R., SMITH H. L., Jr, SWEENEY F. J., Jr An evaluation of intestinal fluids in the pathogenesis of cholera. J Infect Dis. 1961 Jul-Aug;109:35–42. doi: 10.1093/infdis/109.1.35. [DOI] [PubMed] [Google Scholar]
- Freter R. Interactions between mechanisms controlling the intestinal microflora. Am J Clin Nutr. 1974 Dec;27(12):1409–1416. doi: 10.1093/ajcn/27.12.1409. [DOI] [PubMed] [Google Scholar]
- Freter R. Mechanism of Action of Intestinal Antibody in Experimental Cholera II. Antibody-Mediated Antibacterial Reaction at the Mucosal Surface. Infect Immun. 1970 Nov;2(5):556–562. doi: 10.1128/iai.2.5.556-562.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fubara E. S., Freter R. Protection against enteric bacterial infection by secretory IgA antibodies. J Immunol. 1973 Aug;111(2):395–403. [PubMed] [Google Scholar]
- Jones G. W., Abrams G. D., Freter R. Adhesive properties of Vibrio cholerae: adhesion to isolated rabbit brush border membranes and hemagglutinating activity. Infect Immun. 1976 Jul;14(1):232–239. doi: 10.1128/iai.14.1.232-239.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Freter R. Adhesive properties of Vibrio cholerae: nature of the interaction with isolated rabbit brush border membranes and human erythrocytes. Infect Immun. 1976 Jul;14(1):240–245. doi: 10.1128/iai.14.1.240-245.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrank G. D., Verwey W. F. Distribution of cholera organisms in experimental Vibrio cholerae infections: proposed mechanisms of pathogenesis and antibacterial immunity. Infect Immun. 1976 Jan;13(1):195–203. doi: 10.1128/iai.13.1.195-203.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. Biochemical challenge of microbial pathogenicity. Bacteriol Rev. 1968 Sep;32(3):164–184. doi: 10.1128/br.32.3.164-184.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]



