Abstract
The pathogenesis of inflammatory bowel disease is orchestrated by specific subsets of cytokine-secreting T cells. The interleukin 9–producing subset of helper T cells contributes to the pathogenesis of inflammatory bowel disease in part by disrupting intestinal barrier function and impairing tissue-repair mechanisms.
Inflammatory bowel disease (IBD), which encompasses Crohn’s disease (CD) and ulcerative colitis (UC), involves sustained inflammation, mucosal barrier defects and frequent intestinal infections, probably as a result of a dysfunctional immune response. CD4+ T cells promote the chronic inflammatory response and probably determine the nature of the disease. Although enhanced responses of the TH17 subset of helper T cells and diminished responses of regulatory T cells correlate with disease in both CD and UC, there is a distinction between the inflammatory milieu of these diseases in that patients with CD have increased amounts of T helper type 1 (TH1)-related cytokines, while patients with UC exhibit a more pronounced T helper type 2 (TH2)-related immune response1,2. In a study in this issue of Nature Immunology, Gerlach et al. define the important role that anothersubset of helper T cells, interleukin 9 (IL-9)-producing TH9 cells, serves in the pathogenesis of IBD, particularly UC, by regulating intestinal barrier integrity and immunological function3.
Although IL-9 is a pleiotropic cytokine, serving as a growth factor for various cell types, activating mast cells and triggering mucous production by epithelial cells4, it remains an understudied cytokine in many disease models. TH9 cells are major producers of IL-9 and develop in vitro after naive T cells are activated in the presence of IL-4 and transforming growth factor-β (TGF-β)5,6 (Fig. 1). TH9 differentiation requires transcription factors that include PU.1, IRF4 and BATF7–9. IL-9 and TH9 cells have been shown to provide protection against helminth infection, to mediate allergic inflammation and to promote antitumor immunity10. The function of TH9 cells in autoimmune disease is less clear. The initial description of TH9 cells noted that the transfer of TH9 cells into lymphopenic recipient mice deficient in the RAG recombinase worsens colitis5, although the mechanism of this exacerbation and how IL-9 might contribute to the pathogenesis of the colitis were not detailed. Gerlach et al. address these issues by examining the role of IL-9 in mouse models of colitis and by demonstrating that IL-9 expression is associated with the severity of human gastrointestinal disease3.
Figure 1.
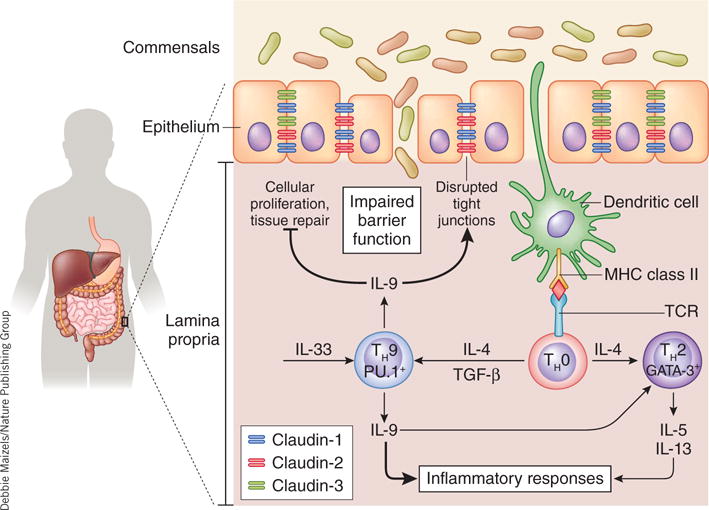
IL-9-mediated pathogenesis during UC. IL-9-producing TH9 cells develop after the cells encounter specific complexes of major histocompatibility complex (MHC) class II and antigen in the presence of TGF-β and IL-4. Local production of IL-33 may also promote IL-9 production. Interactions of IL-9 with the intestinal epithelial layer can inhibit cellular proliferation, impair tissue-repair mechanisms in response to cellular damage and alter the tight-junction composition (for example, claudins). These cumulative effects may be responsible for the IL-9-dependent disruption of intestinal barrier function; this results in the translocation of bacteria into the lamina propria, which triggers more inflammation and gut pathology. Furthermore, IL-9 augments the inflammatory response (for example, the release of cytokines and the intracellular generation of reactive oxygen species) in part by enhancing the TH2-like responses typical of UC. GATA-3, transcription factor.
Initially, Gerlach et al. examine the expression of genes encoding proinflammatory cytokines in colonic biopsies obtained from patients with active or inactive IBD3. IL9 expression is significantly elevated in patients with active UC and is highest in patients with the greater disease severity. Interestingly, increased expression of IL9 mRNA does not simply ‘track with’ intestinal inflammation, as patients with active CD do not exhibit higher IL9 expression. Because IL-9 can be derived from multiple cell types, the authors demonstrate, through the use of surgical resections from patients with IBD and control patients, that IL-9-producing cells in patients with active UC are CD4+. Furthermore, patients with active UC have more CD4+PU.1+ T cells in the intestine than do control patients or patients with CD. Expression of the receptor for IL-9 (IL-9R) is higher on the intestinal epithelial cells of patients with UC than on those of control patients, which suggests a role for signaling by IL-9 in IBD pathogenesis.
To address the function of IL-9 and TH9 cells in IBD experimentally, Gerlach et al. study mouse models of acute and chronic colitis3. Similar to the observations obtained with human patients with active UC, IL-9-producing cells and IL-9R expression on intestinal epithelial cells are increased during colitis. Gerlach et al. use a novel IL-9 reporter mouse to identify CD4+ cells, rather than alternative cell types such as innate lymphoid cells, as the principal source of this cytokine in vivo3. To address the function of IL-9, they induce colitis in mice given a neutralizing antibody to IL-9 or in mice with genetic deficiency in IL-9. In both models, colitis is reduced considerably in the absence of IL-9, as measured by weight loss, the generation of reactive oxygen species and clinical scores; this suggests that IL-9 is a pivotal cytokine in the promotion of colitis disease. Furthermore, PU.1 deficiency in CD4+ T cells, which blocks the differentiation of TH9 cells7, protects mice from colitis, which indicates TH9 cells are also vital to the pathogenesis of colitis.
Gerlach et al. proceed to explore potential mechanisms by which IL-9 might promote colonic disease3. The authors note alterations in tight junctions in the intestinal wall in both patients with active UC and colitis models. In oxazolone-induced colitis (a form of colitis with TH2 characteristics), expression of the pore-forming factors claudin-1 and claudin-2 is increased, in contrast to the diminished expression of claudin-3 and occludin, which are known to increase barrier function. Because IL-9R expression is enhanced on intestinal epithelial cells, the authors hypothesize that stimulation of the intestinal epithelial cells with IL-9 might impair intestinal barrier function. Indeed, in the colitis models, intestinal membrane permeability (as measured by the translocation of bacteria or with the permeability tracer fluorescein isothiocyanate–dextran) is markedly diminished in the absence of IL-9. The authors then investigate whether IL-9 directly affects barrier function in vivo. Using confocal laser endomicroscopy, the authors apply recombinant IL-9 to the intestinal wall. By using the translocation of labeled commensal bacteria as a ‘readout’, Gerlach et al. demonstrate that exposure of the intestinal wall to IL-9 leads to decreased barrier function in vivo3. It is still unclear whether the effects of IL-9 on barrier function are due to the engagement of IL-9R on intestinal epithelial cells in vivo, a proposal supported by the evidence of IL-9-induced expression of claudin-2 in vivo and in intestinal cells in vitro, or whether some effects are mediated indirectly, as observed in a model of anaphylaxis induced by an antigen administered orally showing that IL-9-mediated changes in intestinal permeability are dependent on mast cells11.
To further investigate the effect of IL-9 on the intestinal tract, Gerlach et al. treat an ‘organoid’ culture (three-dimensional organ buds grown in vitro) of intestinal crypts with recombinant IL-9 (ref. 3). While they note no effect on apoptosis, recombinant IL-9 does diminish overall cellular proliferation and bud formation, which indicates that IL-9 potentially limits cellular growth and development in vivo. To investigate this, the authors study the healing of mucosal wounds inflicted through the use of forceps to surgically damage the intestinal wall of mice. While mice treated with saline at the wound display notable tissue repair within 2 days of surgery, mice given recombinant IL-9 at the site of damage exhibit a significant time lag in tissue repair. IL-9 also affects the local intestinal immune response. IL-9 deficiency in the models of acute and chronic colitis leads to a significant reduction in the production and expression of mRNA encoding many proinflammatory cytokines, particularly those associated with TH2 immunity3. Although IL-9 has been shown to promote the differentiation of TH2 cells4, how it does this is still unclear. It is also not yet possible to distinguish the relative importance of IL-9 signaling on immune response versus that of the IL-9R+ intestinal epithelium in the context of the overall colitis pathogenesis. However, IL-9 probably promotes IBD by augmenting inflammation and impairing local tissue integrity and repair (Fig. 1).
Another issue that remains to be explored is the basis for the ‘preferential’ expansion of the IL-9-secreting T cell population. TH2-skewed immune responses and elevated TGF-β production are typical of patients with UC, which indicates the gastrointestinal tract of patients with UC may be supportive of local differentiation of TH9 cells2,3. Increased IL-9 expression and numbers of CD4+IL-9+ T cells are not observed in samples from patients with CD, which could be due to either a more TH1 cell–centric immune response in patients with CD that negates the development of TH9 cells or the alternative environment present in patients with UC3. In the mouse models of colitis, Gerlach et al. note that IL-33, rather than IL-4 and TGF-β, promotes IL-9 production in lamina propria T cells, which may be explained in part by lower expression of the receptors for IL-4 and TGF-β on intestinal T cells than on splenic T cells3. In parallel, IL-33 production is greater in patients with UC than those with CD12, and IL-33 may promote the differentiation of TH9 cells independently of IL-4 signaling13. It will be important to identify what factors ultimately initiate and/or maintain TH9 immune responses in vivo to modify immune responses in patients with UC through the use of specific therapeutics.
The most important implications of this report are that IL-9 promotes inflammation in the intestinal milieu, as well as the therapeutic potential of targeting IL-9 activity in patients with UC. IL-9 not only seems to have a direct effect on intestinal epithelial biology but may also promote the pathogenic TH2-like immune responses associated with UC. IL-9 also has a modest but significant effect on production of the proinflammatory cytokine tumor-necrosis factor, which potentially links IL-9 to the efficacy of treating patients with UC with a neutralizing antibody to tumor-necrosis factor, already a commonly used therapeutic1,2. Therapies that modulate the activity of IL-9 might provide a similar benefit to patients already suffering from IBD or patients who are refractory to established treatments.
Footnotes
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Baumgart DC, Sandborn WJ. Lancet. 2012;380:1590–1605. doi: 10.1016/S0140-6736(12)60026-9. [DOI] [PubMed] [Google Scholar]
- 2.Ordás I, Eckmann L, Talamini M, Baumgart DC, Sandborn WJ. Lancet. 2012;380:1606–1619. doi: 10.1016/S0140-6736(12)60150-0. [DOI] [PubMed] [Google Scholar]
- 3.Gerlach K, et al. Nat Immunol. 2014;15:676–686. doi: 10.1038/ni.2920. [DOI] [PubMed] [Google Scholar]
- 4.Goswami R, Kaplan MH. J Immunol. 2011;186:3283–3288. doi: 10.4049/jimmunol.1003049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dardalhon V, et al. Nat Immunol. 2008;9:1347–1355. doi: 10.1038/ni.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Veldhoen M, et al. Nat Immunol. 2008;9:1341–1346. doi: 10.1038/ni.1659. [DOI] [PubMed] [Google Scholar]
- 7.Chang HC, et al. Nat Immunol. 2010;11:527–534. doi: 10.1038/ni.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabeen R, et al. J Clin Invest. 2013;123:4641–4653. doi: 10.1172/JCI69489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Staudt V, et al. Immunity. 2010;33:192–202. doi: 10.1016/j.immuni.2010.07.014. [DOI] [PubMed] [Google Scholar]
- 10.Kaplan MH. Immunol Rev. 2013;252:104–115. doi: 10.1111/imr.12028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Forbes EE, et al. J Exp Med. 2008;205:897–913. doi: 10.1084/jem.20071046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pastorelli L, et al. Proc Natl Acad Sci USA. 2010;107:8017–8022. doi: 10.1073/pnas.0912678107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Uyttenhove C, Brombacher F, Van Snick J. Eur J Immunol. 2010;40:2230–2235. doi: 10.1002/eji.200940281. [DOI] [PubMed] [Google Scholar]


