Abstract
HDV is a defective RNA pathogen requiring the simultaneous presence of HBV to complete its life cycle. Two major specific patterns of infection have been described: the coinfection with HDV and HBV of a susceptible, anti-HBs-negative individual, or the HDV superinfection of a chronic HBV carrier. Coinfection mostly leads to the eradication of both agents, whereas the majority of patients with HDV superinfection evolve to chronic HDV infection and hepatitis. Chronic HDV infection worsens the preexisting HBV-related liver damage. HDV-associated chronic liver disease (chronic hepatitis D) is characterized by necroinflammation and the relentless deposition of collagen culminating, within a few decades, into the development of cirrhosis and hepatocellular carcinoma.
Patients coinfected by hepatitis D virus (HDV) and hepatitis B virus (HBV) generally eradicate both pathogens, whereas chronic HBV carriers later infected by HDV develop chronic HDV infection and more severe liver damage.
Hepatitis D virus (HDV) infection characterizes a subgroup of hepatitis B surface antigen (HBsAg)-positive patients affected by a frequently aggressive form of chronic liver damage (hepatitis D). Because HDV particle assembly and release are dependent on the obligatory presence of HBV within the same hepatocytes, a productive HDV infection is invariably associated with HBV infection. Two major patterns of infection have been described: coinfection with HBV and HDV, or superinfection with HDV of a person chronically infected with HBV. A third minor pattern or helper-independent HDV infection has been reported in the liver transplant setting. Although its existence is questioned, it will be briefly discussed in view of its historical interest.
ACUTE HEPATITIS D
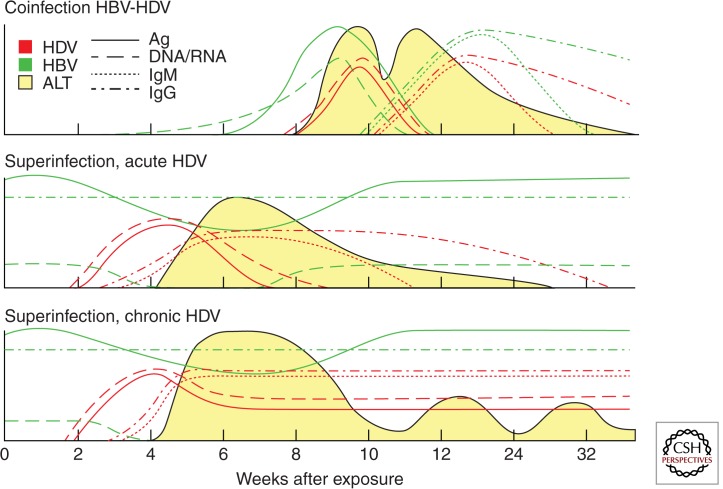
Acute hepatitis D caused by HBV/HDV coinfection occurs on the simultaneous infection with both HBV and HDV of an individual who is susceptible to HBV (and therefore anti-HBs negative) (Fig. 1). From a clinical standpoint, this entity is indistinguishable from an acute hepatitis B (Smedile et al. 1982). Acute hepatitis D occurs after an incubation time of 1–2 mo. The preicteric phase is characterized by nonspecific symptoms such as fatigue, lethargy, digestive symptoms (anorexia, nausea), and the appearance of the usual biochemical markers, such as elevated serum alanine aminotransferase (ALT) and aspartate aminotransferase (AST). The icteric phase, which is not always observed, is characterized by elevated levels of serum bilirubin. In adults, HDV/HBV coinfection is usually transient and self-limited, as the rate of progression to chronicity is the same as that of HBV monoinfection (i.e., less than 5%) (Caredda et al. 1983). A more severe clinical course is frequent, however, and two peaks of serum ALT and AST may be observed. An increased risk of acute liver failure has been reported among patients with HBV monoinfection, especially in drug addicts (Smedile et al. 1982). This is characterized by a massive hepatocyte necrosis that leads to death in 80% of the patients, unless urgent liver transplantation is performed.
Figure 1.
Serologic patterns of hepatitis D. Coinfection with HBV and HDV leading to eradication of both viruses (top panel), self-limited superinfection with HDV of a chronic HBV carrier (middle panel), and superinfection with HDV of a chronic HBV carrier leading to persistent HDV infection (bottom panel). The expression levels of antigens, DNA or RNA, IgM, and IgG for both HDV and HBV and ALT are indicated. (From Pascarella and Negro 2011; reprinted, with permission, from Wiley © 2011.)
To establish diagnosis, assessing the presence of HBsAg is necessary before investigating any other marker because HDV is dependent on HBV (Table 1). HDAg, the only antigen encoded by HDV genome, is detected early but also vanishes quickly. Thus, its transient appearance requires repeated testing (Buti et al. 1986). Serum HDAg lasts longer only in immunodeficient patients because of their slow and weak immune response (Grippon et al. 1987). HDV genomic RNA is an early and sensitive marker of HDV replication in acute HDV infection (Tang et al. 1993). Serum HDV RNA can be detected by RT-PCR testing, which has superseded classical hybridization-based assays owing to its increased sensitivity, with a lower detection limit of 10 genomes per mL (5–11). However, a crucial diagnostic marker is represented by high-titered IgM anti-HBc antibodies, which disappear along with the clinical resolution. IgM anti-HBc are absent in chronic HBV infection, thus enabling to distinguish the acute HBV/HDV coinfection from an acute HDV superinfection of a chronic HBV carrier or from an established chronic HDV infection. On the other hand, the antibody response to HDAg can be detected in coinfected patients. However, anti-HD antibodies of the IgM class are not specific of acute hepatitis D, and anti-HD IgG are low titered and appear late. They may be the only detectable marker in patients who present late because of paucity of symptoms, thus rendering a proper diagnosis difficult. Serum HDAg, anti-HD IgM, and IgG can be detected by enzyme-linked immunosorbent assay (ELISA) (Shattock and Morgan 1984) or radioimmunoassay (RIA), but testing for all of these markers are not available in all countries.
Table 1.
Comparison between the clinical and diagnostic features of HDV infection according to the two patterns of coinfection and superinfection
| Coinfection | Superinfection | |
|---|---|---|
| HBV infection | Acute | Chronic |
| Outcome | Usually recovery with viral eradication (<5% chronicity) | Usually persistent infection |
| HBsAg | Present, early, and transient | Preexisting and persistent |
| IgM anti-HBc | Positive | Negative |
| Anti-HBs | Appears during the convalescence phase | Negative |
| HDV infection | Acute | Acute or chronic |
| Outcome | Usually recovery with viral eradication (<5% chronicity) | Usually persistent infection (80% progress to chronicity) |
| Serum HDAg | Early and short-lived | Transient and later undetectable because of complexing with antibodies |
| Liver HDAg | Positive and short-lived | Positive but 50% sensitivity at late stages |
| Serum HDV RNA | Early positive and transient | Early positive and persistent |
| Anti-HDV | Late and low titered | Rapidly increasing titers and persistent |
| IgM anti-HDV | Positive, transient | Positive, high titered |
Superinfection is the HDV infection of an individual chronically infected with HBV. This pattern of infection causes a severe acute hepatitis that may be self-limited (Fig. 1) but that in most cases (up to 80%) progresses to chronicity (Smedile et al. 1982). Once chronic HDV infection is established, it usually exacerbates the preexisting chronic hepatitis B (Smedile et al. 1981). On the other hand, HBV replication is usually suppressed by HDV, and this suppression becomes persistent in the case of a chronic HDV infection (Table 1) (Krogsgaard et al. 1987; Farci et al. 1988). In the setting of superinfection, the serum level of HDV RNA can reach 1012 copies/mL within a few weeks from infection. Increasing titers of anti-HD IgG appear late, but the seroconversion confirms the diagnosis in the absence of other tests. IgM anti-HBc are typically absent (Table 1).
HELPER-INDEPENDENT HDV INFECTION
Helper-independent infection of HDV was initially reported after liver transplantation (Ottobrelli et al. 1991). HBV infection of the transplanted liver is usually prevented by the administration of anti–hepatitis B surface antigen immunoglobulins. When poorly sensitive molecular hybridization assays were used to monitor HDV RNA in serum, it was observed that patients were HDV RNA–negative in serum despite the rare occurrence of HDAg in the hepatocyte nuclei—as detected by immunohistochemistry—well before HBV recurrence. This was interpreted as consistent with the infrequent evasion of neutralizing antibodies by some HDV particles, thus capable of infecting hepatocytes. Without the concomitant infection of HBV, a productive cycle of HDV could not be completed. This view was corroborated by the notion that the helper virus is necessary for particle formation but not for viral replication (Kuo et al. 1989). In these patients, circulating HDV RNA was only detected—by molecular hybridization assays—several months after transplantation, for instance, when residual HBV had evaded neutralization and coinfected hepatocytes harboring replicating HDV, thus allowing for HDV rescue and cell-to-cell spread (Ottobrelli et al. 1991). This pattern of infection, however, has been revisited with the use of more sensitive RT-PCR–based techniques for detecting HDV RNA. In addition, experiments on chimpanzees first infected with HDV and later challenged with HBV have shown that the rescue of HDV by HBV is possible only when the challenge with the helper virus is performed very early (i.e., after 1 wk from HDV infection) but not later (i.e., after 1 mo) (Smedile et al. 1998). Thus, helper-independent HDV infection is now believed clinically irrelevant and probably of limited virological significance.
CHRONIC HEPATITIS D
HDV induces a usually severe form of chronic hepatitis, although the range of clinical manifestations is very broad, and HDV infection can be associated with asymptomatic cases as well as rapidly progressive hepatitis (Smedile et al. 1981; Rizzetto et al. 1983; Govindarajan et al. 1986a). The diagnosis is often fortuitous, or may follow the appearance of late complications at the cirrhosis stage. ALT and AST levels are persistently elevated in most patients. In chronic hepatitis D, HDAg is complexed with its corresponding anti-HD antibodies, typically present at high titer. Thus, HDAg cannot be detected unless under denaturing conditions (e.g., by immunoblot assay) (Bonino et al. 1986), which is not practical for routine testing, albeit very sensitive (Buti et al. 1989). Even the detection of HDAg by immunohistochemistry in infected livers is out of reach of most routine pathology laboratories. And it is poorly sensitive as the nuclei stained positive only in ∼50% of patients, especially at late stages of the disease (Negro et al. 1988; Wu et al. 1995a). Conversely, HDV RNA is easily detectable in the serum by sensitive RT-PCR-based assays, currently used not only for diagnostic purposes but also to follow the viral RNA titer and kinetics during antiviral treatment (Castelnau et al. 2006; Erhardt et al. 2006; Niro et al. 2006; Mederacke et al. 2010; Schaper et al. 2010; Wedemeyer et al. 2011). Interestingly, anti-HD of the IgM class also persists after the acute infection throughout the chronic phase, at variance with other viral infections (Table 1). Thus, to establish the diagnosis of chronic hepatitis D, the screening assay used should be the detection of anti-HD antibodies by ELISA. The diagnosis of ongoing infection is then confirmed by the immunohistochemical staining for HDAg in the liver or the detection of HDV RNA in the serum. If the HDV infection is confirmed, the next step is to evaluate liver grading and staging to determine whether the patient may be a candidate for antiviral treatment.
Clinically, once chronic HDV infection is established, it usually exacerbates the preexisting liver disease associated with HBV (Smedile et al. 1981). Progression toward cirrhosis may be rapid (Rizzetto et al. 1983; Govindarajan et al. 1986a), although HDV-associated chronic liver disease may run an indolent course (Bonino et al. 1987), and even asymptomatic HDV carriers have been reported in some geographical areas (Hadziyannis et al. 1991). Older studies had reported that 70%–80% of chronic hepatitis D patients developed cirrhosis within 5 to 10 yr (Rizzetto et al. 1983; Govindarajan et al. 1986a) and 15% within 1–2 yr (Saracco et al. 1987). A recent retrospective study (Romeo et al. 2009) followed 299 patients for a mean period of 233 mo. At enrollment, seven had acute hepatitis and 104 had cirrhosis. Among the noncirrhotic, 82 patients developed cirrhosis during follow-up at a yearly rate of 4%. Persistent HDV replication predicted the development of cirrhosis. Overall, the relative risk of developing cirrhosis during follow-up in patients coinfected with HBV and HDV seems twofold compared with patients infected with HBV alone (Fattovich et al. 2008). Once established, however, cirrhosis caused by HDV may remain stable for years before progressing to liver failure or developing a hepatocellular carcinoma (HCC). Patients with HDV-associated cirrhosis have a probability of survival at 5 and 10 yr of 49% and 40%, respectively (Rosina et al. 1999).
The impact of HDV infection on the rate of HCC development in HBV-positive patients is controversial. A retrospective study conducted in Western Europe on 200 patients with compensated HBV-related cirrhosis, of whom 20% were anti-HDV positive, found that HDV infection increased the risk of HCC threefold and mortality twofold (Fattovich et al. 2000) compared with HBV monoinfection. After adjustment for clinical and serological features at baseline, the estimated 5-yr risk for developing HCC was 13%, 4%, and 2% for anti-HDV positive/HBeAg negative, antiHDV negative/HBeAg negative, and anti-HDV negative/HBeAg positive patients, respectively. The corresponding figures for hepatic decompensation were 18%, 8%, and 14%, respectively, and for survival 90%, 95%, and 93%, respectively (Fattovich et al. 2000). The incidence of HCC according to the retrospective study mentioned above was also 2.8% per year (Romeo et al. 2009). Thus, persistent HDV replication predicts the development of HCC and liver-related mortality (Romeo et al. 2009).
FACTORS INFLUENCING HEPATITIS D PROGRESSION
Many factors can influence the outcome of hepatitis D, the single most important being the pattern of infection with HBV, for instance, coinfection versus superinfection as described above.
The severity of hepatitis D is influenced by the HDV genotype. HDV isolates show up to 39% heterogeneity, and the different sequences have been classified into eight HDV genotypes (Dény 2006). Although infection with multiple genotypes may occur in patients at high risk of repeated exposure such as injecting drug users, a single genotype usually predominates with >10% of the viral load being represented by the minor strain (Wu et al. 1999). In the Western world, where most natural history studies detailed in the previous paragraph have been conducted, HDV genotype 1 is prevailing (Dény 2006). In Taiwan, where the predominant genotype is 2, acute HDV infection evolves less frequently toward acute liver failure, and even chronic hepatitis D seems less rapidly progressive (Wu et al. 1995a,b). On the other hand, infection with genotype 3, which is prevalent in South America, induces a severe form of hepatitis. Indeed, severe outbreaks of acute hepatitis D with a high incidence of liver failure have been reported among the Yucpa Indians of Venezuela (Hadler et al. 1992), the Sierra Nevada de Santa Marta in Colombia (Popper et al. 1983), and some areas of the Brazilian (Bensabath et al. 1987) and Peruvian (Casey et al. 1993) Amazonian forest. A viral factor potentially involved in influencing disease outcome is the occurrence of specific HDAg species that have been reported in acute liver failure cases (Tang et al. 1993).
Among the factors related to the helper virus, the HBV genotype modulates the HDV viral load and correlates with adverse outcomes (Su et al. 2006; Kiesslich et al. 2009). High levels of HBV replication are associated with more severe liver damage, and a more ominous course toward liver decompensation has been documented in patients with ongoing replication of both HBV and HDV (Smedile et al. 1991).
PATHOGENESIS OF HEPATITIS D
HDV only replicates in the liver, and therefore pathologic changes are limited to this organ. Liver damage in HDV infection is thought to be mostly immune mediated, although initial data from experimentally infected chimpanzees had suggested a direct cytopathic effect of HDV on hepatocytes, particularly during the primary infection (Kamimura et al. 1983; Canese et al. 1984; Govindarajan et al. 1986b). It was observed that in acute hepatitis D, infected hepatocytes were undergoing degenerative changes characterized by shrunken eosinophilic cytoplasm and pyknotic nuclei, with minimal inflammatory cells in the liver parenchyma, consistent with a cytopathic hepatocellular damage. These findings were reported both in vitro (cell culture system) (Cole et al. 1991) and in human studies (Popper et al. 1983; Lefkowitch et al. 1987). The small isoform of HDAg expressed was suggested to be responsible for this direct cytopathic effect of HDV (Cole et al. 1991). However, other results and in vivo observations, such as the presence of inflammatory cells surrounding the infected hepatocytes and the presence of various autoantibodies in the serum of patients, argue for a mostly immune-mediated liver damage. On the other hand, because HBV replication is usually suppressed by HDV, liver damage is believed to be induced mostly by HDV rather than by HBV.
Variation in immune-mediated responses during acute and chronic HDV infection has been noticed (Casey et al. 2006; Fiedler and Roggendorf 2006), which may explain the variability of the clinical course of HDV infection. Cytotoxic T lymphocytes are mainly responsible for clearing the virus by destroying HDV-infected cells. Delayed and insufficient immune response with the ability to recognize only limited viral epitopes has been implicated in failure to clear the infection coupled with establishment of chronic infection. An exaggerated immune response, particularly a cell-mediated one, is proposed to be involved in causing massive hepatocyte necrosis and liver damage in acute liver failure (Hansson et al. 1991). Thus, a vigorous immune response involving HDAg-specific T-cell response and cytotoxic killing of HDV-infected cells leads to both viral clearance and increased liver damage.
The fine details of the pathogenesis of HDV-induced immune damage are largely unknown, as they have been addressed by only a few studies. Response to HDV involves the activation of antigen-specific helper T cells secreting a variety of cytokines, including interleukin (IL)-2, IL-2 receptor, IL-10, and interferon (IFN)-γ (Magrin et al. 1989; Nisini et al. 1997; Grabowski et al. 2011). IL-2, in turn, stimulates both additional HDV-specific helper T cells and CD8+ cytotoxic T cells that target infected hepatocytes. HDV-specific Th1 and cytotoxic T cells produce large amounts of IFN-γ (Nisini et al. 1997), which, in addition to its known immunological effects (among others, the induction of class I and class II MHC proteins at the surface of hepatocytes) (Franco et al. 1988), may also inhibit viral replication (Magrin et al. 1989). HDAg-specific T-cell responses in peripheral blood of HDV-infected individuals is associated with reduced HDV replication levels (Nisini et al. 1997). IFN-γ also stimulates the secretion of IFN-γ-induced protein-10 (CXCL-10), a chemoattractant that recruits natural killer (NK) cells, which add to the cell damage.
In addition to the immune mediated liver damage, several interactions between HDV and the cell machinery have been reported that may have pathophysiological consequences and clinical impact. Like many viruses capable of establishing persistent infections, HDV has developed a strategy to counter endogenous IFN-α. HDV directly inhibits the activation of the IFN-α signaling pathways by interfering with the early steps of the Janus kinase (JAK)/signal transducers and activators of transcription (STAT) signal transduction pathway. There is an inhibition of the tyrosine kinase 2 (Tyk2), STAT1, and STAT2 phosphorylation and a transcription impairment of several interferon-stimulated genes, including the myxovirus resistance-A (MxA), the 2′,5′-oligoadenylate synthetase (2′,5′-OAS) and protein kinase R in the presence of the virus (Pugnale et al. 2009). Furthermore, the large isoform of HDAg up-regulates MxA transcription, and this may account for the suppression of HBV replication (Williams et al. 2009). In addition, HDV seems to sensitize cells to inflammatory stimuli. The large isoform of HDAg increases the tumor necrosis factor (TNF)-α-induced nuclear factor (NF)-κB signaling, probably via a direct interaction with the TNF-receptor-associated factor 2 (TRAF2), a factor involved in early signal transduction (Park et al. 2009). More generally, HDV products—encompassing the two HDAg isoforms and the various RNA species—have been shown to interact at several levels with the host cell proteome (Mota et al. 2008, 2009), including factors involved in the regulation of cell metabolism and energetic homeostasis, nucleic acid and protein metabolism, and apoptosis and cell growth. Both HDAg isoforms enhance clusterin gene expression via an increased histone H3 acetylation within the clusterin promoter (Liao et al. 2009). These epigenetic changes, common to several other viral infections, may play a role in oncogenesis and thus favor the development of HCC in HDV-infected individuals.
CONCLUSIONS
Because HDV requires the simultaneous presence of HBV to complete its life cycle, two specific patterns of infection may occur: a coinfection with HDV and HBV of a susceptible, anti-HBs-negative individual, or the HDV superinfection of a chronic HBV carrier. Although coinfection mostly leads to the eradication of both viruses, the majority of patients with HDV superinfection progress to chronic HDV infection and hepatitis, usually worsening the preexisting HBV-related liver damage. Chronic hepatitis D is characterized by typical hallmarks of all chronic hepatitides, for instance, necroinflammation and fibrosis. This process may lead to the development of cirrhosis, liver failure, and HCC within decades.
Footnotes
Editors: Christoph Seeger and Stephen Locarnini
Additional Perspectives on Hepatitis B and Delta Viruses available at www.perspectivesinmedicine.org
REFERENCES
- Bensabath G, Hadler SC, Soares MC, Fields H, Dias LB, Popper H, Maynard JE. 1987. Hepatitis Delta virus infection and Labrea hepatitis. Prevalence and role in fulminant hepatitis in the Amazon Basin. JAMA 258: 479–483 [DOI] [PubMed] [Google Scholar]
- Bonino F, Heermann KH, Rizzetto M, Gerlich WH. 1986. Hepatitis Delta virus: Protein composition of Delta antigen and its hepatitis B virus-derived envelope. J Virol 58: 945–950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonino F, Negro F, Baldi M, Brunetto MR, Chiaberge E, Capalbo M, Maran E, Lavarini C, Rocca N, Rocca G. 1987. The natural history of chronic Delta hepatitis. Prog Clin Biol Res 234: 145–152 [PubMed] [Google Scholar]
- Buti M, Esteban R, Jardi R, Esteban JI, Guardia J. 1986. Serological diagnosis of acute Delta hepatitis. J Med Virol 18: 81–85 [DOI] [PubMed] [Google Scholar]
- Buti M, Esteban R, Jardi R, Rodriguez-Frias F, Casacuberta J, Esteban JI, Allende E, Guardia J. 1989. Chronic Delta hepatitis: Detection of hepatitis Delta virus antigen in serum by immunoblot and correlation with other markers of Delta viral replication. Hepatology 10: 907–910 [DOI] [PubMed] [Google Scholar]
- Canese MG, Rizzetto M, Novara R, London WT, Purcell RH. 1984. Experimental infection of chimpanzees with the HBsAg-associated delta (δ dL) agent: An ultrastructural study. J Med Virol 13: 63–72 [DOI] [PubMed] [Google Scholar]
- Caredda F, d’Arminio Monforte A, Rossi E, Farci P, Smedile A, Tappero G, Moroni M. 1983. Prospective study of epidemic Delta infection in drug addicts. Prog Clin Biol Res 143: 245–250 [PubMed] [Google Scholar]
- Casey JL, Brown TL, Colan EJ, Wignall FS, Gerin JL. 1993. A genotype of hepatitis D virus that occurs in northern South America. Proc Natl Acad Sci 90: 9016–9020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey JL, Tennant BC, Gerin JL. 2006. Genetic changes in hepatitis Delta virus from acutely and chronically infected woodchucks. J Virol 80: 6469–6477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castelnau C, Le Gal F, Ripault MP, Gordien E, Martinot-Peignoux M, Boyer N, Pham BN, Maylin S, Bedossa P, Dény P, et al. 2006. Efficacy of peginterferon α-2b in chronic hepatitis Delta: Relevance of quantitative RT-PCR for follow-up. Hepatology 44: 728–735 [DOI] [PubMed] [Google Scholar]
- Cole SM, Gowans EJ, Macnaughton TB, Hall PD, Burrell CJ. 1991. Direct evidence for cytotoxicity associated with expression of hepatitis Delta virus antigen. Hepatology 13: 845–851 [PubMed] [Google Scholar]
- Dény P. 2006. Hepatitis Delta virus genetic variability: From genotypes I, II, III to eight major clades? Curr Top Microbiol Immunol 307: 151–171 [DOI] [PubMed] [Google Scholar]
- Erhardt A, Gerlich W, Starke C, Wend U, Donner A, Sagir A, Heintges T, Häussinger D. 2006. Treatment of chronic hepatitis Delta with pegylated interferon-α2b. Liver Int 26: 805–810 [DOI] [PubMed] [Google Scholar]
- Farci P, Karayiannis P, Lai ME, Marongiu F, Orgiana G, Balestrieri A, Thomas HC. 1988. Acute and chronic hepatitis Delta virus infection: Direct or indirect effect on hepatitis B virus replication? J Med Virol 26: 279–288 [DOI] [PubMed] [Google Scholar]
- Fattovich G, Giustina G, Christensen E, Pantalena M, Zagni I, Realdi G, Schalm SW. 2000. Influence of hepatitis Delta virus infection on morbidity and mortality in compensated cirrhosis type B. The European Concerted Action on Viral Hepatitis (Eurohep). Gut 46: 420–426 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fattovich G, Bortolotti F, Donato F. 2008. Natural history of chronic hepatitis B: Special emphasis on disease progression and prognostic factors. J Hepatol 48: 335–352 [DOI] [PubMed] [Google Scholar]
- Fiedler M, Roggendorf M. 2006. Immunology of HDV infection. Curr Top Microbiol Immunol 307: 187–209 [DOI] [PubMed] [Google Scholar]
- Franco A, Barnaba V, Natali P, Balsano C, Musca A, Balsano F. 1988. Expression of class I and class II major histocompatibility complex antigens on human hepatocytes. Hepatology 8: 449–454 [DOI] [PubMed] [Google Scholar]
- Govindarajan S, De Cock KM, Redeker AG. 1986a. Natural course of Delta superinfection in chronic hepatitis B virus-infected patients: Histopathologic study with multiple liver biopsies. Hepatology 6: 640–644 [DOI] [PubMed] [Google Scholar]
- Govindarajan S, Fields HA, Humphrey CD, Margolis HS. 1986b. Pathologic and ultrastructural changes of acute and chronic Delta hepatitis in an experimentally infected chimpanzee. Am J Pathol 122: 315–322 [PMC free article] [PubMed] [Google Scholar]
- Grabowski J, Yurdaydìn C, Zachou K, Buggisch P, Hofmann WP, Jaroszewicz J, Schlaphoff V, Manns MP, Cornberg M, Wedemeyer H. 2011. Hepatitis D virus-specific cytokine responses in patients with chronic hepatitis Delta before and during interferon alfa-treatment. Liver Int 31: 1395–1405 [DOI] [PubMed] [Google Scholar]
- Grippon P, Ribiere O, Cadranel JF, Pelletier S, Pillot B, Emerit J, Opolon P. 1987. Long-term Delta antigenaemia without appearance of Delta antibody in two immunodeficient patients. Lancet 1: 1031. [DOI] [PubMed] [Google Scholar]
- Hadler SC, Alcala De Monzon M, Rivero D, Perez M, Bracho A, Fields H. 1992. Epidemiology and long-term consequences of hepatitis Delta virus infection in the Yucpa Indians of Venezuela. Am J Epidemiol 136: 1507–1516 [DOI] [PubMed] [Google Scholar]
- Hadziyannis SJ, Papaioannou C, Alexopoulou A. 1991. The role of the hepatitis Delta virus in acute hepatitis and in chronic liver disease in Greece. Prog Clin Biol Res 364: 51–62 [PubMed] [Google Scholar]
- Hansson BG, Riesbeck K, Nordenfelt E, Weiland O. 1991. Successful treatment of fulminant hepatitis B and fulminant hepatitis B and D coinfection explained by inhibitory effect on the immune response? Prog Clin Biol Res 364: 421–427 [PubMed] [Google Scholar]
- Kamimura T, Ponzetto A, Bonino F, Feinstone SM, Gerin JL, Purcell RH. 1983. Cytoplasmic tubular structures in liver of HBsAg carrier chimpanzees infected with Delta agent and comparison with cytoplasmic structures in non-A, non-B hepatitis. Hepatology 3: 631–637 [DOI] [PubMed] [Google Scholar]
- Kiesslich D, Crispim MA, Santos C, Ferreira Fde L, Fraiji NA, Komninakis SV, Diaz RS. 2009. Influence of hepatitis B virus (HBV) genotype on the clinical course of disease in patients coinfected with HBV and hepatitis Delta virus. J Infect Dis 199: 1608–1611 [DOI] [PubMed] [Google Scholar]
- Krogsgaard K, Kryger P, Aldershvile J, Andersson P, Sorensen TI, Nielsen JO. 1987. Delta-infection and suppression of hepatitis B virus replication in chronic HBsAg carriers. Hepatology 7: 42–45 [DOI] [PubMed] [Google Scholar]
- Kuo MY, Chao M, Taylor J. 1989. Initiation of replication of the human hepatitis Delta virus genome from cloned DNA: Role of Delta antigen. J Virol 63: 1945–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefkowitch JH, Goldstein H, Yatto R, Gerber MA. 1987. Cytopathic liver injury in acute Delta virus hepatitis. Gastroenterology 92: 1262–1266 [DOI] [PubMed] [Google Scholar]
- Liao FT, Lee YJ, Ko JL, Tsai CC, Tseng CJ, Sheu GT. 2009. Hepatitis Delta virus epigenetically enhances clusterin expression via histone acetylation in human hepatocellular carcinoma cells. J Gen Virol 90: 1124–1134 [DOI] [PubMed] [Google Scholar]
- Magrin S, Craxì A, Carini C, Colombo P, di Blasi F, Spinelli G, Fratazzi C, Messina MC, Antonelli G, Ausiello C. 1989. Interleukin-2, interleukin-2 receptor and γ-interferon synthesis by peripheral blood mononuclear cells in chronic hepatitis Delta virus infection. J Hepatol 8: 358–366 [DOI] [PubMed] [Google Scholar]
- Mederacke I, Bremer B, Heidrich B, Kirschner J, Deterding K, Bock T, Wursthorn K, Manns MP, Wedemeyer H. 2010. Establishment of a novel quantitative hepatitis D virus (HDV) RNA assay using the Cobas TaqMan platform to study HDV RNA kinetics. J Clin Microbiol 48: 2022–2029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mota S, Mendes M, Penque D, Coelho AV, Cunha C. 2008. Changes in the proteome of Huh7 cells induced by transient expression of hepatitis D virus RNA and antigens. J Proteomics 71: 71–79 [DOI] [PubMed] [Google Scholar]
- Mota S, Mendes M, Freitas N, Penque D, Coelho AV, Cunha C. 2009. Proteome analysis of a human liver carcinoma cell line stably expressing hepatitis Delta virus ribonucleoproteins. J Proteomics 72: 616–627 [DOI] [PubMed] [Google Scholar]
- Negro F, Baldi M, Bonino F, Rocca G, Demartini A, Passarino G, Maran E, Lavarini C, Rizzetto M, Verme G. 1988. Chronic HDV (hepatitis Delta virus) hepatitis: Intrahepatic expression of Delta antigen, histologic activity and outcome of liver disease. J Hepatol 6: 8–14 [DOI] [PubMed] [Google Scholar]
- Niro GA, Ciancio A, Gaeta GB, Smedile A, Marrone A, Olivero A, Stanzione M, David E, Brancaccio G, Fontana R, et al. 2006. Pegylated interferon α-2b as monotherapy or in combination with ribavirin in chronic hepatitis Delta. Hepatology 44: 713–720 [DOI] [PubMed] [Google Scholar]
- Nisini R, Paroli M, Accapezzato D, Bonino F, Rosina F, Santantonio T, Sallusto F, Amoroso A, Houghton M, Barnaba V. 1997. Human CD4+ T-cell response to hepatitis Delta virus: Identification of multiple epitopes and characterization of T-helper cytokine profiles. J Virol 71: 2241–2251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottobrelli A, Marzano A, Smedile A, Recchia S, Salizzoni M, Cornu C, Lamy ME, Otte JB, De Hemptinne B, Geubel A, et al. 1991. Patterns of hepatitis Delta virus reinfection and disease in liver transplantation. Gastroenterology 101: 1649–1655 [DOI] [PubMed] [Google Scholar]
- Park CY, Oh SH, Kang SM, Lim YS, Hwang SB. 2009. Hepatitis Delta virus large antigen sensitizes to TNF-α-induced NF-κB signaling. Mol Cells 28: 49–55 [DOI] [PubMed] [Google Scholar]
- Pascarella S, Negro F. 2011. Hepatitis D virus: An update. Liver Int 31: 7–21 [DOI] [PubMed] [Google Scholar]
- Popper H, Thung SN, Gerber MA, Hadler SC, de Monzon M, Ponzetto A, Anzola E, Rivera D, Mondolfi A, Bracho A, et al. 1983. Histologic studies of severe Delta agent infection in Venezuelan Indians. Hepatology 3: 906–912 [DOI] [PubMed] [Google Scholar]
- Pugnale P, Pazienza V, Guilloux K, Negro F. 2009. Hepatitis Delta virus inhibits α interferon signaling. Hepatology 49: 398–406 [DOI] [PubMed] [Google Scholar]
- Rizzetto M, Verme G, Recchia S, Bonino F, Farci P, Aricò S, Calzia R, Picciotto A, Colombo M, Popper H. 1983. Chronic hepatitis in carriers of hepatitis B surface antigen, with intrahepatic expression of the Delta antigen. An active and progressive disease unresponsive to immunosuppressive treatment. Ann Intern Med 98: 437–441 [DOI] [PubMed] [Google Scholar]
- Romeo R, Del Ninno E, Rumi M, Russo A, Sangiovanni A, de Franchis R, Ronchi G, Colombo M. 2009. A 28-year study of the course of hepatitis Delta infection: A risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 136: 1629–1638 [DOI] [PubMed] [Google Scholar]
- Rosina F, Conoscitore P, Cuppone R, Rocca G, Giuliani A, Cozzolongo R, Niro G, Smedile A, Saracco G, Andriulli A, et al. 1999. Changing pattern of chronic hepatitis D in Southern Europe. Gastroenterology 117: 161–166 [DOI] [PubMed] [Google Scholar]
- Saracco G, Rosina F, Brunetto MR, Amoroso P, Caredda F, Farci P, Piantino P, Bonino F, Rizzetto M. 1987. Rapidly progressive HBsAg-positive hepatitis in Italy. The role of hepatitis Delta virus infection. J Hepatol 5: 274–281 [DOI] [PubMed] [Google Scholar]
- Schaper M, Rodriguez-Frias F, Jardi R, Tabernero D, Homs M, Ruiz G, Quer J, Esteban R, Buti M. 2010. Quantitative longitudinal evaluations of hepatitis Delta virus RNA and hepatitis B virus DNA shows a dynamic, complex replicative profile in chronic hepatitis B and D. J Hepatol 52: 658–664 [DOI] [PubMed] [Google Scholar]
- Shattock AG, Morgan BM. 1984. Sensitive enzyme immunoassay for the detection of Delta antigen and anti-Delta, using serum as the Delta antigen source. J Med Virol 13: 73–82 [DOI] [PubMed] [Google Scholar]
- Smedile A, Dentico P, Zanetti A, Sagnelli E, Nordenfelt E, Actis GC, Rizzetto M. 1981. Infection with the Delta agent in chronic HBsAg carriers. Gastroenterology 81: 992–997 [PubMed] [Google Scholar]
- Smedile A, Farci P, Verme G, Caredda F, Cargnel A, Caporaso N, Dentico P, Trepo C, Opolon P, Gimson A, et al. 1982. Influence of Delta infection on severity of hepatitis B. Lancet 2: 945–947 [DOI] [PubMed] [Google Scholar]
- Smedile A, Rosina F, Saracco G, Chiaberge E, Lattore V, Fabiano A, Brunetto MR, Verme G, Rizzetto M, Bonino F. 1991. Hepatitis B virus replication modulates pathogenesis of hepatitis D virus in chronic hepatitis D. Hepatology 13: 413–416 [PubMed] [Google Scholar]
- Smedile A, Casey JL, Cote PJ, Durazzo M, Lavezzo B, Purcell RH, Rizzetto M, Gerin JL. 1998. Hepatitis D viremia following orthotopic liver transplantation involves a typical HDV virion with a hepatitis B surface antigen envelope. Hepatology 27: 1723–1729 [DOI] [PubMed] [Google Scholar]
- Su CW, Huang YH, Huo TI, Shih HH, Sheen IJ, Chen SW, Lee PC, Lee SD, Wu JC. 2006. Genotypes and viremia of hepatitis B and D viruses are associated with outcomes of chronic hepatitis D patients. Gastroenterology 130: 1625–1635 [DOI] [PubMed] [Google Scholar]
- Tang JR, Hantz O, Vitvitski L, Lamelin JP, Parana R, Cova L, Lesbordes JL, Trépo C. 1993. Discovery of a novel point mutation changing the HDAg expression of a hepatitis Delta virus isolate from Central African Republic. J Gen Virol 74: 1827–1835 [DOI] [PubMed] [Google Scholar]
- Wedemeyer H, Yurdaydìn C, Dalekos GN, Erhardt A, Çakaloğlu Y, Değertekin H, Gürel S, Zeuzem S, Zachou K, Bozkaya H, et al. 2011. Peginterferon plus adefovir versus either drug alone for hepatitis Delta. N Engl J Med 364: 322–331 [DOI] [PubMed] [Google Scholar]
- Williams V, Brichler S, Radjef N, Lebon P, Goffard A, Hober D, Fagard R, Kremsdorf D, Dény P, Gordien E. 2009. Hepatitis Delta virus proteins repress hepatitis B virus enhancers and activate the α/β interferon-inducible MxA gene. J Gen Virol 90: 2759–2767 [DOI] [PubMed] [Google Scholar]
- Wu JC, Chen TZ, Huang YS, Yen FS, Ting LT, Sheng WY, Tsay SH, Lee SD. 1995a. Natural history of hepatitis D viral superinfection: Significance of viremia detected by polymerase chain reaction. Gastroenterology 108: 796–802 [DOI] [PubMed] [Google Scholar]
- Wu JC, Choo KB, Chen CM, Chen TZ, Huo TI, Lee SD. 1995b. Genotyping of hepatitis D virus by restriction-fragment length polymorphism and relation to outcome of hepatitis D. Lancet 346: 939–941 [DOI] [PubMed] [Google Scholar]
- Wu JC, Huang IA, Huang YH, Chen JY, Sheen IJ. 1999. Mixed genotypes infection with hepatitis D virus. J Med Virol 57: 64–67 [PubMed] [Google Scholar]