Abstract
The peripherin-2 (PRPH2) gene encodes a photoreceptor-specific tetraspanin protein called peripherin-2/retinal degeneration slow (RDS), which is critical for the formation and maintenance of rod and cone outer segments. Over 90 different disease-causing mutations in PRPH2 have been identified, which cause a variety of forms of retinitis pigmentosa and macular degeneration. Given the disease burden associated with PRPH2 mutations, the gene has long been a focus for preclinical gene therapy studies. Adeno-associated viruses and compacted DNA nanoparticles carrying PRPH2 have been successfully used to mediate improvement in the rds−/− and rds+/− mouse models. However, complexities in the pathogenic mechanism for PRPH2-associated macular disease coupled with the need for a precise dose of peripherin-2 to combat a severe haploinsufficiency phenotype have delayed the development of clinically viable genetic treatments. Here we discuss the progress and prospects for PRPH2-associated gene therapy.
More than 90 mutations in PRPH2 cause various forms of retinitis pigmentosa and macular degeneration. Several obstacles have delayed the development of clinically viable genetic treatments.
The peripherin-2 (PRPH2) gene, previously known as retinal degeneration slow (RDS), encodes a photoreceptor-specific glycoprotein, which is essential for the morphogenesis of rod and cone outer segments (OSs). Although primarily a structural protein, it is absolutely required for vision. Because of the convergence of a combination of factors, this gene has been a target for ocular gene therapy for more than two decades. First and foremost, more than 90 different mutations in PRPH2 are known to cause varying forms of rod- and cone-dominant, blinding retinal degeneration in patients, making PRPH2 a logical therapeutic target. In addition, there are several extremely well-characterized animal models for PRPH2 mutations, with ocular pathologies that mimic many of the patient disease phenotypes, making preclinical testing straightforward. Finally, the PRPH2 cDNA is relatively small (∼1.1 kb coding region), making it easy to deliver using a variety of different therapeutic approaches. However, in spite of these resources and the length of time researchers have dedicated to PRPH2 gene therapy, the complex nature of peripherin-2 protein function and regulation, combined with variability in disease mechanism, have thus far precluded development of clinically viable PRPH2 genetic treatments. Here we discuss these complexities, consider the significant advancements that have been made, and explore barriers that will be overcome as next-generation genetic therapies are developed.
THE ROLE OF PERIPHERIN-2 PROTEIN IN PHOTORECEPTOR MORPHOGENESIS AND FUNCTION
Peripherin-2 is a ∼39 kDa member of the tetraspanin superfamily of proteins. In common with other family members, it has four transmembrane domains, cytoplasmic amino- and carboxy-termini, and a large extracellular loop (called EC2 or D2). Whereas most tetraspanins are located on the plasma membrane, peripherin-2 localization is restricted to the rim region of rod and cone discs/lamellae. Because the rod discs are completely separate from the plasma membrane, the peripherin-2 D2 loop is intradiscal rather than extracellular in rods. After synthesis in the photoreceptor inner segment, peripherin-2 forms noncovalently linked tetramers with itself and its nonglycosylated homolog rod outer segment membrane protein-1 (ROM1) (Goldberg and Molday 1996; Chakraborty et al. 2008a). These complexes subsequently assemble into covalently linked, larger hetero-octamers and peripherin-2 homo-oligomers that localize to the OS rim region (Goldberg et al. 1998; Loewen and Molday 2000; Chakraborty et al. 2008a).
Peripherin-2 is required for the morphogenesis and maintenance of the OS, specifically the rim region. Although the complete role of peripherin-2 in this process is not yet fully elucidated, the carboxy-terminal domain of peripherin-2 has been shown to play a role in membrane fusion (Boesze-Battagliaa and Stefano 2002, 2003), initiation of rim membrane curvature (Khattree et al. 2013), and OS targeting (Tam et al. 2004; Tian et al. 2014). The D2 loop and covalently linked peripherin-2 complexes are required for the maintenance of the flattened rim morphology (Wrigley et al. 2000; Chakraborty et al. 2009, 2010). Peripherin-2/ROM1 complexes are also thought to play a role in regulating disc size and alignment (Cheng et al. 1997; Kedzierski et al. 1997), and, in common with other tetraspanins, are hypothesized to organize a protein microdomain in the rim region (Conley et al. 2011). Interestingly, evidence from disease phenotypes and animal models (discussed below) suggests that peripherin-2 may be differentially required for rods and cones, thus adding a complicating factor for gene therapy.
RETINAL DISEASE PHENOTYPES ASSOCIATED WITH THE PRPH2 GENE
Mutations in PRPH2 lead to a wide variety of disease phenotypes (excellently reviewed in Boon et al. 2008). PRPH2-associated disease is dominantly inherited and, depending on the mutation, can manifest as autosomal-dominant retinitis pigmentosa (ADRP), digenic ADRP, pattern dystrophy (including butterfly shaped pigment dystrophy and adult vitelliform macular dystrophy), central areolar choroidal dystrophy, and other forms of late-onset macular degeneration (MD) (see http://www.retina-international.org/sci-news/rdsmut.htm). PRPH2-associated monogenic and digenic forms of ADRP primarily target rod photoreceptors; patients usually present with night blindness and progressive loss of peripheral vision. They typically exhibit standard clinical signs of retinitis pigmentosa, including decline of the rod electroretinogram (ERG) response, appearance of bone spicules in the fundus and attenuation of retinal arterioles (Boon et al. 2008). Although the age of onset can be as early as the first to third decade of life in a few cases (Farrar et al. 1991; Gruning et al. 1994), disease presentation typically occurs between the third and fifth decades (Saga et al. 1993; Gruning et al. 1994).
The age of onset for macular and cone-dominant diseases is often similar to that for ADRP, but clinical signs differ dramatically. Although there is a wide spectrum of clinical phenotypes, patients with cone-dominant disease often experience central vision loss (Wickham et al. 2009) and have abnormal multifocal or pattern ERGs, even in the presence of a normal full-field ERG. When full-field ERG defects are present, consistent with the cone-dominant nature of the disease, cone function is usually affected earlier and more severely than rod function (Boon et al. 2008). Common clinical signs include abnormal fundus phenotypes characterized by central retinal pigmentation changes, regions of hyperfluorescence and hypofluorescence, and macular defects (Michaelides et al. 2005; Wickham et al. 2009). In addition, patients often exhibit atrophy of the retinal pigment epithelium (RPE) and choriocapillaris, and sometimes choroidal neovascularization (Boon et al. 2007b; Vaclavik et al. 2012), phenotypes that are thought to contribute to the severity of the vision loss (Moshfeghi et al. 2006; Vaclavik et al. 2012).
One difficulty in defining disease type is that there is often transition from one clinical classification to another; for example, patients that begin by presenting with a purely MD phenotype will later transition to a more general cone-rod or rod-cone dystrophy (Boon et al. 2008). In addition, there can be pronounced inter- and intra-familial phenotypic variability, even among patients all carrying the same mutant allele (Michaelides et al. 2005; Boon et al. 2007a; Vaclavik et al. 2012). Recently, it has been suggested that some of this variability may be a result of the presence of other genetic modifiers. Two genes have thus far been proposed as genetic modifiers: ROM1, mutations in which have only thus far associated with digenic RP (with PRPH2 [Kajiwara et al. 1994]), and ABCA4, mutations in which are typically associated with autosomal recessive Stargardt dystrophy (Koenekoop 2003), but which have also been reported to cause some visual impairment in patients carrying only one mutant allele (Maia-Lopes et al. 2008). One study assessing potential modifiers for PRPH2-associated disease reported that patients carrying only the R172W PRPH2 mutation (i.e., no alterations in ROM1 or ABCA4) exhibited well-characterized MD, while those carrying only sequence alterations in ROM1 or ABCA4 exhibited only mild phenotypic abnormalities, such as slight lowering of multifocal ERG amplitudes in the central retina (Poloschek et al. 2010). However, clinical phenotypes were worsened and accelerated when mutations in ROM1 and/or ABCA4 accompanied the R172W PRPH2 mutation (Poloschek et al. 2010). However, in other families with PRPH2-associated disease phenotypes, changes in ROM1 have been ruled out as a modifying factor (Leroy et al. 2007), underlining the fact that this issue remains under exploration, and the presence of modifying factors is likely to be highly variable. The multiplicity of mutant alleles and potential modifiers combined with the variability of disease phenotypes contributes to the difficulty in designing rational treatment strategies for PRPH2-associated diseases.
ANIMAL MODELS FOR PERIPHERIN-2 ASSOCIATED DISEASES
One of the greatest assets for the development of PRPH2-associated genetic therapies is the availability of a plethora of well-characterized animal models, which mimic a variety of the human disease phenotypes. In addition to serving as systems for preclinical testing, these models have also been critical for our understanding of the way peripherin-2 functions in rods and cones, both normally and in the presence of disease-causing mutations. The first, and still predominant, peripherin-2 animal model is the naturally occurring rds mutant mouse (also known as rd2). It was first described in 1978 (van Nie et al. 1978) as a model exhibiting slow retinal degeneration (Jansen and Sanyal 1984; Reuter and Sanyal 1984; Sanyal and Zeilmaker 1984; Hawkins et al. 1985), and, subsequently, the genetic and molecular defect was localized to the peripherin-2 gene (Travis et al. 1989, 1991; Arikawa et al. 1992). This mouse carries a large insertion in the first intron of the gene and no detectable peripherin-2 protein is produced (Ma et al. 1995; Ding et al. 2004), making it a functional knockout model. In addition to slow photoreceptor cell loss, mice homozygous for the mutant allele (rds−/−) form no OSs (Jansen and Sanyal 1984). Instead, the connecting cilium terminates with a small membranous bleb, and the subretinal space is filled with abnormal membranous material. In the absence of OSs, the photopigment rhodopsin accumulates in the inner segment and in these abnormal extracellular membranes (Usukura and Bok 1987), a phenomenon thought to contribute to retinal degeneration in the rds−/− model. In addition to these severe structural defects, the mouse exhibits no significant rod or cone photoreceptor ERG signal (Reuter and Sanyal 1984; Chakraborty et al. 2009), emphasizing the role of peripherin-2 in OS morphogenesis and the importance of the elaborated OS structures for proper vision.
Mice heterozygous for the rds mutant allele exhibit a striking haploinsufficiency phenotype similar to that seen in patients with ADRP (Cheng et al. 1997). In contrast to the rds−/− mice, rds+/− mice do form OSs, but they are short and highly abnormal, characterized by whorls of disc membranes rather than properly formed and aligned discs. The rds+/− mice also exhibit gradual photoreceptor degeneration, but it is much slower than in the rds−/− animals. These structural defects are associated with severe, early onset loss of rod function, detectable as early as ERGs are recorded, with only late-onset cone dysfunction, first appearing ∼4 mo of age (Cheng et al. 1997). Because of the genetic (i.e., one wild-type, WT, allele and one mutant allele) and phenotypic (early rod functional deficits followed by late cone deficits) characteristics, the rds+/− mouse has been extensively used as a model for PRPH2-associated ADRP.
Because PRPH2 mutations are also associated with cone-dominant macular diseases but the WT mouse has very few cones, a cone-dominant peripherin-2 knockout was generated by crossing rds−/− mice onto the Nrl−/− background. Nrl is a transcription factor required for the development of rod cells, and in its absence developing rods adopt a cone-like fate (Mears et al. 2001). Studies in rds−/−/Nrl−/− mice have highlighted the differential role of peripherin-2 in rods and cones. In contrast to rods lacking peripherin-2 (in the WT background), cones lacking peripherin-2 (in the rds−/−/Nrl−/− mice) form OSs, albeit dysmorphic ones (Farjo et al. 2006b, 2007). These OSs lack the elaborate folded lamellae structure of normal cones and have no rims at all, but nonetheless retain the ability to mediate phototransduction.
In addition to knockout models, several transgenic models that express different disease-causing-mutations in peripherin-2 have been generated. These include models carrying the ADRP mutations C214S (Stricker et al. 2005; Nour et al. 2008) and P216L (Kedzierski et al. 1997, 2001), and the MD mutation R172W (Ding et al. 2004; Conley et al. 2007, 2014). By crossing these mice onto different rds backgrounds, researchers have been able to study the associated disease mechanisms. Expression of the C214S allele on a WT background causes no defects; however, when expressed on the rds+/− background (as would be the case in patients), the phenotype is quite similar to that of the nontransgenic rds+/− (Stricker et al. 2005). This observation suggests that the C214S mutation is a loss-of-function allele, and other biochemical studies have shown that the C214S protein is unstable, possibly misfolded, and cannot bind to ROM1, likely leading to aggregation in the inner segment and subsequent degradation (Goldberg et al. 1998; Stricker et al. 2005; Conley et al. 2010). In contrast, expression of the MD mutation R172W causes severe, dominant structural and functional degeneration of cones, on either the WT or rds+/− background. Rod function, on the other hand, is improved by the presence of the R172W transgene (i.e., R172W/rds+/− vs. rds+/−) (Ding et al. 2004). On a biochemical level, the R172W protein traffics normally to the OS and assembles into complexes with ROM1, although these complexes are not quite normal, especially in cones (Ding et al. 2004; Conley et al. 2014). Combined, these data have led to an overarching hypothesis postulating that rods are more sensitive to the total amount of peripherin-2, whereas cones are more sensitive to having properly assembled peripherin-2 complexes. The corollary to this is that rod-dominant, PRPH2-associated disease (e.g., ADRP) is caused by haploinsufficiency, whereas cone-dominant disease is caused by toxic dominant-negative mutations.
Although this hypothesis may be generally useful, the situation is likely more complicated. In the first place, transgenic mice carrying the P216L ADRP mutation exhibit some dominant-negative rod defects such as worsening in OS ultrastructure in P216L versus WT eyes and faster degeneration in P216L/rds+/− eyes than in rds+/− eyes (Kedzierski et al. 1997). This observation suggests that while haploinsufficiency certainly causes rod-dominant disease, some ADRP mutant alleles may also have toxic gain-of-function or dominant-negative effects in rods. In addition, the mechanism by which R172W (and possibly other PRPH2 mutations) causes MD is likely more complex than a primary defect in cones. A key phenotype of PRPH2-associated MD (including that associated with R172W) is maculopathy and the development of atrophic patches in the RPE, which are visible by fundus examination (Downes et al. 1999) and often appear before ERG changes or vision loss are apparent. The severe MD coupled with choroidal/RPE atrophy seen in PRPH2-associated MD patients is therefore likely the result of a combination of primary molecular defects in cone photoreceptors (caused by mutant peripherin-2 protein) coupled with secondary sequellae that impact the RPE as well as the choriocapillaris and choroid. These phenotypes likely present first in the macula, due to the concentration of cones in that region and the metabolic demands on the RPE there, but at a molecular level probably occur throughout the retina (Boon et al. 2008). Exploring the connection between the primary photoreceptor defect and the development of macular changes in the RPE/choroid will be critical for the development of effective genetic therapies. Unfortunately, because the mouse lacks a macula, modeling this interaction is not necessarily straightforward.
GENE THERAPY APPROACHES
There are at least four different gene therapy approaches for the treatment of PRPH2-associated retinal disease: (1) gene replacement therapy; (2) gene knockdown and replacement therapy; (3) delivery of neurotrophic factors; and (4) genetic treatment of disease sequellae such as neovascularization. Typical gene delivery requires packaging of the genetic material of interest (DNA or RNA) into either viral or nonviral particles, as DNA alone is not readily taken up by photoreceptors (Farjo et al. 2006a; Cai et al. 2009). Delivery is usually by subretinal injection to promote access to photoreceptors; however, the invasive nature of this approach makes development of effective alternatives a high research priority. There is great variety in the types of nucleic acid packaging approaches that can be used for ocular gene therapy, and as they have been reviewed elsewhere (e.g., Conley and Naash 2010; Han et al. 2011) we will not discuss them here.
Gene Replacement Therapy with Transgenes in Peripherin-2 Models
The earliest proof-of-principle for PRPH2 gene replacement therapy consisted of attempts to correct the degenerative phenotypes in the rds−/− and rds+/− by transgenic expression of WT peripherin-2. These attempts were highly successful; expression of a WT peripherin-2 transgene in rods driven by the rod-opsin promoter on the rds−/− background preserved photoreceptors and rod OS ultrastructure (Travis et al. 1992). Similarly, subsequent studies showed that expression of a WT peripherin-2 transgene in rods and cones driven by the interphotoreceptor retinoid binding protein (IRBP) promoter rescued rod and cone OS structure and function when expressed on the rds+/− background (Nour et al. 2004). These studies were later extended to show that transgenic expression of WT peripherin-2 could mediate structural and functional improvement in the presence of disease-causing mutations associated with ADRP (C214S) and MD (R172W) (Conley et al. 2007; Nour et al. 2008). Several observations that arose from these studies have a direct bearing on the success of future gene therapy studies. The first was that the dose of the transgene was absolutely critical. This is not surprising given the haploinsufficiency phenotype associated with ADRP in patients, but was reemphasized in the transgenic studies. Models carrying anywhere from 10% (Travis et al. 1992) to 150% (Nour et al. 2004) of WT peripherin-2 were characterized and the amount of peripherin-2 correlated with the severity of the phenotype. Mice expressing ∼80% of WT levels had significant structural and functional improvement compared with haploinsufficient rds+/− mice, but photoreceptors still exhibited some defects. Lower levels of expression resulted in correspondingly more severe phenotypes. Positively, overexpression of peripherin-2 elicited no toxic outcomes; so while having too little peripherin-2 results in incomplete rescue, having too much is not detrimental (Nour et al. 2004). The second interesting observation was that WT peripherin-2 expression mediated structural and functional improvements even in the presence of dominant-negative mutations such as R172W (Conley et al. 2007). Unfortunately, although some improvement was noted, it was not to the same magnitude and did not last as long as the improvement observed when WT peripherin-2 was expressed in the presence of loss-of-function mutations (e.g., C214S) associated with ADRP (Nour et al. 2008).
The partial rescue we observe with transgenesis in R172W animals suggests that the R172W mutation behaves as a dominant-negative (rather than a toxic gain-of-function mutation) and thus may be at least somewhat amenable to rescue via gene supplementation. However, it is not clear whether other dominantly inherited MD mutations in PRPH2 are gain-of-function or dominant-negatives. Traditional gain-of-function mutations would not likely be rescued by gene supplementation, and the only partial rescue seen in the R172W model suggests that that full rescue for dominant-negative or gain-of-function mutations may require concurrent knockdown of the mutant allele.
Gene Replacement Therapy with Adeno-Associated Viruses (AAV) in Peripherin-2 Models
In the last 15 years, gene replacement therapy for peripherin-2 has been attempted using both viral and nonviral approaches. Initial studies by Dr. Robin Ali’s group used recombinant AAV (rAAV) carrying a 2.2 kb fragment of the rod-opsin promoter and the murine peripherin-2 cDNA (rAAV.rho.prph2) (Ali et al. 2000). This approach has been quite promising for other ocular gene therapy studies, and AAVs are currently in clinical trials for the treatment of RPE-associated Leber congenital amaurosis (recently reviewed in Boye et al. 2013). Initially, rAAV.rho.prph2 was delivered subretinally to rds−/− animals at postnatal day (P) 10, and animals were assessed at times ranging from 3–9 wk postinjection (Ali et al. 2000). The investigators reported exciting improvements in scotopic ERG amplitudes and rod OS structure (Ali et al. 2000), although levels did not meet those seen in WT or rds+/− animals. Subsequent studies evaluated the efficacy of treatment at multiple ages (ranging from P5 to P95), with evaluation at 1 mo postinjection (Sarra et al. 2001). Although the best improvements in OS structure were observed when treatment was delivered early (P5 or P10), some minor improvements were noted at later delivery ages. Interestingly, in spite of transducing ∼30%–40% of photoreceptors, there was no reduction in the rate or magnitude of photoreceptor cell loss after treatment with rAAV.rho.prph2. The investigators hypothesize that this is tied to the fact that the onset of expression of the rAAV is ∼2 wk postinjection (Sarra et al. 2001), a time point at which the rds−/− retina is already undergoing significant degeneration. Although this provides another argument for early intervention and the development of treatments with rapid-onset expression, it is worth remembering that degeneration in the rds+/− retina (which more closely mimics that in patients) is much slower than in the rds−/− retina.
In a final study, researchers asked whether multiple injections could enhance the beneficial effect and how long the benefits of AAV-associated therapy would last (Schlichtenbrede et al. 2003). Repeat injection 5 d after the initial treatment (at P15) resulted in better rescue than was achieved with a single injection, but in both cases improvements in scotopic b-wave amplitude peaked at 5 wk postinjection, and by 15 wk postinjection retinal function in treated rds−/− mice was comparable to levels observed in untreated rds−/− animals (Schlichtenbrede et al. 2003). Although these results were promising, the magnitude of the improvement was fairly small; maximum b-wave amplitudes in animals treated with AAV were ∼150 µV (improved from ∼60 µV in uninjected rds−/− mice) compared to 400–500 µV in age-matched WT animals (Schlichtenbrede et al. 2003). Furthermore, significant improvements in scotopic a-wave amplitudes, a more direct measure of rod photoreceptor function, were not reported. Excitingly, the AAVs appeared to be well-tolerated and there was no sign of an inflammatory response in any of the eyes tested (Ali et al. 2000).
The reasons for this incomplete rescue may be tied to the partial transduction (investigators estimate ∼30% transduction) or the levels of expression, but also highlight some issues related to the rds−/− model. This model has the benefit of “starting from zero”; that is, because there are no detectable OSs and no detectable function or protein, small improvements are easy to detect. On the other hand, the absence of even baseline OS structure/function and the ongoing degeneration may make it more difficult to initiate OS formation by exogenous therapeutic delivery. More importantly, this model does not accurately mimic the case in patients who have haploinsufficiency-associated disease. A more clinically relevant model would be the rds+/− mouse, which has a much slower rate of degeneration and exhibits OSs, albeit highly malformed. However, it is not clear whether improvements on the scale reported with AAV would be detectable in that model.
Gene Replacement Therapy with Nanoparticles in Peripherin-2 Models
Based on the positive initial results using AAV to deliver peripherin-2, we have more recently explored the use of compacted DNA nanoparticles (NPs) to deliver WT peripherin-2. These NPs are composed of polyethylene glycol-conjugated polylysine (CK30-PEG) and a plasmid DNA, and we have shown that they are capable of transducing a variety of retinal cell types (Farjo et al. 2006a; Han et al. 2012a). In addition to our murine studies on PRPH2-associated ADRP (discussed further below), we have showed that these NPs can mediate long-term ocular phenotypic improvements in mouse models of RPE65-associated Leber congenital amaurosis (Koirala et al. 2013a,b) and ABCA4-associated Stargardt macular dystrophy (Han et al. 2012b). Importantly, these NPs are well-tolerated in the retina, even after repeat injections (Ding et al. 2009; Cai et al. 2010; Han et al. 2012a,c), and have a large DNA capacity (tested up to 14 kb in the eye [Han et al. 2012b] and 20 kb in the lung [Fink et al. 2006]). To determine whether they were capable of mediating improvements in the PRPH2-associated retinitis pigmentosa phenotype, we generated NPs carrying the full-length mouse peripherin-2 cDNA (called NMP for normal mouse peripherin-2) under the control of the rod- and cone-specific IRBP promoter or the ubiquitously expressed chicken beta actin (CBA) promoter (Cai et al. 2009). We elected to use the rds+/− model, as it more closely mimics the patient situation, and subretinally delivered the NPs (or controls) at P5. Gene expression and disease phenotypes were tracked for up to 4 mo postinjection. We observed appreciable expression levels of the transferred gene message as early as 2 d postinjection, and by 1 wk postinjection, levels of peripherin-2 message in treated eyes had stabilized at levels two- to threefold higher than was observed in uninjected eyes, and these levels persisted throughout the study (Cai et al. 2009). As controls, we injected eyes with naked (i.e., uncompacted) plasmid; no increases in gene expression were observed in eyes treated with naked DNA compared to uninjected controls. The NP-mediated expression of peripherin-2 resulted in improved photoreceptor OS structure at both 1 and 4 mo postinjection, detected mostly in the region near the site of injection. Functionally we observed small but nonsignificant improvements in scotopic a-wave, and significant improvement in photopic (cone)-b waves, for NP-injected eyes compared to naked DNA injected eyes (Cai et al. 2009). Interestingly, there was no significant difference in efficacy for the two promoters.
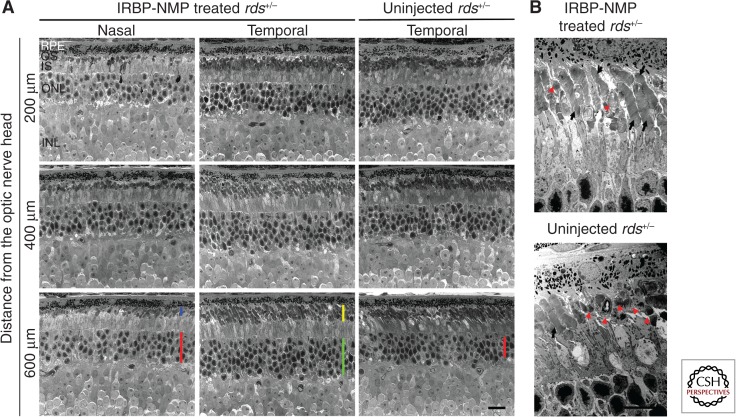
To qualitatively assess the longevity of phenotypic improvement, a small subset of rds+/− animals injected at P5 with IRBP-NMP NPs were aged out to 15 mo postinjection. We observed pronounced preservation of outer nuclear layer thickness in the treated eye compared to a matched region from the uninjected contralateral eye (Fig. 1A, green bar vs. red bar in lower panels). This preservation was most pronounced in the peripheral retina and on the side of the eye at which injection had occurred (see the red bars in the lower left column of Fig. 1A). We also observed that the thickness of the OS layer was better preserved on the side of the eye that had been injected compared to the opposing side (Fig. 1A, yellow vs. blue bar in lower panels). This preservation was reflected on the ultrastructural level as well (Fig. 1B) in the region of injection. Whereas nicely stacked (black arrows) and highly dysmorphic (red arrowheads) OSs were observed in both the IRBP-NMP NP-treated eye and the uninjected control, overall ultrastructure was improved in the NP-treated eye (Fig. 1B). These data suggest that compacted-DNA NPs can mediate long-term improvements in retinal structure, but only in a limited region of the treated retina.
Figure 1.
IRBP NPs carrying peripherin-2 mediate improvements in retinal function at 15 mo postinjection. At P5, rds+/− animals were injected in one eye with compacted DNA NPs carrying the peripherin-2 cDNA (NMP) under the control of the IRBP promoter. The contralateral eye served as an uninjected control. Fifteen months later, eyes were harvested for histological analysis. (A) Light micrographs showing improved outer nuclear layer (green and red bars) and OS thickness (blue and yellow bars) in the peripheral retina on the side of the eye that received the injection. NMP, peripherin-2-carrying nanoparticles; RPE, retinal pigment epithelium; OS, outer segment; IS, inner segment, ONL, outer nuclear layer; INL, inner nuclear layer. Scale bar, 25 µm. (B) Transmission electron micrographs of the same eyes taken from the temporal region. Black arrows identify nicely stacked OSs whereas red arrowheads show the dysmorphic swirl OS typical of rds+/− eyes. Scale bar, 10 µm.
In common with those testing AAVs, we also asked whether treatment of rds+/− at a later age (P22) could mediate improvements in photoreceptor structure and function. For these studies, we elected to use NPs carrying the murine peripherin-2 cDNA (NMP) under the control of a 221 bp fragment of the mouse opsin promoter (MOP), which is expressed in rods and cones (Glushakova et al. 2006). As before, we observed only small, nonsignificant improvements in the scotopic a-wave after treatment with MOP-NMP NPs irrespective of treatment age (P5 or P22), although modest improvements in the scotopic b-wave were observed after treatment at either age. Again, we observed significant improvement in cone function (photopic b-wave) up to 4 mo posttreatment—the longest period tested—compared to controls injected with naked DNA. Although some functional improvements were detected after treatment at P22, structural improvements were quite minor compared to those seen after treatment at P5, confirming that earlier intervention yields better results. Because ERG does not always correlate with vision, we also tested visual acuity and contrast sensitivity under photopic conditions using the OptoMotry system in a subset of eyes treated at P5 with MOP-NMP NPs (Cai et al. 2010). Assessments were performed at 9–10 mo postinjection; treated animals exhibited visual acuities and contrast sensitivities similar to those observed with WT animals, whereas saline-injected eyes were not different from age-matched uninjected controls.
Combined, these studies suggest that gene augmentation with either NPs or AAV is feasible for PRPH2-associated RP; however, achieving full rescue has been difficult, likely because of the requirements of a precise dose of peripherin-2 to support OS structure and the difficulty in transfecting the whole retina.
Gene Knockdown Therapy in Peripherin-2 Models
Because PRPH2-associated disease is often caused by gain-of-function or dominant-negative alleles (such as R172W) and pure gene replacement (e.g., with transgenes) improved but did not completely correct the dominant phenotype in mice carrying that mutation (Conley et al. 2007), knockdown approaches may need to be developed to eliminate the mutant allele. From a practical standpoint, these will need to be combined with gene replacement therapy, as peripherin-2 haploinsufficiency makes knockdown of the mutant allele alone unlikely to be successful. Furthermore, the sheer number of different mutant alleles combined with the infrequency of any given allele means that developing a knockdown strategy for each mutation would be both time consuming and economically disadvantageous. As a result, development of a mutation-independent knockdown approach is desirable. This approach has been explored with relative success for rhodopsin gene therapy. Like PRPH2, there are a plethora of different dominant, disease-causing mutant rhodopsin alleles that cause inherited retinal degeneration, and knockdown has been attempted in vitro and in vivo with varying degrees of success using multiple methods, including ribozymes (Chakraborty et al. 2008b), siRNA (Teusner et al. 2006; Gorbatyuk et al. 2007; O’Reilly et al. 2007; Mao et al. 2012), and zinc finger nucleases (Mussolino et al. 2011). Thus far, shRNA is the most well-developed approach, and recent results suggest that a single AAV vector carrying an shRNA capable of knocking down WT- and mutant rhodopsin, coupled with a WT rhodopsin that is resistant to silencing, results in long-term (up to 9 mo of age) preservation of photoreceptor structure and function in the P23H mouse model of rhodopsin-associated ADRP (Mao et al. 2012).
Recently, the same group that has led the field in the development of rhodopsin-associated knockdown has begun to apply this approach to peripherin-2 (Petrs-Silva et al. 2012). After identifying two different siRNAs that could knockdown WT peripherin-2 in vitro, researchers packaged the shRNAs into rAAV vectors and delivered them to the subretinal space of WT mice. Peripherin-2 message was reduced by ∼75% in shRNA-treated eyes, and a corresponding 30%–50% decrease in the scotopic a-wave was reported. Subsequently, a version of peripherin-2 resistant to the shRNAs was placed under control of the CBA promoter in an AAV vector and codelivered with the shRNA AAV into WT mice. Codelivery of the two viruses led to rescue of the functional defect caused by the shRNA knockdown and partial recovery of total peripherin-2 protein levels (Petrs-Silva et al. 2012). A similar approach was taken by different investigators, who injected WT mice with a shRNA vector and a resistant WT peripherin-2 gene, using electroporation rather than viral compaction. They reported knockdown of the endogenous WT allele and stable expression of the resistant, exogenously delivered allele (Palfi et al. 2006). Although the efficacy of this approach in disease models has yet to be evaluated, these exciting studies show proof of principle for allele-independent knockdown combined with gene supplementation to combat PRPH2-associated disease.
Gene-Based Delivery of Neurotrophic Factors in the Peripherin-2 Model
Although gene augmentation therapy is the most obvious strategy for PRPH2-associated disease, delivery of neurotrophic factors was actually the first option to be explored. This approach does not correct the underlying genetic defect, but it has the benefit of being inherently allele- and gene-independent and therefore potentially applicable to a variety of forms of degeneration. In 1998, a French group delivered ciliary neurotrophic factor (CNTF) to the intravitreal space of rds−/− mice using an adenoviral vector (Cayouette et al. 1998). Excitingly, they reported preservation of outer nuclear layer thickness at 2 wk and 2 mo postinjection. However, they reported only very small improvements in rod function and no improvements in cone function. The retardation of retinal degeneration was enough to promote further research in that direction, however, and a separate group reported similar preservation of structure but not function after AAV-mediated delivery of CNTF in the rds−/− model (Liang et al. 2001). In later work, rAAV was also used to deliver CNTF in the P216L/rds+/− ADRP model. A rescue of retinal degeneration similar to that seen with the rds−/− mouse was reported, but the investigators also observed dose-dependent abnormalities in photoreceptor nuclei and a decrease in rod and cone function (Bok et al. 2002). Subsequent studies using this model showed that CNTF significantly altered retinal signaling pathways and down-regulated many important phototransduction genes, including cone opsins (Rhee et al. 2007). Although this toxicity precludes the further use of CNTF for PRPH2-associated disease, other neurotrophic factors have also been tested. Lentivirus carrying either human fibroblast growth factor-2 (FGF-2) or human pigment epithelial-derived factor (PEDF) was reported to improve scotopic a- and b-waves in rds−/− mice, but it did not improve photoreceptor survival (Miyazaki et al. 2008). Conclusions from that study are complicated, however, by significant inconsistencies between the baseline ERG values reported (Miyazaki et al. 2008) and those from other groups using the same model (Reuter and Sanyal 1984; Ali et al. 2000; Chakraborty et al. 2009). Many neurotrophic factors have been explored for the prevention of degeneration in other forms of retinal degeneration, and one may be identified which is clearly beneficial for PRPH2-associated disease. However, it is evident that extensive testing in the right models will be required to identify a clinically relevant choice.
Other drugs and hormones which do not fall into the category of traditional neurotrophic factors have also been used to provide some level of neuroprotection (i.e., retardation of retinal degeneration) and, in some cases, improved function, in the rds−/− and rds+/− models. These include the hormone erythropoietin (Rex et al. 2004, 2009), the calcium channel blocker nilvadipine (Takeuchi et al. 2008), and the monoamine oxidase inhibitor rasagiline (Eigeldinger-Berthou et al. 2012). It is possible that genetic interference in the pathways regulated by these compounds may prove to be a beneficial approach, or that simple delivery of pharmacologic agents in conjunction with gene supplementation therapy may be effective. Further studies will be needed to assess the safety and efficacy of this method.
Antineovascularization Therapy for Peripherin-2-Associated Disease
The final type of potential genetic therapy is targeted at the specific clinical signs of disease. Much like neurotrophic therapy, this approach does not correct the underlying genetic defect, and it is also allele- and gene-independent. As in most age-related MD, vision loss in many forms of PRPH2-associated macular- or pattern dystrophy is caused or exacerbated by neovascularization of the retina or choroid (Moshfeghi et al. 2006; Boon et al. 2008). In a patient carrying the PRPH2-pattern dystrophy mutation Y141C who exhibited choroidal neovascularization, repeat injections with the anti-VEGF drug ranibizumab ameliorated the neovascularization and improved visual acuity (Vaclavik et al. 2012). Benefits have also been reported in other studies using anti-VEGF drugs (bevacizumab or ranibizumab) to treat various forms of pattern dystrophy-associated choroidal neovascularization (Parodi et al. 2010; Gallego-Pinazo et al. 2011). Because repeated injections of this type of drug are usually required for efficacy, age-related MD research has focused on development of effective, long-lasting genetic therapies that can inhibit VEGF-associated neovascularization (e.g., Askou et al. 2012; Pihlmann et al. 2012), and has resulted in the initiation of several clinical trials (NCT01494805, NCT01367444, NCT01301443, NCT01024998). This approach has not been directly tested in rds models, but utilization of anti-VEGF drugs in PRPH2 patients with neovascularization may have merit.
CONCLUDING REMARKS
Here we have discussed a variety of different options for the genetic treatment of PRPH2-associated ADRP and MD. Because of the differential requirements for peripherin-2 for OS structure and function in rods and cones, effective gene augmentation for loss-of-function alleles or gene knockdown plus supplementation for gain-of-function alleles will likely be a mainstay of any truly curative therapy. However, given that vision loss is sometimes associated with secondary (i.e., nonphotoreceptor) defects such as choroidal neovascularization, combinatorial therapy that includes a neuroprotective or antineovascular agent could increase treatment efficacy.
Several obstacles to the development of targeted genetic therapies for peripherin-2 remain. These include the lack of understanding of the actual disease mechanism for PRPH2-associated macular disease, and specifically a lack of understanding of the connection between primary photoreceptor defects that are well-tolerated for several decades and secondary RPE/choroidal defects that lead to late-onset vision loss. The lack of a mouse model with a macula and the large clinical and genetic heterogeneity in peripherin-2 patient populations further confound studies of this disease mechanism. Another complicating factor is the structural nature of the peripherin-2 protein, and the associated requirement for a very precise dose. Peripherin-2 haploinsufficiency means that effective disease treatment must generate a fairly large quantity of protein, and a result of constant OS renewal, effective treatments must result in prolonged, elevated gene expression. In addition, early treatment is clearly optimal, but the age at which treatment of mice is most effective (P5 to P10) has an in utero developmental equivalent in humans; that is, by birth the human retina has already passed the stage that has been identified as optimal for gene delivery in mice. This, combined with the fact that disease presentation often follows, rather than precedes, the onset of degeneration, means that early intervention is nearly impossible. Finally, there are obstacles that affect many forms of photoreceptor-associated gene therapy, such as the difficulty in achieving pan-retinal transfection and the difficulty of transfecting photoreceptors to any extent. Although both AAVs and compacted DNA NPs can transfect photoreceptors, they are not nearly as easy to target as other cell types (such as the adjacent RPE). This has implications for the development and targeting of delivery strategies. In addition, expression is usually limited to the region of retina detached during the subretinal injection procedure (i.e., the retinal bleb). Because retinal detachment in itself can be harmful, development of effective intravitreal gene delivery vehicles would be ideal.
Despite these limitations, advances in vector engineering to increase, prolong, and regulate gene expression, combined with advances in delivery technology, such as precisely engineered AAV capsids and targeted NPs, make the development of a clinically applicable peripherin-2 gene therapy more likely than ever before. Given that current vectors (both AAVs and NPs) are able to drive per cell gene expression at fairly high levels; key targets for immediate preclinical research in this field include increasing distribution of gene expression throughout the retina and development of therapies that are effective after intravitreal delivery.
ACKNOWLEDGEMENTS
The authors thank Ms. Barb Nagel for her excellent technical assistance with the histology. This work was supported by the National Eye Institute (EY10609, EY022778, EY01865 to M.I.N.), the Foundation Fighting Blindness (M.I.N.), and the Oklahoma Center for the Advancement of Science and Technology (M.I.N. and S.M.C.).
Footnotes
Editors: Eric A. Pierce, Richard H. Masland, and Joan W. Miller
Additional Perspectives on Retinal Disorders: Genetic Approaches to Diagnosis and Treatment available at www.perspectivesinmedicine.org
REFERENCES
- Ali RR, Sarra GM, Stephens C, Alwis MD, Bainbridge JW, Munro PM, Fauser S, Reichel MB, Kinnon C, Hunt DM, et al. 2000. Restoration of photoreceptor ultrastructure and function in retinal degeneration slow mice by gene therapy. Nat Genet 25: 306–310 [DOI] [PubMed] [Google Scholar]
- Arikawa K, Molday LL, Molday RS, Williams DS. 1992. Localization of peripherin/rds in the disk membranes of cone and rod photoreceptors: Relationship to disk membrane morphogenesis and retinal degeneration. J Cell Biol 116: 659–667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askou AL, Pournaras JA, Pihlmann M, Svalgaard JD, Arsenijevic Y, Kostic C, Bek T, Dagnaes-Hansen F, Mikkelsen JG, Jensen TG, et al. 2012. Reduction of choroidal neovascularization in mice by adeno-associated virus-delivered anti-vascular endothelial growth factor short hairpin RNA. J Gene Med 14: 632–641 [DOI] [PubMed] [Google Scholar]
- Boesze-Battagliaa K, Stefano FP. 2002. Peripherin/rds fusogenic function correlates with subunit assembly. Exp Eye Res 75: 227–231 [DOI] [PubMed] [Google Scholar]
- Boesze-Battaglia K, Goldberg AF, Dispoto J, Katragadda M, Cesarone G, Albert AD. 2003. A soluble peripherin/Rds C-terminal polypeptide promotes membrane fusion and changes conformation upon membrane association. Exp Eye Res 77: 505–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bok D, Yasumura D, Matthes MT, Ruiz A, Duncan JL, Chappelow AV, Zolutukhin S, Hauswirth W, LaVail MM. 2002. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp Eye Res 74: 719–735 [DOI] [PubMed] [Google Scholar]
- Boon CJ, Klevering BJ, den Hollander AI, Zonneveld MN, Theelen T, Cremers FP, Hoyng CB. 2007a. Clinical and genetic heterogeneity in multifocal vitelliform dystrophy. Arch Ophthalmol 125: 1100–1106 [DOI] [PubMed] [Google Scholar]
- Boon CJ, van Schooneveld MJ, den Hollander AI, van Lith-Verhoeven JJ, Zonneveld-Vrieling MN, Theelen T, Cremers FP, Hoyng CB, Klevering BJ. 2007b. Mutations in the peripherin/RDS gene are an important cause of multifocal pattern dystrophy simulating STGD1/fundus flavimaculatus. Br J Ophthalmol 91: 1504–1511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon CJ, den Hollander AI, Hoyng CB, Cremers FP, Klevering BJ, Keunen JE. 2008. The spectrum of retinal dystrophies caused by mutations in the peripherin/RDS gene. Prog Retin Eye Res 27: 213–235 [DOI] [PubMed] [Google Scholar]
- Boye SE, Boye SL, Lewin AS, Hauswirth WW. 2013. A comprehensive review of retinal gene therapy. Mol Ther 21: 509–519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Nash Z, Conley SM, Fliesler SJ, Cooper MJ, Naash MI. 2009. A partial structural and functional rescue of a retinitis pigmentosa model with compacted DNA nanoparticles. PLoS ONE 4: e5290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X, Conley SM, Nash Z, Fliesler SJ, Cooper MJ, Naash MI. 2010. Gene delivery to mitotic and postmitotic photoreceptors via compacted DNA nanoparticles results in improved phenotype in a mouse model of retinitis pigmentosa. FASEB J 24: 1178–1191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cayouette M, Behn D, Sendtner M, Lachapelle P, Gravel C. 1998. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci 18: 9282–9293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty D, Ding XQ, Fliesler SJ, Naash MI. 2008a. Outer segment oligomerization of Rds: Evidence from mouse models and subcellular fractionation. Biochemistry 47: 1144–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty D, Whalen P, Lewin AS, Naash MI. 2008b. In vitro analysis of ribozyme-mediated knockdown of an ADRP associated rhodopsin mutation. Adv Exp Med Biol 613: 97–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty D, Ding XQ, Conley SM, Fliesler SJ, Naash MI. 2009. Differential requirements for retinal degeneration slow intermolecular disulfide-linked oligomerization in rods versus cones. Hum Mol Genet 18: 797–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty D, Conley SM, Stuck MW, Naash MI. 2010. Differences in RDS trafficking, assembly and function in cones versus rods: Insights from studies of C150S-RDS. Hum Mol Genet 19: 4799–4812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng T, Peachey NS, Li S, Goto Y, Cao Y, Naash MI. 1997. The effect of peripherin/rds haploinsufficiency on rod and cone photoreceptors. J Neurosci 17: 8118–8128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley SM, Naash MI. 2010. Nanoparticles for retinal gene therapy. Prog Retin Eye Res 29: 376–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley S, Nour M, Fliesler SJ, Naash MI. 2007. Late-onset cone photoreceptor degeneration induced by R172W mutation in Rds and partial rescue by gene supplementation. Invest Ophthalmol Vis Sci 48: 5397–5407 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley SM, Stricker HM, Naash MI. 2010. Biochemical analysis of phenotypic diversity associated with mutations in codon 244 of the retinal degeneration slow gene. Biochemistry 49: 905–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley SM, Stuck MW, Naash MI. 2011. Structural and functional relationships between photoreceptor tetraspanins and other superfamily members. Cell Mol Life Sci. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conley SM, Stuck MW, Burnett JL, Chakraborty D, Azadi S, Fliesler SJ, Naash MI. 2014. Insights into the mechanisms of macular degeneration associated with the R172W mutation in RDS. Hum Mol Genet. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding XQ, Nour M, Ritter LM, Goldberg AF, Fliesler SJ, Naash MI. 2004. The R172W mutation in peripherin/rds causes a cone–rod dystrophy in transgenic mice. Hum Mol Genet 13: 2075–2087 [DOI] [PubMed] [Google Scholar]
- Ding XQ, Quiambao AB, Fitzgerald JB, Cooper MJ, Conley SM, Naash MI. 2009. Ocular delivery of compacted DNA-nanoparticles does not elicit toxicity in the mouse retina. PLoS ONE 4: e7410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downes SM, Fitzke FW, Holder GE, Payne AM, Bessant DA, Bhattacharya SS, Bird AC. 1999. Clinical features of codon 172 RDS macular dystrophy: Similar phenotype in 12 families. Arch Ophthalmol 117: 1373–1383 [DOI] [PubMed] [Google Scholar]
- Eigeldinger-Berthou S, Meier C, Zulliger R, Lecaude S, Enzmann V, Sarra GM. 2012. Rasagiline interferes with neurodegeneration in the Prph2/rds mouse. Retina 32: 617–628 [DOI] [PubMed] [Google Scholar]
- Farjo R, Skaggs J, Quiambao AB, Cooper MJ, Naash MI. 2006a. Efficient non-viral ocular gene transfer with compacted DNA nanoparticles. PLoS ONE 1: e38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farjo R, Skaggs JS, Nagel BA, Quiambao AB, Nash ZA, Fliesler SJ, Naash MI. 2006b. Retention of function without normal disc morphogenesis occurs in cone but not rod photoreceptors. J Cell Biol 173: 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrar GJ, Kenna P, Jordan SA, Kumar-Singh R, Humphries MM, Sharp EM, Sheils DM, Humphries P. 1991. A three-base-pair deletion in the peripherin–RDS gene in one form of retinitis pigmentosa. Nature 354: 478–480 [DOI] [PubMed] [Google Scholar]
- Farjo R, Fliesler SJ, Naash MI. 2007. Effect of Rds abundance on cone outer segment morphogenesis, photoreceptor gene expression, and outer limiting membrane integrity. J Comp Neurol 504: 619–630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fink TL, Klepcyk PJ, Oette SM, Gedeon CR, Hyatt SL, Kowalczyk TH, Moen RC, Cooper MJ. 2006. Plasmid size up to 20 kbp does not limit effective in vivo lung gene transfer using compacted DNA nanoparticles. Gene Ther 13: 1048–1051 [DOI] [PubMed] [Google Scholar]
- Gallego-Pinazo R, Dolz-Marco R, Pardo-Lopez D, Arevalo JF, Diaz-Llopis M. 2011. Primary intravitreal ranibizumab for adult-onset foveomacular vitelliform dystrophy. Graefes Arch Clin Exp Ophthalmol 249: 455–458 [DOI] [PubMed] [Google Scholar]
- Glushakova LG, Timmers AM, Issa TM, Cortez NG, Pang J, Teusner JT, Hauswirth WW. 2006. Does recombinant adeno-associated virus-vectored proximal region of mouse rhodopsin promoter support only rod-type specific expression in vivo? Mol Vis 12: 298–309 [PubMed] [Google Scholar]
- Goldberg AF, Molday RS. 1996. Subunit composition of the peripherin/rds-rom-1 disk rim complex from rod photoreceptors: Hydrodynamic evidence for a tetrameric quaternary structure. Biochemistry 35: 6144–6149 [DOI] [PubMed] [Google Scholar]
- Goldberg AF, Loewen CJ, Molday RS. 1998. Cysteine residues of photoreceptor peripherin/rds: Role in subunit assembly and autosomal dominant retinitis pigmentosa. Biochemistry 37: 680–685 [DOI] [PubMed] [Google Scholar]
- Gorbatyuk M, Justilien V, Liu J, Hauswirth WW, Lewin AS. 2007. Suppression of mouse rhodopsin expression in vivo by AAV mediated siRNA delivery. Vision Res 47: 1202–1208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruning G, Millan JM, Meins M, Beneyto M, Caballero M, Apfelstedt-Sylla E, Bosch R, Zrenner E, Prieto F, Gal A. 1994. Mutations in the human peripherin/RDS gene associated with autosomal dominant retinitis pigmentosa. Hum Mutat 3: 321–323 [DOI] [PubMed] [Google Scholar]
- Han Z, Conley SM, Naash MI. 2011. AAV and compacted DNA nanoparticles for the treatment of retinal disorders: Challenges and future prospects. Invest Ophthalmol Vis Sci 52: 3051–3059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Conley SM, Makkia R, Guo J, Cooper MJ, Naash MI. 2012a. Comparative analysis of DNA nanoparticles and AAVs for ocular gene delivery. PLoS ONE 7: e52189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Conley SM, Makkia RS, Cooper MJ, Naash MI. 2012b. DNA nanoparticle-mediated ABCA4 delivery rescues Stargardt dystrophy in mice. J Clin Invest 122: 3221–3226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han Z, Koirala A, Makkia R, Cooper MJ, Naash MI. 2012c. Direct gene transfer with compacted DNA nanoparticles in retinal pigment epithelial cells: Expression, repeat delivery and lack of toxicity. Nanomedicine (Lond) 7: 521–539 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawkins RK, Jansen HG, Sanyal S. 1985. Development and degeneration of retina in rds mutant mice: Photoreceptor abnormalities in the heterozygotes. Exp Eye Res 41: 701–720 [DOI] [PubMed] [Google Scholar]
- Jansen HG, Sanyal S. 1984. Development and degeneration of retina in rds mutant mice: electron microscopy. J Comp Neurol 224: 71–84 [DOI] [PubMed] [Google Scholar]
- Kajiwara K, Berson EL, Dryja TP. 1994. Digenic retinitis pigmentosa due to mutations at the unlinked peripherin/RDS and ROM1 loci. Science 264: 1604–1608 [DOI] [PubMed] [Google Scholar]
- Kedzierski W, Lloyd M, Birch DG, Bok D, Travis GH. 1997. Generation and analysis of transgenic mice expressing P216L-substituted rds/peripherin in rod photoreceptors. Invest Ophthalmol Vis Sci 38: 498–509 [PubMed] [Google Scholar]
- Kedzierski W, Nusinowitz S, Birch D, Clarke G, McInnes RR, Bok D, Travis GH. 2001. Deficiency of rds/peripherin causes photoreceptor death in mouse models of digenic and dominant retinitis pigmentosa. Proc Natl Acad Sci 98: 7718–7723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khattree N, Ritter LM, Goldberg AF. 2013. Membrane curvature generation by a C-terminal amphipathic helix in peripherin-2/rds, a tetraspanin required for photoreceptor sensory cilium morphogenesis. J Cell Sci 126: 4659–4670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenekoop RK. 2003. The gene for Stargardt disease, ABCA4, is a major retinal gene: A mini-review. Ophthalmic Genet 24: 75–80 [DOI] [PubMed] [Google Scholar]
- Koirala A, Conley SM, Makkia R, Liu Z, Cooper MJ, Sparrow JR, Naash MI. 2013a. Persistence of non-viral vector mediated RPE65 expression: Case for viability as a gene transfer therapy for RPE-based diseases. J Control Release 172: 745–752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koirala A, Makkia RS, Conley SM, Cooper MJ, Naash MI. 2013b. S/MAR-containing DNA nanoparticles promote persistent RPE gene expression and improvement in RPE65-associated LCA. Hum Mol Genet 22: 1632–1642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leroy BP, Kailasanathan A, De Laey JJ, Black GC, Manson FD. 2007. Intrafamilial phenotypic variability in families with RDS mutations: Exclusion of ROM1 as a genetic modifier for those with retinitis pigmentosa. Br J Ophthalmol 91: 89–93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FQ, Aleman TS, Dejneka NS, Dudus L, Fisher KJ, Maguire AM, Jacobson SG, Bennett J. 2001. Long-term protection of retinal structure but not function using RAAV.CNTF in animal models of retinitis pigmentosa. Mol Ther 4: 461–472 [DOI] [PubMed] [Google Scholar]
- Loewen CJ, Molday RS. 2000. Disulfide-mediated oligomerization of Peripherin/Rds and Rom-1 in photoreceptor disk membranes. Implications for photoreceptor outer segment morphogenesis and degeneration. J Biol Chem 275: 5370–5378 [DOI] [PubMed] [Google Scholar]
- Ma J, Norton JC, Allen AC, Burns JB, Hasel KW, Burns JL, Sutcliffe JG, Travis GH. 1995. Retinal degeneration slow (rds) in mouse results from simple insertion of a t haplotype-specific element into protein-coding exon II. Genomics 28: 212–219 [DOI] [PubMed] [Google Scholar]
- Maia-Lopes S, Silva ED, Silva MF, Reis A, Faria P, Castelo-Branco M. 2008. Evidence of widespread retinal dysfunction in patients with stargardt disease and morphologically unaffected carrier relatives. Invest Ophthalmol Vis Sci 49: 1191–1199 [DOI] [PubMed] [Google Scholar]
- Mao H, Gorbatyuk MS, Rossmiller B, Hauswirth WW, Lewin AS. 2012. Long-term rescue of retinal structure and function by rhodopsin RNA replacement with a single adeno-associated viral vector in P23H RHO transgenic mice. Hum Gene Ther 23: 356–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mears AJ, Kondo M, Swain PK, Takada Y, Bush RA, Saunders TL, Sieving PA, Swaroop A. 2001. Nrl is required for rod photoreceptor development. Nat Genet 29: 447–452 [DOI] [PubMed] [Google Scholar]
- Michaelides M, Holder GE, Bradshaw K, Hunt DM, Moore AT. 2005. Cone–rod dystrophy, intrafamilial variability, and incomplete penetrance associated with the R172W mutation in the peripherin/RDS gene. Ophthalmology 112: 1592–1598 [DOI] [PubMed] [Google Scholar]
- Miyazaki M, Ikeda Y, Yonemitsu Y, Goto Y, Kohno R, Murakami Y, Inoue M, Ueda Y, Hasegawa M, Tobimatsu S, et al. 2008. Synergistic neuroprotective effect via simian lentiviral vector-mediated simultaneous gene transfer of human pigment epithelium-derived factor and human fibroblast growth factor-2 in rodent models of retinitis pigmentosa. J Gene Med 10: 1273–1281 [DOI] [PubMed] [Google Scholar]
- Moshfeghi DM, Yang Z, Faulkner ND, Karan G, Thirumalaichary S, Pearson E, Zhao Y, Tsai T, Zhang K. 2006. Choroidal neovascularization in patients with adult-onset foveomacular dystrophy caused by mutations in the RDS/peripherin gene. Adv Exp Med Biol 572: 35–40 [DOI] [PubMed] [Google Scholar]
- Mussolino C, Sanges D, Marrocco E, Bonetti C, Di Vicino U, Marigo V, Auricchio A, Meroni G, Surace EM. 2011. Zinc-finger-based transcriptional repression of rhodopsin in a model of dominant retinitis pigmentosa. EMBO Mol Med 3: 118–128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour M, Ding XQ, Stricker H, Fliesler SJ, Naash MI. 2004. Modulating expression of peripherin/rds in transgenic mice: Critical levels and the effect of overexpression. Invest Ophthalmol Vis Sci 45: 2514–2521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nour M, Fliesler SJ, Naash MI. 2008. Genetic supplementation of RDS alleviates a loss-of-function phenotype in C214S model of retinitis pigmentosa. Adv Exp Med Biol 613: 129–138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Reilly M, Palfi A, Chadderton N, Millington-Ward S, Ader M, Cronin T, Tuohy T, Auricchio A, Hildinger M, Tivnan A, et al. 2007. RNA interference-mediated suppression and replacement of human rhodopsin in vivo. Am J Hum Genet 81: 127–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palfi A, Ader M, Kiang AS, Millington-Ward S, Clark G, O’Reilly M, McMahon HP, Kenna PF, Humphries P, Farrar GJ. 2006. RNAi-based suppression and replacement of rds-peripherin in retinal organotypic culture. Hum Mutat 27: 260–268 [DOI] [PubMed] [Google Scholar]
- Parodi MB, Iacono P, Cascavilla M, Zucchiatti I, Kontadakis DS, Bandello F. 2010. Intravitreal bevacizumab for subfoveal choroidal neovascularization associated with pattern dystrophy. Invest Ophthalmol Vis Sci 51: 4358–4361 [DOI] [PubMed] [Google Scholar]
- Petrs-Silva H, Yasumura D, Matthes MT, LaVail MM, Lewin AS, Hauswirth WW. 2012. Suppression of rds expression by siRNA and gene replacement strategies for gene therapy using rAAV vector. Adv Exp Med Biol 723: 215–223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pihlmann M, Askou AL, Aagaard L, Bruun GH, Svalgaard JD, Holm-Nielsen MH, Dagnaes-Hansen F, Bek T, Mikkelsen JG, Jensen TG, et al. 2012. Adeno-associated virus-delivered polycistronic microRNA-clusters for knockdown of vascular endothelial growth factor in vivo. J Gene Med 14: 328–338 [DOI] [PubMed] [Google Scholar]
- Poloschek CM, Bach M, Lagreze WA, Glaus E, Lemke JR, Berger W, Neidhardt J. 2010. ABCA4 and ROM1: Implications for modification of the PRPH2-associated macular dystrophy phenotype. Invest Ophthalmol Vis Sci 51: 4253–4265 [DOI] [PubMed] [Google Scholar]
- Reuter JH, Sanyal S. 1984. Development and degeneration of retina in rds mutant mice: The electroretinogram. Neurosci Lett 48: 231–237 [DOI] [PubMed] [Google Scholar]
- Rex TS, Allocca M, Domenici L, Surace EM, Maguire AM, Lyubarsky A, Cellerino A, Bennett J, Auricchio A. 2004. Systemic but not intraocular Epo gene transfer protects the retina from light- and genetic-induced degeneration. Mol Ther 10: 855–861 [DOI] [PubMed] [Google Scholar]
- Rex TS, Wong Y, Kodali K, Merry S. 2009. Neuroprotection of photoreceptors by direct delivery of erythropoietin to the retina of the retinal degeneration slow mouse. Exp Eye Res 89: 735–740 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee KD, Ruiz A, Duncan JL, Hauswirth WW, Lavail MM, Bok D, Yang XJ. 2007. Molecular and cellular alterations induced by sustained expression of ciliary neurotrophic factor in a mouse model of retinitis pigmentosa. Invest Ophthalmol Vis Sci 48: 1389–1400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saga M, Mashima Y, Akeo K, Oguchi Y, Kudoh J, Shimizu N. 1993. A novel Cys-214-Ser mutation in the peripherin/RDS gene in a Japanese family with autosomal dominant retinitis pigmentosa. Hum Genet 92: 519–521 [DOI] [PubMed] [Google Scholar]
- Sanyal S, Zeilmaker GH. 1984. Development and degeneration of retina in rds mutant mice: Light and electron microscopic observations in experimental chimaeras. Exp Eye Res 39: 231–246 [DOI] [PubMed] [Google Scholar]
- Sarra GM, Stephens C, de Alwis M, Bainbridge JW, Smith AJ, Thrasher AJ, Ali RR. 2001. Gene replacement therapy in the retinal degeneration slow (rds) mouse: The effect on retinal degeneration following partial transduction of the retina. Hum Mol Genet 10: 2353–2361 [DOI] [PubMed] [Google Scholar]
- Schlichtenbrede FC, da Cruz L, Stephens C, Smith AJ, Georgiadis A, Thrasher AJ, Bainbridge JW, Seeliger MW, Ali RR. 2003. Long-term evaluation of retinal function in Prph2Rd2/Rd2 mice following AAV-mediated gene replacement therapy. J Gene Med 5: 757–764 [DOI] [PubMed] [Google Scholar]
- Stricker HM, Ding XQ, Quiambao A, Fliesler SJ, Naash MI. 2005. The Cys214→Ser mutation in peripherin/rds causes a loss-of-function phenotype in transgenic mice. Biochem J 388: 605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeuchi K, Nakazawa M, Mizukoshi S. 2008. Systemic administration of nilvadipine delays photoreceptor degeneration of heterozygous retinal degeneration slow (rds) mouse. Exp Eye Res 86: 60–69 [DOI] [PubMed] [Google Scholar]
- Tam BM, Moritz OL, Papermaster DS. 2004. The C terminus of peripherin/rds participates in rod outer segment targeting and alignment of disk incisures. Mol Biol Cell 15: 2027–2037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teusner JT, Lewin AS, Hauswirth WW. 2006. Down-regulation of rhodopsin gene expression by AAV-vectored short interfering RNA. Adv Exp Med Biol 572: 233–238 [DOI] [PubMed] [Google Scholar]
- Tian G, Ropelewski P, Nemet I, Lee R, Lodowski KH, Imanishi Y. 2014. An unconventional secretory pathway mediates the cilia targeting of peripherin/rds. J Neurosci 34: 992–1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Travis GH, Brennan MB, Danielson PE, Kozak CA, Sutcliffe JG. 1989. Identification of a photoreceptor-specific mRNA encoded by the gene responsible for retinal degeneration slow (rds). Nature 338: 70–73 [DOI] [PubMed] [Google Scholar]
- Travis GH, Sutcliffe JG, Bok D. 1991. The retinal degeneration slow (rds) gene product is a photoreceptor disc membrane-associated glycoprotein. Neuron 6: 61–70 [DOI] [PubMed] [Google Scholar]
- Travis GH, Groshan KR, Lloyd M, Bok D. 1992. Complete rescue of photoreceptor dysplasia and degeneration in transgenic retinal degeneration slow (rds) mice. Neuron 9: 113–119 [DOI] [PubMed] [Google Scholar]
- Usukura J, Bok D. 1987. Opsin localization and glycosylation in the developing BALB/c and rds mouse retina. Prog Clin Biol Res 247: 195–207 [PubMed] [Google Scholar]
- Vaclavik V, Tran HV, Gaillard MC, Schorderet DF, Munier FL. 2012. Pattern dystrophy with high intrafamilial variability associated with Y141C mutation in the peripherin/RDS gene and successful treatment of subfoveal CNV related to multifocal pattern type with anti-VEGF (ranibizumab) intravitreal injections. Retina 32: 1942–1949 [DOI] [PubMed] [Google Scholar]
- van Nie R, Ivanyi D, Demant P. 1978. A new H-2-linked mutation, rds, causing retinal degeneration in the mouse. Tissue Antigens 12: 106–108 [DOI] [PubMed] [Google Scholar]
- Wickham L, Chen FK, Lewis GP, Uppal GS, Neveu MM, Wright GA, Robson AG, Webster AR, Grierson I, Hiscott P, et al. 2009. Clinicopathological case series of four patients with inherited macular disease. Invest Ophthalmol Vis Sci 50: 3553–3561 [DOI] [PubMed] [Google Scholar]
- Wrigley JD, Ahmed T, Nevett CL, Findlay JB. 2000. Peripherin/rds influences membrane vesicle morphology. Implications for retinopathies. J Biol Chem 275: 13191–13194 [DOI] [PubMed] [Google Scholar]