Abstract
Background
Pediatric neurologists and neonatologists are often asked to prognosticate about cognitive outcome after perinatal brain injury (including likely memory and learning outcomes); however, relatively little data exists upon which accurate predictions can be made. Further, while the consequences of brain injury on hippocampal volume and memory performance have been studied extensively in adults, little work has been done in children.
Methods
We measured the volume of the hippocampus in 27 children with perinatal stroke and 19 controls, and measured their performance on standardized verbal and non-verbal memory tests.
Results
(i) As a group, children with perinatal stroke had smaller left and right hippocampi compared to controls. (ii) Individually, children with perinatal stroke demonstrated one of 3 findings: no hippocampal loss, unilateral hippocampal loss, or bilateral hippocampal volume loss compared to controls. (iii) Hippocampal volume inversely correlated with memory test performance in the perinatal stroke group, with smaller left and right hippocampal volumes related to poorer verbal and non-verbal memory test performance, respectively. (iv) Seizures played a significant role in determining the presence of memory deficit and extent of hippocampal volume reduction in patients with perinatal stroke.
Conclusions
These findings support the view that, in the developing brain, the left and right hippocampi preferentially support verbal and non-verbal memory respectively, a consistent finding in the adult literature but a subject of debate in the pediatric literature. This is the first work to report that children with focal brain injury incurred from perinatal stroke have volume reduction in the hippocampus and impairments in certain aspects of declarative memory.
Keywords: Hippocampus, memory, stroke, pediatrics, epilepsy
Introduction
Ample evidence demonstrates that adults who sustain damage to the hippocampus and other medial temporal lobe structures incur profound, life-long declarative (i.e., episodic and semantic) memory impairment 1-4. One consistent finding has been that patients with left-sided brain lesions tend to be more impaired at verbal memory tasks, whereas patients with right-sided brain lesions tend to be more impaired at non-verbal memory tasks 5-13. Similarly, evidence from fMRI, PET imaging, and behavioral testing of adult patients with epilepsy suggests that the left hippocampus is more involved in verbal memory tasks while the right hippocampus is move involved in non-verbal memory tasks 6,10,12-19. Further, patients with bilateral hippocampal lesions are much more impaired than patients with unilateral hippocampal lesions, such that patients with bilateral lesions have difficulties holding jobs and managing their own affairs while patients with unilateral lesions often learn to function independently using compensatory strategies 9,20.
In contrast to this extensive literature in the adult, comparatively little work has been done investigating the neuroanatomical substrates of memory in children. The few previous studies that examine memory in children with perinatal stroke or with localization-related epilepsy have found conflicting results, with some studies finding that lesion laterality was important in the presence and type of memory deficit while others found no difference in memory impairment based on the side of the lesion 21-26. Notably, none of the previous studies has examined structure-function relationships with hippocampal volume and memory measures.
Studies of memory function in children are vitally important for a variety of reasons. First, such studies offer a unique opportunity to investigate the developing brain. Second, pediatric brain injury is much more common than previously thought; in particular, current estimates suggest that the rate of perinatal stroke is about 1 in 2500 to 1 in 4000 live births 27. Third, children with memory impairment secondary to brain injury need medical and educational interventions to reach their maximum cognitive and intellectual potential, but again there is insufficient data upon which to base the selection of appropriate treatment modalities. The young brain has remarkable potential for plasticity and functional compensation, but this potential cannot be fully harnessed without a better understanding of the consequences of brain injury during the critical period of development.
As part of a larger study of cognitive function and the role of seizures in children with perinatal stroke, we analyzed hippocampal volume in children with perinatal stroke and correlated hippocampal volume with verbal and non-verbal memory function. We hypothesized that there would be a direct relationship between hippocampal volume and memory function in perinatal stroke patients. Additionally, we hypothesized that seizures in this population would have an adverse effect on hippocampal volume and memory.
Methods
Participants
Forty-six children and adolescents participated in the study. Twenty-seven subjects had a single unilateral brain lesion caused by a perinatal stroke (age range 6-16 years, mean 10.7 +/− 0.6 years, 14 male and 13 female). Twelve patients had right-hemisphere strokes and 15 had left hemisphere strokes. Subjects were recruited from pediatric neurology clinics in San Diego County. Perinatal stroke was defined as a single, unilateral brain lesion in a arterial vascular distribution, either identified in the neonatal period with neuroimaging, or identified later in infancy after presentation with a hemiparesis and imaging documentation of an old unilateral infarct (presumed perinatal stroke). Children were excluded if they have bilateral lesions or evidence of more widespread brain damage such as a history of hypoxic-ischemic encephalopathy. The presence of seizures was determined by review of the medical record and family interview; patients were classified as having seizures if they had a seizure after 28 days of life. 59% of children in the stroke group had hemiparetic cerebral palsy; all had normal function of their non-affected hand and arm. Exclusionary criteria included a history of hypoxic ischemic encephalopathy, central nervous system infection, in utero drug exposure, significant closed head injury, or any other condition that might have caused brain damage other than from the stroke. Nineteen control subjects (7-14 years [9.3 +/− 0.5 years], 9 male and 10 female) were recruited from the community through advertisements and by word of mouth. Complete medical and family histories were obtained for all control children. All controls included in the study had normal developmental and medical histories and were free of learning and behavioral problems. The stroke and control populations were group-matched for socioeconomic status. Demographic information for the children is shown in Table 1. All ages are the age at which the participant underwent the MRI that was used for this study.
Table 1.
Demographics, characteristics and lesion/neurological information
| Subject | Age (years) |
Gender | Race | Group | IQ | Seizures | Hemiparesis | Lesion Side |
Lesion Site |
|---|---|---|---|---|---|---|---|---|---|
| PT1 | 16 | M | C | Patient | 101 | NO | NO | Right | FTPOSW |
| PT2 | 14 | M | C | Patient | 121 | NO | NO | Right | FP |
| PT3 | 15 | F | C | Patient | 118 | NO | NO | Left | F |
| PT4 | 13 | M | C | Patient | 71 | YES | YES | Right | FTPBW |
| PT5 | 7 | M | C | Patient | 83 | YES | YES | Left | FTPOSGBW |
| PT6 | 7 | F | c | Patient | 95 | YES | NO | Right | FS |
| PT7 | 8 | M | c | Patient | 104 | NO | YES | Right | B |
| PT8 | 8 | M | H | Patient | 114 | NO | YES | Left | F |
| PT9 | 10 | M | C | Patient | 89 | YES | NO | Right | FSG |
| PT10 | 12 | F | C | Patient | 58 | YES | YES | Right | FTPOSB |
| PT11 | 11 | F | C | Patient | 82 | NO | YES | Left | M |
| PT12 | 8 | F | H | Patient | 100 | YES | NO | Left | P |
| PT13 | 14 | F | C | Patient | 82 | YES | NO | Left | P |
| PT14 | 14 | F | C | Patient | 75 | NO | YES | Left | T |
| PT15 | 7 | F | H | Patient | 63 | YES | YES | Left | FTPSBW |
| PT16 | 14 | M | C | Patient | 99 | YES | YES | Right | FTP |
| PT17 | 12 | M | H | Patient | 86 | NO | YES | Left | FPSG |
| PT18 | 12 | M | C | Patient | 135 | NO | NO | Left | PS |
| PT19 | 8 | M | C | Patient | 68 | YES | YES | Right | FTPSMBW |
| PT20 | 6 | F | C | Patient | 66 | YES | YES | Left | P |
| PT21 | 8 | F | C | Patient | 62 | YES | YES | Right | FTPOSBW |
| PT22 | 9 | M | C | Patient | 89 | YES | NO | Left | FP |
| PT23 | 9 | F | C | Patient | 86 | YES | YES | Right | FTPSGBW |
| PT24 | 14 | F | C | Patient | 57 | NO | NO | Left | FTP |
| PT25 | 13 | M | C | Patient | 112 | NO | NO | Left | FS |
| PT26 | 11 | F | C | Patient | 101 | NO | YES | Left | TPSW |
| PT27 | 12 | M | C | Patient | 67 | YES | YES | Right | FTPSGBW |
| CON1 | 10 | M | H | Control | 107 | NO | NO | Normal | None |
| CON2 | 8 | F | C | Control | 107 | NO | NO | Normal | None |
| CON3 | 8 | M | C | Control | 112 | NO | NO | Normal | None |
| CON4 | 7 | M | C | Control | 117 | NO | NO | Normal | None |
| CON5 | 13 | M | AA | Control | 90 | NO | NO | Normal | None |
| CON6 | 8 | F | C | Control | 113 | NO | NO | Normal | None |
| CON7 | 12 | F | H | Control | 120 | NO | NO | Normal | None |
| CON8 | 10 | F | C | Control | 113 | NO | NO | Normal | None |
| CON9 | 7 | F | C | Control | 131 | NO | NO | Normal | None |
| CONIO | 13 | M | C | Control | 133 | NO | NO | Normal | None |
| CON11 | 10 | M | C | Control | 122 | NO | NO | Normal | None |
| CON12 | 8 | M | A | Control | 135 | NO | NO | Normal | None |
| CON13 | 8 | M | A | Control | 135 | NO | NO | Normal | None |
| CON14 | 7 | F | C | Control | 128 | NO | NO | Normal | None |
| CON15 | 9 | F | C | Control | 106 | NO | NO | Normal | None |
| CON16 | 9 | M | C | Control | 113 | NO | NO | Normal | None |
| CON17 | 14 | F | C | Control | 107 | NO | NO | Normal | None |
| CON18 | 8 | F | C | Control | 121 | NO | NO | Normal | None |
| CON19 | 7 | F | C | Control | 110 | NO | NO | Normal | None |
C= Caucasian; H = Hispanic; A = Asian; AA = African American; F = Frontal; T = Tempora Parietal; O = Occipital; S = Subcortial; M = Thalamus; G = Basal Ganglia; B = Broca’s area; Wernicke’s Area Severity scores range from 0 to 5 (see Methods of details)
Structural MRI
All participants were imaged without sedation in a 3-Tesla GE Signa magnet. After scout images, field maps, and alignment scans were performed, four whole-brain image series were collected for all participants (3D sagittal T2-weighted, 3D sagittal T1-weighted, DWI, FLAIR). Hippocampal measurements were performed on the reconstructed sagittal T1 images (see below). These T1 images were acquired using an SPGR pulse sequence with the following scanner settings: TE = 3.1, Flip angle = 120, FOV = 25 cm, slice thickness = 1.2 mm, matrix 256×256, total 120 images.
A clinical neuroradiologist, blinded to cognitive and clinical status, performed a clinical assessment of each neuroimaging (MRI) study, providing indication of the presence of a single unilateral brain lesion as well as documentation of lesion location (i.e., designation of cortical, subcortical, and lobar involvement). Lesion severity was rated by a grading system described previously with grades from 1 = minimal ventricular dilation or atrophy to 5 = large porencephaly involving multiple lobes 28,29. Fifteen patients had lesions assigned a severity score of 5 (9 of these patients had a right-sided lesion, 6 of these patients had a left-sided lesion); one of these patients had a lesion described as a “destructive lesion of the left temporal and parietal lobes” and this patient had no remaining left hippocampus. Every other patient in this study had some hippocampal tissue bilaterally. Eight patients had lesions assigned a severity score of 4 (all were left-sided lesions), one patient had a severity score of 3 (right-sided lesion), and three patients had a severity score of 2 (2 right-sided lesions, 1 left-sided lesion). No patients in this study were assigned a severity score of 1. Severity scores are summarized in Table 2.
Table 2.
Severity scores for Derinatal stroke Datients
| Severity | Right-sided lesior | Left-sided lesior | Total patients |
|---|---|---|---|
| 2 | 2 | 1 | 3 |
| 3 | 1 | 0 | 1 |
| 4 | 0 | 8 | 8 |
| 5 | 9 | 6 | 15 |
|
| |||
| Total patients | 12 | 15 | 27 |
Lesion severity was rated by a grading system described previously (Trauner et al., 2001; Vargha-Khadem et al., 1985) with grades from 1 = minimal ventricular dilation or atrophy to 5 = large porencephaly involving multiple lobes.
Using the AFNI suite of analysis tools 30, each patient’s MRI images were aligned along the anterior commissure to posterior commissure axis, and voxels were linearly resampled to 1 mm by 1 mm by 1 mm. Using methods developed and validated by our group 31, the left and right hippocampal regions (hippocampus proper, dentate gyrus, and subicular complex) were measured in the sagittal view, beginning laterally at the appearance of hippocampal tissue within the lateral ventricle. The drawing continued medially, observing the separation between the hippocampal region and the amygdala. Measurements were then reevaluated in the coronal view to ensure complete separation between the hippocampus and the posterior aspect of the pulvinar, the separation between the subicular complex and entorhinal cortex, and white matter/gray matter segmentation.
Behavioral testing
Every child received the Wechsler Intelligence Scale for Children, Third Edition (WISC-III) to assess global cognitive functioning. To assess memory, we administered selected subtests from a broad-based and relatively comprehensive standardized test to assess various aspects of memory. Subjects were administered the Dots and Stories subtests of the Children’s Memory Scales (CMS), which measures learning and retrieval of non-verbal and verbal material, respectively 32-35. In the Dot Locations subtest of non-verbal memory, children were presented with a card showing black dots arranged on a grid. The children’s task was to recreate the location of the dots on the grid using plastic chips they could place on a replica of the grid. During each of three learning trials, children were shown the target grid for 5 seconds and then were scored based on how many chips they correctly placed on the grid (summed number of correctly placed dots during the three learning trials = Dots – Learning). Children were then shown a distracter grid and were asked to recreate this grid as well (unscored). Children were then asked to reproduce the original target grid, and their performance on the learning trials was added to their performance on this short-delay recall trial to generate a total score (Dots – Total). After 20 minutes, children were given a final opportunity to recreate the original target grid (Dots – Recall). In the Stories subtest of verbal memory, children were presented with a story and then were asked to recount it (Stories - Immediate Recall). After a delay (typically about 20 minutes), children were again asked to recall the story (Stories - Delayed Recall) and then took a recognition memory test for the words and themes of the stories (Stories - Delayed Recognition). This process was repeated so that each child was tested using two stories.
Analysis
SPSS for Windows was used for all analyses. Comparisons of hippocampal volumes between patients and controls, as well as comparisons between scores on CMS subtests, were carried out using two-tailed t-tests and reported as significant if p<0.05. Correlations between lesion volume and performance on CMS subtests were carried out using linear regression analysis and evaluated with Pearson coefficients that were reported as significant if p<0.05. Mann Whitney U test was also used to compare severity scores (1-5) between the group of patients with seizures and the group of patients without seizures; these results were also reported as significant if p<0.05.
Results
Demographics
Although the focal lesion subjects had a slightly higher mean age than controls, there was no significant difference (control mean 9.31 +/− 0.5 years, patient mean 10.7 +/− 0.6 years, p = 0.08). Full-scale IQ was significantly lower in the focal lesion group compared with controls (Table 3). Patients with right hemisphere lesions (n=12) were demographically similar to patients with left hemisphere lesions (n=15). Patients had similar ages (right hemisphere lesion patient mean 10.8 +/− 0.8 years, left hemisphere lesion patient mean 10.6 +/− 0.8 years, p = 0.85), IQs (right hemisphere lesion patients mean 85.0 +/− 6, left hemisphere lesion patients mean 91 +/− 6, p = 0.49), and lesion severity scores (right hemisphere lesion patients mean 4.3 +/− 0.4, left hemisphere lesion patients mean 4.3 +/− 0.2, p = 0.87).
Table 3.
IQ scores of patients and control
| IQ | |
|---|---|
| Controls (n = 19) | 117 +/− 2.7 |
| All patients (n = 27) | 88 +/− 4.0 |
| No seizures (n = 12) | 100 +/− 6.4 |
| Seizures (n = 15) | 78 +/− 3.7 |
IQ mean +/− SEM. By definition, the mean IQ of the general population is
There was no difference between the control and perinatal stroke subjects in socioeconomic status nor on maternal level of education.
Patients without seizures were older than patients with seizures (with seizures [n=15] mean 9.5 +/− 0.7 years, without seizures [n=12] mean 12.2 +/− 0.7 years, p = 0.01).
Hippocampal volumes
As a group, patients had smaller hippocampi than controls. All 27 patients and 19 controls were measured. On average, patients with right hemisphere lesions had smaller right hippocampi than controls (control mean 3797 +/− 92 mm3, patient mean 2840 +/− 325 mm3, p = 0.002) and patients with left hemisphere lesions had smaller left hippocampi than controls (control mean 3532 +/− 108 mm3, patient mean 2756 +/− 236 mm3, p = 0.003) (Table 4). Hippocampal volume was not correlated with age in the range of subjects in this study (control right hippocampal volume vs age R = 0.01, p = 0.98; control left hippocampal volume vs age R = 0.24, p = 0.32) 36. Similarly, there was no correlation between hippocampal volume and IQ (control right hippocampal volume vs IQ R = −0.12, p = 0.62; control left hippocampal volume vs IQ R = −0.19, p = 0.44). Further, neither right nor left hippocampal volume was correlated with performance on any subset of the CMS in the control population. Finally, the right hippocampi of the control subjects were somewhat larger than the left hippocampi of the control subjects, which is a well-established finding in MRI measurements of hippocampal volume37; for this reason, comparison between the volume of the left and right hippocampi of the patients and controls were not performed.
Table 4.
Volume of the hippocampal region
| Right | Right p-value Left | Left p-value | |
|---|---|---|---|
| Controls (n = 19) | 3797 +/− 92 | 3532 +/− 108 | |
| All patients (n = 27) | 3109 +/− 184 | 0.005 2922 +/− 159 | 0.006 |
| Left hemisphere lesions (n = 15) | 3324 +/− 199 | 0.03 2756 +/− 236 | 0.003 |
| Right hemisphere lesions (n = 12) | 2840 +/− 325 | 0.002 3131 +/− 197 | 0.06 |
Volumes in mm^3 +/− SEM. p-values = two-tailed t-test vs. Controls.
To investigate the effect of focal (i.e., unilateral) brain injury on the contralateral hippocampus, we decided a priori to consider an individual patient’s hippocampal volume to be significantly reduced from control if the patient’s hippocampal volume was more than 1.5 SD below the control mean. Of the 12 patients with right-sided lesions, 5 had significant unilateral hippocampal volume reduction, 4 had significant bilateral hippocampal volume reduction, and 3 had no significant hippocampal volume reduction. Of the 15 patients with left-sided lesions, 4 had significant unilateral hippocampal volume reduction, 4 had significant bilateral hippocampal volume reduction, and 7 had no significant hippocampal volume reduction. Thus, a subset of the children with right hemisphere lesions and a subset of children with left hemisphere lesions had significantly smaller hippocampi on the side contralateral to the lesion as well as ipsilateral to the lesion. The pattern of volume reduction was not related to the severity score assigned to the brain lesion.
Behavioral testing
Perinatal stroke subjects as a group had significantly lower IQ scores than did controls (control mean 117 +/− 2.7, patient mean 88 +/− 4.0, p <0.001). Patients without seizures (mean 100 +/− 6.4) had IQ within the range of normal, whereas patients with seizures (mean 78 +/− 6.4) had IQ more than 1 standard deviation from the population mean (mean 100 +/− standard deviation of 15) (Table 3).
All 27 patients and 19 controls participated in all 6 measures of memory tested by the CMS, except that one patient did not receive the Stories – Delayed Recognition test and was excluded from that analysis only. Perinatal stroke subjects as a group were impaired on all 6 measures of the Dots and Stories subtests of the CMS relative to controls (Table 5). Patients with right and left hemisphere lesions were statistically similar on all 6 measures of the Stories and Dot Locations subtests of the CMS. Patients without seizures scored significantly better than patients with seizures on all measures of the Dot Locations subtest and the Delayed Recall measure of the Dot Locations subtest. Patients without seizures scored better than patients with seizures on the Immediate Recall and Delayed Recognition measures of the Stories subtest as well, but the difference in scores did not reach statistical significance (Table 5).
Table 5.
Children’s Memory Scale performance
| Test | Controls Mean +/− SEM |
All patients Mean +/− SEM |
Controls vs All patients p-value |
Patients with seizures Mean +/− SEM |
Patients without seizures Mean +/− SEM |
Patients with sz vs Patients without sz p-value |
Pt with lesions in right hemisphere Mean +/− SEM |
Pt with lesions in left hemisphere Mean +/− SEM |
Right hemisphere vs Left hemisphere p-value |
|---|---|---|---|---|---|---|---|---|---|
| Stories | |||||||||
| Immediate Recall | 13.5 +/− 0.7 | 8.4 +/− 0.8 | <0.001 | 7.0 +/− 0.8 | 10.1 +/− 1.4 | 0.06 | 7.8 +/− 1.1 | 8.9 +/− 1.2 | 0.51 |
| Delayed Recall | 13.9 +/− 0.8 | 7.9 +/− 0.8 | <0.001 | 6.2 +/− 0.9 | 10.0 +/− 1.2 | 0.02 | 7.3 +/− 1.1 | 8.3 +/− 1.2 | 0.56 |
| Delayed Recognition | 11.5 +/− 0.5 | 8.0 +/− 0.8 | 0.001 | 7.1 +/− 1.1 | 9.2 +/− 0.9 | 0.17 | 8.3 +/− 1.4 | 7.9 +/− 0.9 | 0.80 |
| Dots | |||||||||
| Learning | 10.9 +/− 0.5 | 8.9 +/− 0.8 | 0.05 | 7.6 +/− 1.1 | 10.6 +/− 0.8 | 0.05 | 9.3 +/− 1.4 | 8.7 +/− 0.9 | 0.71 |
| Total | 11.8 +/− 0.5 | 9.0 +/− 0.7 | 0.003 | 7.8 +/− 0.9 | 10.6 +/− 0.9 | 0.04 | 9.2 +/− 1.2 | 8.9 +/− 0.8 | 0.87 |
| Delayed Recall | 12.6 +/− 0.4 | 10.0 +/− 0.5 | <0.001 | 8.8 +/− 0.5 | 11.4 +/− 0.8 | 0.009 | 9.7 +/− 0.7 | 10.2 +/− 0.7 | 0.62 |
Control n = 19 for all tests. All patient n = 27 (seizures n = 15, no seizures n = 12, right hemisphere lesions n = 12, left hemisphere lesions n = 15), except that one patient did not receive the Delayed Recognition measure of the Stories subtest (patient n = 26, seizures n = 14, right hemisphere lesions n = 11). p-value = two-tailed t-test.
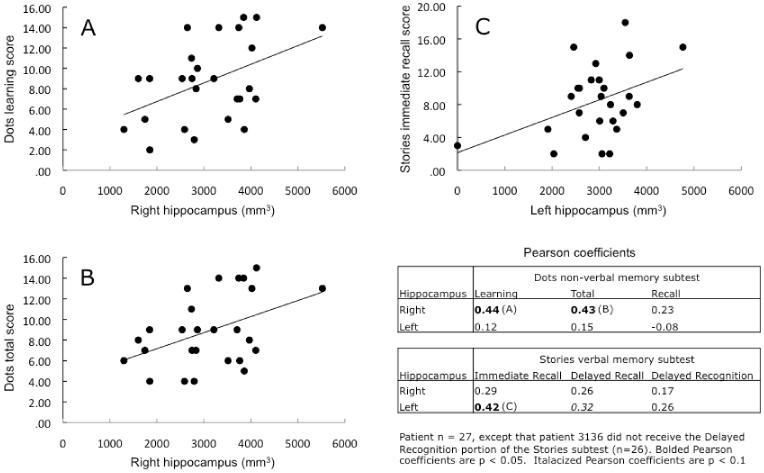
We calculated Pearson coefficients of correlation between hippocampal volume (right and left) and performance on each subtest of the Children’s Memory Scale for the patient group (Figure 1). Statistically significant correlations were: (I) the volume of the right hippocampus vs. performance on the Learning measure of the Dots subtest, R = 0.44, p = 0.02 (Figure 1A); (ii) the volume of the right hippocampus vs. performance on the Total measure of the Dots subtest, R = 0.43, p = 0.02 (Figure 1B); (ii) the volume of the left hippocampus vs. performance on the Immediate Recall measure of the Stories subtest, R = 0.42, p = 0.03 (Figure 1C). The correlation of the volume of the left hippocampus vs performance on the Delayed Recall measure of the Stories subtest trended toward but did not reach significance (R = 0.32, p = 0.1). Finally, we calculated all of the Pearson coefficients of correlation analysis again, controlling for IQ. The volume of the left hippocampus vs. performance on the Immediate Recall measure of the Stories subtest remained significantly correlated, and the correlation became slightly stronger (R = 0.49, p = 0.01); similarly, the correlation of the volume of the left hippocampus vs performance on the Delayed Recall measure of the Stories subtest trended even closer to significance (R = 0.38, p = 0.06). The correlations between the volume of the right hippocampus vs performance on the Learning measure of the Dots subtest (R = 0.31) and the volume of the right hippocampus vs performance on the Total measure of the Dots subtest (R = 0.30) no longer reached statistical significance (p > 0.1).
Figure 1.
Patient n=27, except that patient 3136 did not receive the Delayed Recognition portion of the Stories Subtest (n=26). Bolded Pearson co-efficients are p < 0.05. Italicized Pearson co-efficients are p < 0.1.
We calculated Pearson coefficients of correlation between hippocampal volume (right and left) and performance on each subtest of the Children’s Memory Scale for the control group. We also calculated Pearson coefficients of correlation between IQ and performance on each subtest of the Children’s Memory Scale for the control group. No correlations neared statistical significance (p > 0.1 for all comparisons).
Seizures, lesion severity, and hippocampal volume
The group of 15 patients with seizures had significant hippocampal volume reduction relative to controls (right mean 2708 +/− 232 mm3, p < .001; left mean 2913 +/− 96 mm3, < .001). As a group, the 12 patients without seizures had no significant volume reduction of the right hippocampus and near-significant hippocampal volume reduction of the left hippocampus (right mean 3609 +/− 233 mm3, p = 0.39; left mean 2934 +/− 346 mm3, p = 0.06) compared with controls. In part, this was due to one patient with a left hippocampal volume of 0 mm3 as mentioned in the Methods section; if this patient were excluded from the hippocampal volume analysis then the trend toward statistical significance lessened (left mean 3200 +/− 242 mm3, p = 0.16).
Lesion severity was not related to the presence of seizures. Of the patients without seizures, two had severity scores of 2, five had severity scores of 4, and five had severity scores of 5 whereas of the patients with seizures one had a severity score of 2, one had a severity score of 3, three had a severity score of 4, and 10 had a severity score of 5; this difference is not statistically different (non-parametric Mann-Whitney U test, p = 0.31).
Discussion
This is the first study to quantify hippocampal volume loss after unilateral brain injury in the perinatal period, correlate memory impairment with the location and extent of hippocampal volume reduction, and study the role of seizures in both hippocampal volume reduction and memory impairment. We found that stroke in the perinatal period may have variable effects on the hippocampus. It is clear that seizure beyond the neonatal period plays an important role in determining the nature and extent of hippocampal volume loss and also the pattern of memory impairment. The degree of hippocampal volume reduction was correlated with poorer performance on memory tests, with left hippocampal volume reduction related to poorer performance on a verbal memory test and right hippocampal volume reduction related to poorer performance on a non-verbal memory test. This pattern of memory impairment is congruent with the extensive literature on adult memory, but at odds with a previous study that found disparate patterns of memory impairment after brain injury in children and adults 21. However, interpretation of that study is complicated for a number of reasons: (a) the study combined patients with perinatal strokes and strokes up to 1 year of age, and also included a group of pediatric patients with strokes after 1 year of age; (b) the memory test was the California Verbal Learning Test–Children’s Version, which tests verbal but not non-verbal memory, making interpretation of a memory deficit as opposed to a language deficit difficult; (c) the influence of seizures was not considered. Our findings strongly support the view that, as in adults, the left and right hippocampi in children preferentially support verbal and non-verbal memory, respectively.
It is notable that there was a wide range of hippocampal damage following focal stroke. As a group, patients had significant reduction in hippocampal volume bilaterally; some individuals had no significant reduction in hippocampal volume relative to controls, others had unilateral hippocampal volume reduction, while others had bilateral hippocampal volume reduction. One likely explanation is that the hippocampus is particularly susceptible to damage in patients with seizures, and that patients with seizures are more likely to have hippocampal volume loss (both ipsalateral and contralateral to a brain lesion) than patients without seizures 18,38-43. Children with seizures show evidence of damage to the hippocampus and other temporal lobe areas, and some have concluded that hippocampal injury is often secondary to a remote seizure focus 44-46. In this study, patients with seizures were more likely to have any hippocampal volume reduction (either unilateral or bilateral) than patients who did not have seizures; this was true regardless of the size of the lesion (that is, higher severity scores were not significantly associated with the presence of greater hippocampal volume reduction). Using severity score as an indicator of lesion size, we did not find that children with larger lesions were more likely to have seizures than children with smaller lesions. Thus, it appears that a history of seizures, rather than the size of the lesion, is most important in determining whether there will be hippocampal volume reduction after perinatal stroke.
Factors other than seizures may also be related to hippocampal volume reduction after early focal brain injury. For example, network models suggest that damage to one hippocampus especially early in life can result in changes in synaptic physiology in the contralateral hippocampus 47. Further work investigating the correlation between hippocampal volume loss and memory performance in children with stroke beyond the perinatal period, as well as children with seizures from causes other than perinatal stroke, is needed to clarify the relationship between stroke, seizure, hippocampal volume loss, and memory performance.
Several interesting trends emerged when our data were analyzed based on the presence or absence of seizures. First, as we have reported elsewhere, patients with seizures had significantly lower IQ than patients who did not have seizures 48. In fact, in the present study patients with seizures had IQ more than one standard deviation below the population mean, whereas patients without seizures had normal IQ. Second, patients with seizures had significantly smaller hippocampi than controls, whereas the hippocampi of patients without seizures were not significantly smaller than controls. Finally, patients with seizures were impaired relative to controls on all 6 measures of the CMS; further, patients with seizures were impaired relative to patients without seizures on 4/6 measures of the CMS (and there was a trend toward better performance by the patients without seizures on the other two measures).
We recognize that the IQ of our control group was more than one standard deviation greater than the population mean, while the IQ of the patient group was below the population mean. To the extent that subjects with higher IQ may perform better on memory tests generally, this may have caused us to overestimate the magnitude of the memory impairment in the patients relative to the control group due to factors other than IQ. However, performance on the memory tests was not correlated with IQ in the control group in our study. Controlling for IQ within the patient group strengthened some correlations between performance on the memory tests and hippocampal volume while weakening other correlations. Thus, our data suggest that IQ did not have a strong impact on memory performance as measured by the CMS within either the control or the patient group.
This is the first study to show that children who suffer stroke in the perinatal period can have volume reduction in the hippocampus relative to age-matched controls, and that the perinatal stroke children exhibit different patterns of memory impairment (verbal vs. non-verbal) correlated with the side of hippocampal volume loss. Specifically, our findings indicate that (i) children who suffer focal brain lesions early in life may demonstrate no hippocampal loss, unilateral hippocampal loss, or bilateral hippocampal volume loss; (ii) the degree of hippocampal volume loss is correlated with the degree of impairment on memory tests; (iii) damage to the left hippocampus is more likely to result in impairment in verbal memory while damage to the right hippocampus is more likely to result in impairment in non-verbal memory; (iv) the pattern of memory deficit (verbal or non-verbal) that is found in the context of volume loss in the hippocampus (left, right, or bilateral) in patients with perinatal-onset focal brain damage may be similar to those patterns observed after injury to the hippocampus in adults; (iv) seizures play a significant role in determining the presence of memory deficit and extent of hippocampal volume reduction in patients with perinatal-onset focal brain injury; (v) MRI imaging and volumetric analysis of the hippocampus may predict memory impairment after perinatal focal stroke.
The pediatric brain has remarkable capacity for plasticity. Our results caution against dire prognostication about cognitive and memory outcomes after focal stroke in the perinatal period. Based on our findings, it seems likely that seizures may limit the natural post-injury plasticity and prevent full functional recovery. This is critically important, because it suggests that aggressive seizure control beyond the perinatal period may improve cognitive and memory outcomes in children who suffer perinatal stroke. Additional work is needed to determine whether optimal seizure control may affect outcome after perinatal stroke.
Acknowledgements
This research was supported by National Institute of Health RO1 NS42584 (to D.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors have no financial conflicts of interest to disclose.
References
- 1.Squire LR, Wixted JT. The cognitive neuroscience of human memory since h.m. Annu Rev Neurosci. 2011;34:259–288. doi: 10.1146/annurev-neuro-061010-113720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Squire LR, Zola-Morgan S. Memory: brain systems and behavior. Trends Neurosci. 1988 Apr;11(4):170–175. doi: 10.1016/0166-2236(88)90144-0. [DOI] [PubMed] [Google Scholar]
- 3.Scoville WB, Milner B. Loss of recent memory after bilateral hippocampal lesions. J Neurol Neurosurg Psychiatry. 1957 Feb;20(1):11–21. doi: 10.1136/jnnp.20.1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Corkin S. What’s new with the amnesic patient H.M.? Nat Rev Neurosci. 2002 Feb;3(2):153–160. doi: 10.1038/nrn726. [DOI] [PubMed] [Google Scholar]
- 5.Milner B. Interhemispheric differences in the localization of psychological processes in man. Br Med Bull. 1971 Sep;27(3):272–277. doi: 10.1093/oxfordjournals.bmb.a070866. [DOI] [PubMed] [Google Scholar]
- 6.Richardson MP, Strange BA, Thompson PJ, Baxendale SA, Duncan JS, Dolan RJ. Pre-operative verbal memory fMRI predicts post-operative memory decline after left temporal lobe resection. Brain. 2004 Nov;127(Pt 11):2419–2426. doi: 10.1093/brain/awh293. [DOI] [PubMed] [Google Scholar]
- 7.Spiers HJ, Burgess N, Maguire EA, et al. Unilateral temporal lobectomy patients show lateralized topographical and episodic memory deficits in a virtual town. Brain. 2001 Dec;124(Pt 12):2476–2489. doi: 10.1093/brain/124.12.2476. [DOI] [PubMed] [Google Scholar]
- 8.Frisk V, Milner B. The role of the left hippocampal region in the acquisition and retention of story content. Neuropsychologia. 1990;28(4):349–359. doi: 10.1016/0028-3932(90)90061-r. [DOI] [PubMed] [Google Scholar]
- 9.Smith ML, Milner B. The role of the right hippocampus in the recall of spatial location. Neuropsychologia. 1981;19(6):781–793. doi: 10.1016/0028-3932(81)90090-7. [DOI] [PubMed] [Google Scholar]
- 10.Powell HW, Richardson MP, Symms MR, et al. Preoperative fMRI predicts memory decline following anterior temporal lobe resection. J Neurol Neurosurg Psychiatry. 2008 Jun;79(6):686–693. doi: 10.1136/jnnp.2007.115139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lencz T, McCarthy G, Bronen RA, et al. Quantitative magnetic resonance imaging in temporal lobe epilepsy: relationship to neuropathology and neuropsychological function. Ann Neurol. 1992 Jun;31(6):629–637. doi: 10.1002/ana.410310610. [DOI] [PubMed] [Google Scholar]
- 12.Powell HW, Koepp MJ, Symms MR, et al. Material-specific lateralization of memory encoding in the medial temporal lobe: blocked versus event-related design. Neuroimage. 2005 Aug 1;27(1):231–239. doi: 10.1016/j.neuroimage.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 13.Golby AJ, Poldrack RA, Brewer JB, et al. Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain. 2001 Sep;124(Pt 9):1841–1854. doi: 10.1093/brain/124.9.1841. [DOI] [PubMed] [Google Scholar]
- 14.Strange BA, Fletcher PC, Henson RN, Friston KJ, Dolan RJ. Segregating the functions of human hippocampus. Proc Natl Acad Sci U S A. 1999 Mar 30;96(7):4034–4039. doi: 10.1073/pnas.96.7.4034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kopelman MD, Stevens TG, Foli S, Grasby P. PET activation of the medial temporal lobe in learning. Brain. 1998 May;121(Pt 5):875–887. doi: 10.1093/brain/121.5.875. [DOI] [PubMed] [Google Scholar]
- 16.Tulving E, Markowitsch HJ, Craik FE, Habib R, Houle S. Novelty and familiarity activations in PET studies of memory encoding and retrieval. Cereb Cortex. 1996 Jan-Feb;6(1):71–79. doi: 10.1093/cercor/6.1.71. [DOI] [PubMed] [Google Scholar]
- 17.Wilkinson H, Holdstock JS, Baker G, Herbert A, Clague F, Downes JJ. Long-term accelerated forgetting of verbal and non-verbal information in temporal lobe epilepsy. Cortex. 2011 Mar 10; doi: 10.1016/j.cortex.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 18.Alessio A, Damasceno BP, Camargo CH, Kobayashi E, Guerreiro CA, Cendes F. Differences in memory performance and other clinical characteristics in patients with mesial temporal lobe epilepsy with and without hippocampal atrophy. Epilepsy Behav. 2004 Feb;5(1):22–27. doi: 10.1016/j.yebeh.2003.10.010. [DOI] [PubMed] [Google Scholar]
- 19.Kelley WM, Miezin FM, McDermott KB, et al. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998 May;20(5):927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- 20.Batchelor S, Thompson EO, Miller LA. Retrograde memory after unilateral stroke. Cortex. 2008 Feb;44(2):170–178. doi: 10.1016/j.cortex.2006.05.003. [DOI] [PubMed] [Google Scholar]
- 21.Lansing AE, Max JE, Delis DC, et al. Verbal learning and memory after childhood stroke. J Int Neuropsychol Soc. 2004 Sep;10(5):742–752. doi: 10.1017/S1355617704105122. [DOI] [PubMed] [Google Scholar]
- 22.Mosch SC, Max JE, Tranel D. A matched lesion analysis of childhood versus adult-onset brain injury due to unilateral stroke: another perspective on neural plasticity and recovery of social functioning. Cogn Behav Neurol. 2005 Mar;18(1):5–17. doi: 10.1097/01.wnn.0000152207.80819.3c. [DOI] [PubMed] [Google Scholar]
- 23.Kolk A, Ennok M, Laugesaar R, Kaldoja ML, Talvik T. Long-term cognitive outcomes after pediatric stroke. Pediatr Neurol. 2011 Feb;44(2):101–109. doi: 10.1016/j.pediatrneurol.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 24.Talib TL, Pongonis SJ, Williams LS, et al. Neuropsychologic outcomes in a case series of twins discordant for perinatal stroke. Pediatr Neurol. 2008 Feb;38(2):118–125. doi: 10.1016/j.pediatrneurol.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Westmacott R, Askalan R, MacGregor D, Anderson P, Deveber G. Cognitive outcome following unilateral arterial ischaemic stroke in childhood: effects of age at stroke and lesion location. Dev Med Child Neurol. 2010 Apr;52(4):386–393. doi: 10.1111/j.1469-8749.2009.03403.x. [DOI] [PubMed] [Google Scholar]
- 26.Westmacott R, MacGregor D, Askalan R, deVeber G. Late emergence of cognitive deficits after unilateral neonatal stroke. Stroke. 2009 Jun;40(6):2012–2019. doi: 10.1161/STROKEAHA.108.533976. [DOI] [PubMed] [Google Scholar]
- 27.Darmency-Stamboul V, Chantegret C, Ferdynus C, et al. Antenatal Factors Associated With Perinatal Arterial Ischemic Stroke. Stroke. 2012 Jun 26; doi: 10.1161/STROKEAHA.111.642181. [DOI] [PubMed] [Google Scholar]
- 28.Trauner DA, Nass R, Ballantyne A. Behavioural profiles of children and adolescents after pre- or perinatal unilateral brain damage. Brain. 2001 May;124(Pt 5):995–1002. doi: 10.1093/brain/124.5.995. [DOI] [PubMed] [Google Scholar]
- 29.Vargha-Khadem F, O’Gorman AM, Watters GV. Aphasia and handedness in relation to hemispheric side, age at injury and severity of cerebral lesion during childhood. Brain. 1985 Sep;108(Pt 3):677–696. doi: 10.1093/brain/108.3.677. [DOI] [PubMed] [Google Scholar]
- 30.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996 Jun;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 31.Gold JJ, Squire LR. Quantifying medial temporal lobe damage in memory-impaired patients. Hippocampus. 2005;15(1):79–85. doi: 10.1002/hipo.20032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cohen M. CMS - Children’s Memory Scale Manual. The Psychological Corporation, Harcourt Brace and Company; San Antonio, TX: 1997. [Google Scholar]
- 33.Chang L, Smith LM, LoPresti C, et al. Smaller subcortical volumes and cognitive deficits in children with prenatal methamphetamine exposure. Psychiatry Res. 2004 Dec 15;132(2):95–106. doi: 10.1016/j.pscychresns.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 34.Davidson M, Dorris L, O’Regan M, Zuberi SM. Memory consolidation and accelerated forgetting in children with idiopathic generalized epilepsy. Epilepsy Behav. 2007 Nov;11(3):394–400. doi: 10.1016/j.yebeh.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 35.Lum JA, Conti-Ramsden G, Page D, Ullman MT. Working, declarative and procedural memory in specific language impairment. Cortex. 2011 Jun 12; doi: 10.1016/j.cortex.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Uematsu A, Matsui M, Tanaka C, et al. Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLoS One. 2012;7(10):e46970. doi: 10.1371/journal.pone.0046970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasboun D, Chantome M, Zouaoui A, et al. MR determination of hippocampal volume: comparison of three methods. AJNR Am J Neuroradiol. 1996 Jun-Jul;17(6):1091–1098. [PMC free article] [PubMed] [Google Scholar]
- 38.Kalviainen R, Salmenpera T, Partanen K, Vainio P, Riekkinen P, Pitkanen A. Recurrent seizures may cause hippocampal damage in temporal lobe epilepsy. Neurology. 1998 May;50(5):1377–1382. doi: 10.1212/wnl.50.5.1377. [DOI] [PubMed] [Google Scholar]
- 39.Tasch E, Cendes F, Li LM, Dubeau F, Andermann F, Arnold DL. Neuroimaging evidence of progressive neuronal loss and dysfunction in temporal lobe epilepsy. Ann Neurol. 1999 May;45(5):568–576. doi: 10.1002/1531-8249(199905)45:5<568::aid-ana4>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 40.Theodore WH, Bhatia S, Hatta J, et al. Hippocampal atrophy, epilepsy duration, and febrile seizures in patients with partial seizures. Neurology. 1999 Jan 1;52(1):132–136. doi: 10.1212/wnl.52.1.132. [DOI] [PubMed] [Google Scholar]
- 41.Bernasconi N, Natsume J, Bernasconi A. Progression in temporal lobe epilepsy: differential atrophy in mesial temporal structures. Neurology. 2005 Jul 26;65(2):223–228. doi: 10.1212/01.wnl.0000169066.46912.fa. [DOI] [PubMed] [Google Scholar]
- 42.Shamim S, Hasler G, Liew C, Sato S, Theodore WH. Temporal lobe epilepsy, depression, and hippocampal volume. Epilepsia. 2009 May;50(5):1067–1071. doi: 10.1111/j.1528-1167.2008.01883.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bonilha L, Edwards JC, Kinsman SL, et al. Extrahippocampal gray matter loss and hippocampal deafferentation in patients with temporal lobe epilepsy. Epilepsia. 2010 Apr;51(4):519–528. doi: 10.1111/j.1528-1167.2009.02506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mitchell LA, Harvey AS, Coleman LT, Mandelstam SA, Jackson GD. Anterior temporal changes on MR images of children with hippocampal sclerosis: an effect of seizures on the immature brain? AJNR Am J Neuroradiol. 2003 Sep;24(8):1670–1677. [PMC free article] [PubMed] [Google Scholar]
- 45.Riney CJ, Harding B, Harkness WJ, Scott RC, Cross JH. Hippocampal sclerosis in children with lesional epilepsy is influenced by age at seizure onset. Epilepsia. 2006 Jan;47(1):159–166. doi: 10.1111/j.1528-1167.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 46.Squier W, Salisbury H, Sisodiya S. Stroke in the developing brain and intractable epilepsy: effect of timing on hippocampal sclerosis. Dev Med Child Neurol. 2003 Sep;45(9):580–585. doi: 10.1017/s0012162203001075. [DOI] [PubMed] [Google Scholar]
- 47.van Praag H, Chun D, Black IB, Staubli UV. Unilateral hippocampal ablation at birth causes a reduction in contralateral LTP. Brain Res. 1998 Jun 8;795(1-2):170–178. doi: 10.1016/s0006-8993(98)00287-x. [DOI] [PubMed] [Google Scholar]
- 48.Ballantyne AO, Spilkin AM, Hesselink J, Trauner DA. Plasticity in the developing brain: intellectual, language and academic functions in children with ischaemic perinatal stroke. Brain. 2008 Nov;131(Pt 11):2975–2985. doi: 10.1093/brain/awn176. [DOI] [PMC free article] [PubMed] [Google Scholar]