Abstract
Objective: Spontaneous differentiation of human embryonic stem cell (hESC) cultures is a major concern in stem cell research. Physical removal of differentiated areas in a stem cell colony is the current approach used to keep the cultures in a pluripotent state for a prolonged period of time. All hESCs available for research require unidentified soluble factors secreted from feeder layers to maintain the undifferentiated state and pluripotency. Under experimental conditions, stem cells are grown on various matrices, the most commonly used being Matrigel.
Materials and Methods: We propose an alternative method to prevent spontaneous differentiation of hESCs grown on Matrigel that uses low amounts of recombinant noggin. We make use of the porosity of Matrigel to serve as a matrix that traps noggin and gradually releases it into the culture to antagonize bone morphogenetic proteins (BMP). BMPs are known to initiate differentiation of hESCs and are either present in the conditioned medium or are secreted by hESCs themselves.
Results: hESCs grown on Matrigel supplemented with noggin in conditioned medium from feeder layers (irradiated mouse embryonic fibroblasts) retained both normal karyotype and markers of hESC pluripotency for 14 days. In addition, these cultures were found to have increased cell proliferation of stem cells as compared to hESCs grown on Matrigel alone.
Conclusion: Noggin can be utilized for short term prevention of spontaneous differentiation of stem cells grown on Matrigel.
Introduction
Embryonic stem cells are capable of differentiating into cells of all three germ layers. A key to unlocking their potential in cell replacement therapy and drug discovery is understanding the critical pathways and factors involved in maintenance of pluripotency and self–renewal, as well as differentiation towards specific lineages. To be able to perform such experiments, it is imperative that the starting stem cell population is in a pluripotent state, as spontaneous differentiation of stem cells is a major problem. It has been previously documented that Matrigel, a solubilized basement membrane preparation extracted from Engelbreth‐Holm‐Swarm mouse sarcoma (a tumour rich in extra cellular matrix proteins), favours spontaneous differentiation of stem cells into trophoblast lineage (1).
Traditionally, pluripotency of human embryonic stem cells (hESC) is maintained by growing the cells on irradiated mouse embryonic fibroblast (iMEF) layers (2, 3), cultured on laminin or gelatin‐coated tissue culture plates or on Matrigel, supplemented with conditioned medium from iMEF (4). Present feeder‐free methods use Matrigel to facilitate attachment of hESCs, along with medium conditioned by feeder cells (iMEF) (5) or human fibroblasts (6). Additionally, signals received from the feeder layers do not operate through the leukaemia inhibitory factor/gp130 pathway (7, 8), as is the case with mouse embryonic stem cells. Selection of optimal extracellular matrices and identification of the soluble factors present in conditioned medium are active research areas (9). This is of particular interest in terms of derivation of new hESC cell lines, which may be of clinical relevance. It has been reported that medium conditioned by feeder cells can be replaced by high levels of basic fibroblast growth factor (FGF‐2/bFGF) and noggin (10) or a combination of activin A, nicotinamide and keratinocyte growth factor (11). Ezashi et al. have reported use of low oxygen concentration for maintenance of pluripotency (12).
Noggin is a 232 amino acid‐secreted glycosylated protein, which forms covalently linked homodimers and has high affinity for bone morphogenetic protein 4 (BMP4) (13). Noggin functions to antagonize BMP signalling by blocking the ability of BMP to interact with its receptor (14). Xu et al. used 500 ng/ml noggin for their in vitro experiments to maintain pluripotency, but concluded that 100 ng/ml was sufficient if used together with 40 mg/ml of bFGF (15). In contrast, treatment of HES‐2 and HES‐3 cells with noggin resulted in changes in colony morphology and loss of pluripotency marker GCTM‐2, in addition to appearance of early markers of neural differentiation (3). In a separate study, noggin expressing NIH 3T3 cells were used to provide conditioned medium to grow stem cells in a feeder‐free environment (10).
The goal of the present study was to identify the potential of noggin alone as a mediator of pluripotency and to evaluate hESCs grown on noggin‐containing Matrigel or noggin‐containing medium over a 2‐week period.
Materials and methods
hESC culture
The hESC cell line H1 (WA‐01, WiCell Research Institute, Madison, WI, USA) was maintained on iMEF feeder layers in Dulbecco's modified Eagle's medium (DMEM)/F12 supplemented with 20% knockout serum (Invitrogen, Carlsbad, CA, USA), 1 mm l‐glutamine (Cellgro, Herndon, VA, USA), 1% non‐essential amino acids (Cellgro, Herndon, VA, USA), 0.1 mmβ‐mercaptoethanol (Sigma, St Louis, MO, USA) and 4 ng/ml bFGF (Invitrogen). For differentiation experiments, cells were split using 1 mg/ml collagenase type IV (Invitrogen) and plated onto Matrigel™‐coated plates (BD, Franklin Lakes, NJ, USA) in iMEF conditioned medium. A dose–response curve for noggin (200, 400, 600 and 800 ng/ml) (R&D Systems, Minneapolis, MN, USA) indicated that 400 ng/ml was the optimal concentration to be used for these studies (data not shown). hESCs were treated with noggin (400 ng/ml) for either 14 or 7 days with noggin alone, followed by addition of BMP4 (90 ng/ml; R&D Systems) for another 6–7 days. Control wells received iMEF conditioned medium. In separate experiments, noggin was also incorporated into Matrigel at the time of plating before polymerization. Noggin (400 ng/ml) was added to Matrigel‐containing DMEM/F‐12, plated on 6‐well plates and incubated overnight before use. Medium exchange was performed daily except on day 2 after plating of cultures.
Conditioned medium
iMEF were plated on gelatin‐coated 6‐well plates in DMEM containing 10% foetal bovine serum and 1% penicillin‐streptomycin. On the following day, cultures were washed in phosphate‐buffered saline (PBS) and with hESC medium. Conditioned medium were collected, filter‐sterilized and added to hESC on Matrigel. This procedure was repeated daily.
Reverse transcription–polymerase chain reaction
Total RNA was isolated (Trizol Reagent, Life Technologies Inc., Rockville, MD, USA) from hESCs untreated (control) or treated with noggin, for either 13 days or 7 days, followed by BMP4 for an additional 6 days. Total RNA was then quantified. Two micrograms of total RNA was reverse‐transcribed using oligo dT primers (Invitrogen) and AMV‐Reverse Transcriptase (Sigma) for reverse transcription–polymerase chain reaction (RT‐PCR) analysis. DNA primers specific to GAPDH, Oct4, hHand1, AP2α, BMP4, hCGβ, Flk1, Nodal, Nanog, Cripto and Pax6 (IDT Technologies, Coralville, IA, USA) were used in PCRs (25–30 cycles of amplifications) to determine relative levels of expression of these genes, across the treatment groups. Sequences of forward and reverse primers are listed in Table 1.
Table 1.
Sequence of forward and reverse primers used
| Markers | Forward primer | Reverse primer |
|---|---|---|
| Oct4 | CGTGAAGCTGGAGAAGGAGAAGCTG | CAAGGGCCGCAGCTTACACATGTTC |
| BMP4 | AGGAGCTTCCACCACGAAGAACAT | TTCAGTGGGCACACAACAGGCTTT |
| TFAP2α | TCCCTGTCCAAGTCCAACAGCAAT | TGGCAGGAAATTCGGTTTCGCACA |
| Hand1 | ATTAACAGCGCATTCGCGGAGTTG | TGGTTTAACTCCAGCGCCCAGACTT |
| hCGβ | ACGACTGAGTCTCTGAGGTCACTT | ACAGATGGTGGTGGTGACGGTGAT |
| GAPDH | TGAAGGTCGGAGTCAACGGATTTGGT | CATGTGGGCCATGAGGTCCACCAC |
| Flk1 | ACGCTGACATGTACGGTCTAT | GCCAAGCTTGTACCATGTGAG |
| Nodal | AGACATCATCCGCAGCCTACA | GACCTGGGACAAAGTGACAGTGAA |
| Nanog | ACCAGAACTGTGTTCTCTTCCACC | GGTTGCTCCAGGTTGAATTGTTCC |
| Cripto | CAGAACCTGCTGCCTGAATG | GTAGAAATGCCTGAGGAAAGC |
| Pax6 | AACAGACACAGCCCTCACAAACA | CGGGAACTTGAACTGGAACTGAC |
| Brachyury T | GTGACCAAGAACGGCAGGAGG | TGTTCCGATGAGCATAGGGGC |
Immunocytochemistry
hESCs were grown on Matrigel with noggin (400 ng/ml) or Matrigel alone for 7 days and then subcultured, using collagenase type IV (1 mg/ml; Sigma), on to chamber slides (LabTek, Nalge Nunc International, Rochester, NY) coated with Matrigel (plus or minus noggin). Treatments (presence or absence of BMP4; 90 ng/ml) were initiated and cultures continued for another 6 days. Slides were fixed with 4% paraformaldehyde for 10 min at room temperature, post‐fixed with ice‐cold ethanol–acetic acid mixture for 5 min at –20 °C and subsequently washed with PBS. Following washing with PBS, slides were blocked with 10% normal donkey serum for 1 h at room temperature. Primary antibodies against Oct4 (Santa Cruz Biotechnology, Santa Cruz, CA, USA) and hCGβ (Amersham Biosciences, Piscataway, NJ, USA) were used at a concentration of 10 µg/ml, of 1% normal donkey serum in PBS, and incubated at room temperature for 1 h. Donkey anti‐mouse antibody conjugated with fluorescein isothiocyanate (FITC; 1 : 50 dilution; Jackson ImmunoResearch Laboratories, West Grove, PA, USA) in 1% normal donkey serum in PBS was added for an additional hour at room temperature. Slides were washed three times in PBS and mounted using Prolong Antifade DAPI‐containing Vecta shield (Molecular Probes, Invitrogen) and colonies observed using a confocal fluorescence microscope [Zeiss LSM Pascal confocal imaging system mounted on a Zeiss Axioscope II using UV (405 nm), HeNe (543 nm) and Argon (488 nm) laser excitation]. Additionally, noggin‐treated colonies were stained for Oct4 (FITC) and hCGβ (phycoerythrin) and counterstained with DAPI (Molecular Probes, Invitrogen).
Flow cytometric analysis to quantify undifferentiated cells under different culture conditions
hESCs were cultured for 13 days on Matrigel alone and Matrigel supplemented with noggin. Half of the wells in each set received BMP4 treatment (90 ng/ml) from days 8 to 13 of culture. Cells were harvested using a 5‐min collagenase treatment, followed by preparation of single‐cell suspensions using non‐enzymatic cell dissociation medium (Sigma). Single cell suspensions from each condition were labelled with rabbit anti‐Oct4 (C‐10; Santa Cruz Biotechnology) and mouse anti‐SSEA‐4 (Abcam, Cambridge, MA) and detected with Alexa Fluor 488‐conjugated donkey anti‐rabbit and Cy5‐conjugated donkey anti‐mouse immunoglobulin G (Jackson ImmunoResearch Laboratories), respectively. Cells were analysed using a Becton Dickinson FACScan and cell‐surface antigen expression was quantified using CellQuest software (Becton Dickinson).
Cell proliferation assay
Carboxyfluorescein succinimidyl ester (CFSE; Molecular Probes, Invitrogen) was utilized to measure cell proliferation following exposure to noggin (16, 17). Cells were grown on Matrigel alone or Matrigel containing noggin and exposed to a single pulse of 5 µm CFSE for 10 min at room temperature. Knockout serum (50%) was then added to quench any residual CFSE not taken up by cells. Cells were washed in PBS and iMEF conditioned medium were replaced. A zero time point sample was taken at this time to assess starting CFSE fluorescence intensity. After 7 days, cells were washed and dissociated into single cell suspension using cell dissociation medium (Sigma) and fixed in 2% paraformaldehyde. Rate of decay in CFSE fluorescence was indicative of rate of proliferation. Fluorescence intensity was assessed by flow cytometry and proliferation rates (division index) determined using Flow Jo software (Tree Star, Ashland, OR, USA).
Western blotting
Cytoplasmic and nuclear extracts were prepared (NE‐PER, Pierce Biotechnology, Rockford, IL, USA) from hESCs treated with noggin or BMP4, resolved on 10% SDS‐PAGE precast gels (ISC BioExpress, Kaysville, UT, USA) and transferred on to immobilon‐P membrane (Millipore, Temecula, CA). The blot was probed with Oct4 antibody (1 : 200 dilution, 4 °C overnight). Secondary horseradish peroxidase‐labelled antibody was used at 1 : 10 000 dilution (Jackson ImmunoResearch Laboratories). Peroxidase activity was detected by Super Signal, West Pico chemiluminiscence substrate (Pierce Biotechnology). The blot was exposed to autoradiography film (ISC BioExpress) and developed. Subsequently, immune complexes were removed from the blot using stripping buffer (Pierce Biotechnology) and re‐probed with β‐actin antibody to serve as a loading control.
Karyotype analysis
We determined hESC karyotype by G‐banding (data not shown) at the cytogenetics laboratory of the University of Kansas Medical Center. hESC were plated on Matrigel and cultured in iMEF conditioned medium and exposed to noggin (400 ng/ml) for 2 weeks. At least 15 cells were examined from each sample.
Statistical analysis
Data were analysed by one‐way analysis of variance (anova) to detect statistically significant effects. Statistical differences between means were detected by Fisher's test if the anova indicated a significant difference (P ≤ 0.05).
Results
Exogenous daily addition of noggin
Conditioned medium supplemented with noggin resulted in hESC colonies exhibiting densely packed cells with no apparent signs of differentiation (Fig. 1, right panel). In contrast, flattened areas were observed in the middle and at the edge of hESC colonies grown in conditioned medium without noggin. This resulted in distinct doughnut‐shaped morphology (Fig. 1, left panel).
Figure 1.
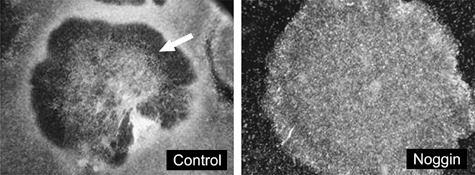
Human embryonic stem cell (hESC) colony under conventional culture conditions for 7 days of culture (left panel). Signs of differentiation such as a doughnut‐shaped colony structure and flattened cells in the middle of the colony (arrow) and on the periphery of the colony are evident. Representative hESC colony (right panel) cultured in media containing noggin (400 ng/ml) showing no signs of differentiation even after 13 days of culture.
We observed that disruption of BMP4 signalling by the use of BMP antagonist noggin, resulted in noggin maintaining cell pluripotency as indicated by Oct4 expression (Fig. 2). Since hESC have previously been reported to spontaneously differentiate into trophoblast, we used hTFAP2α, hCGβ and Hand1 as trophoblast markers. Absence of these trophoblastic markers was evident in noggin‐treated cells (Fig. 2). Interestingly, Oct4 transcript levels did not vary between noggin‐treated and BMP4‐treated cells. Treatment of hESCs for 13 days with noggin seemed to lower BMP4 transcript levels, suggesting that noggin, in addition to physically interacting with BMP4, may also affect BMP4 mRNA levels.
Figure 2.
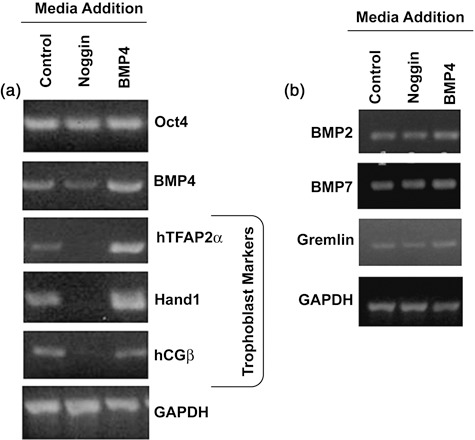
Reverse transcription–polymerase chain reaction analysis of control [irradiated mouse embryonic fibroblasts (iMEF) conditioned medium], noggin‐treated (iMEF conditioned medium containing noggin) and BMP4‐treated cells (iMEF conditioned medium containing BMP4). (a) Treatment with noggin (400 ng/ml) reduced expression of trophoblast differentiation markers. Cells treated with BMP4 (90 ng/ml) to induce differentiation towards trophoblast lineage expressed trophoblast markers (hTFAP2α, Hand1 and hCGβ) as did control cultures containing colonies undergoing spontaneous differentiation. GAPDH served as a loading control. (b) Transcripts for BMP2, BMP7 and BMP antagonist Gremlin remained unchanged across the treatment groups. Transcripts for noggin and chordin were not detected (data not shown).
Previous studies have found that cultures treated with noggin and BMP4 did not express neuronal/ectodermal markers, such as Sox1 (18), nestin (19) or neuroD1 (11). We also assessed levels of BMP2, BMP4 and BMP7 and their antagonists noggin, gremlin and chordin mRNA by RT‐PCR analysis (Fig. 2b) (17). BMP4 was by far the most abundant, followed by comparable levels of BMP2 and BMP7 transcripts. Gremlin was the only detectable BMP antagonist expressed in the cultures.
Oct4 protein levels did not correspond with its transcript levels
Oct4 transcript levels assessed by RT‐PCR appeared to be similar between noggin‐treated samples, untreated control and BMP4‐treated samples (2, 3). This led us to compare Oct4 protein levels by Western blot analysis of nuclear extracts from noggin‐treated cells and from cells where treatment was shifted from noggin to BMP4. Cytoplasmic extract was run as control. Oct4 protein levels were markedly higher in noggin‐treated cells as compared to control cells and were significantly diminished upon switching treatment of hESC from noggin to BMP4 (Fig. 4).
Figure 3.
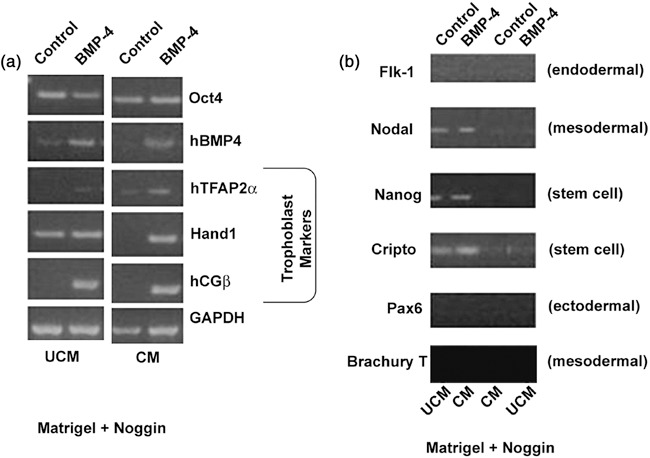
Gene expression in cells grown in irradiated mouse embryonic fibroblast conditioned medium (CM) or unconditioned medium (UCM). Human embryonic stem cells were grown on Matrigel supplemented with noggin (incorporated at the time of Matrigel polymerization on the culture plates) and cultured in UCM or CM for 7 days and subsequently exposed to BMP4 (90 ng/ml) or media alone for 6 additional days. (a) Reverse transcription–polymerase chain reaction (RT‐PCR) analysis of trophoblast‐specific markers, hTFAP2α, hCGβ and Hand1, revealed that their expression was decreased in cultures grown in CM and expression was induced following treatment with BMP4. Oct4 levels seem to be similar between control and BMP4‐treated cells. (b) RT‐PCR analysis for expression of stem cell markers (Nanog and Cripto) as well as germ layer‐specific markers (Flk1‐endodermal, Nodal and Brachury T‐mesodermal, Pax6‐neuronal/ectodermal marker). Total RNA was isolated at the end of the culture period.
Figure 4.
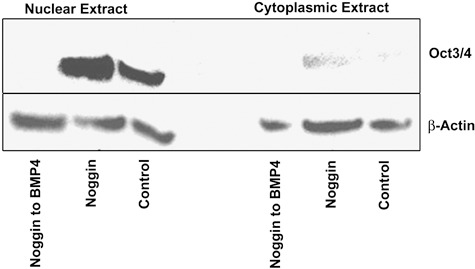
Nuclear and cytoplasmic levels of Oct4 in human embryonic stem cells (hESC). hESCs were grown on Matrigel and exposed to noggin (400 ng/ml) for 7 days followed by an additional 7 days with noggin or alternatively, treatment with BMP4 (90 ng/ml). A third group of cells was grown on Matrigel in the absence of noggin and BMP4 and served as untreated controls. Cells were harvested on day 14 for nuclear and cytosolic extract preparation; proteins were resolved on 8–16% gradient sodium dodecyl sulphate–polyacrylamide gel electrophoresis, transferred to nitrocellulose membrane and probed for Oct4 and β‐actin. Noggin‐treated cells clearly showed much higher levels of Oct4 as compared to control. When treatment was switched from noggin to BMP4, Oct4 protein was completely lost; this thus indicates that ability of cells to differentiate was not lost as a result of noggin treatment.
Noggin added to Matrigel at time of polymerization
As an alternative to daily addition of noggin to the media (due to daily media replacement), we added noggin to Matrigel at the time of its polymerization. We hypothesized that slow release of noggin into the medium would be as effective at repressing spontaneous differentiation as that achieved following direct addition to the medium. This resulted in containment of spontaneous differentiation as demonstrated by colony and cell morphology and by maintenance of high levels of pluripotency markers, such as Oct4, Nanog (11), and Cripto (3), as shown in Fig. 3. This was further supported by absence of lineage‐specific differentiation markers, such as ectodermal marker PAX6 (3), trophoblast markers such as Hand1 (20), hTFAP2α and hCGβ (1), an endodermal marker Flk1 (21), and a mesodermal marker Nodal (22), as shown by RT‐PCR analysis (Fig. 3a,b). We also found variability in BMP4 mRNA levels across the treatment groups.
A comparison between conditioned and unconditioned media was also made to explore the possibility of eliminating use of iMEF conditioned medium. Noggin appeared to reduce BMP4 mRNA in hESC cultured in conditioned medium as compared to unconditioned medium. Control samples from unconditioned medium appeared to express higher BMP4 mRNA levels as compared to controls from conditioned medium. Noggin transcript was not detectable in any of the samples, whereas gremlin mRNA was present in all of them and appeared to be more abundant in hESC grown in unconditioned medium (data not shown). hESC grown in conditioned medium had greatly reduced spontaneous differentiation as compared to hESC grown in unconditioned medium. Noggin inhibited spontaneous differentiation (cell morphology and mRNA levels of Oct4) in both media but was more effective when added to hESC grown in conditioned medium. hCGβ and Hand1 expression were higher in cultures from unconditioned medium as compared to those from conditioned medium (Fig. 3a). In addition, hCGβ and Hand1 expression was higher in control cells grown in unconditioned medium compared to those grown in conditioned medium (Fig. 3a). Sox1, Nestin and NeuroD1 transcripts were undetectable following exposure of hESC to noggin, regardless of their culture medium (conditioned or unconditioned). This clearly indicated that noggin was not involved in initiating differentiation towards a neuronal lineage, which is supported by an earlier report by Li et al. (23).
Oct4 and hCGβ immunocytochemistry
Immunocytochemistry experiments for Oct4 and hCGβ in cells grown on Matrigel with and without noggin revealed that noggin‐treated cells had greater overall Oct4 staining that was more uniform across a colony (Fig. 5a vs. 5c). Cells grown in the presence of noggin appeared to be smaller compared to spontaneously differentiated cells, which were positive for hCGβ and negative for Oct4. This altered morphology and pattern of hCGβ expression is similar to that seen following BMP4‐induced differentiation of hESCs (data not shown). These observations emphasize the role of noggin in maintenance of stem cell pluripotency and inhibition of spontaneous differentiation.
Figure 5.
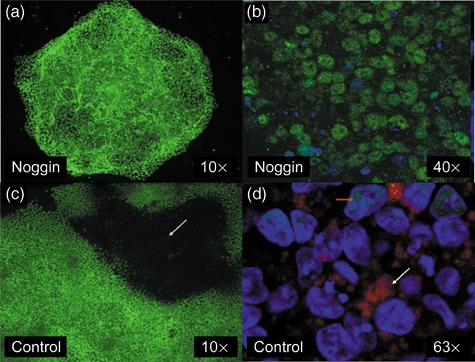
Effect of noggin on colony morphology and protein expression. Cells were cultured in irradiated mouse embryonic fibroblast conditioned medium on Matrigel‐coated chamber slides in the absence (c and d) or presence of noggin (a and b). Human embryonic stem cell colonies cultured in the presence of noggin for 7 days and stained with (a) an Oct4 antibody [fluorescein isothiocyanate (FITC)] or (b) stained for Oct4 (FITC) and hCGβ (phycoerythrin) and counterstained with DAPI. (c) Spontaneously differentiating colony grown on Matrigel showing typical doughnut‐shaped appearance with Oct4‐negative, differentiated cells in the centre of the photograph (dark area indicated by arrow). (d) Spontaneously differentiated cells stained for Oct4 (FITC), and hCGβ (phycoerythrin) and counterstained with DAPI. Orange arrow indicating residual nuclear Oct4 staining (green) in some cells and also much larger nuclei (DAPI) of the differentiated cells. White arrow is shows positive hCGβ (red) staining.
FACS analysis of pluripotency markers
Flow cytometric analysis for pluripotency surface marker SSEA4 (24) and transcription factor Oct4 revealed that a higher proportion of the cells expressed these markers when grown on noggin‐containing Matrigel compared to those grown on Matrigel alone. Exposure of hESCs to BMP4 (90 ng/ml) reduced the percentage of cells expressing SSEA4 and Oct4 regardless of presence or absence of noggin (Fig. 6). Thus, these findings indicate that hESCs grown on Matrigel supplemented with noggin can undergo BMP4‐induced differentiation (that is, suppression of expression of pluripotency markers) when administered to the media.
Figure 6.
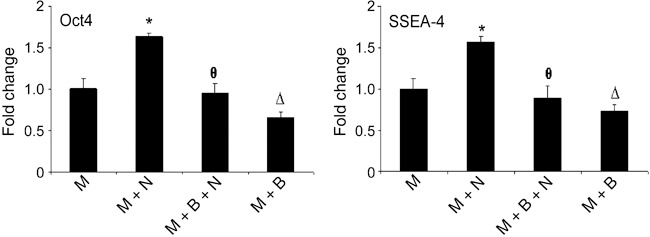
Regulation of Oct4 and SSEA4 protein levels by noggin and BMP4. Level of expression of Oct4 and SSEA4 was assessed by FACS analysis and data presented as fold change in average mean intensity. Level of Oct4 [fluorescein isothiocyanate (FITC)] and SSEA4 (Cy5) in hESCs grown on Matrigel supplemented with noggin (M + N) was enhanced compared to cells grown on Matrigel (M) alone. Additional cultures were treated with BMP4 in the presence (M + N + B) or absence (M + B) of noggin. Treatment of cells with BMP4 suppressed levels of both Oct4 and SSEA4 in cells grown in the presence or absence of noggin. Data are presented as percentage of cells that stained positively for Oct4 (FITC) and SSEA4 (Cy5). * represents P < 0.05 for M + N vs. M, θ represents P < 0.05 for M + N vs. M + N + B, and Δ represents P < 0.05 for M vs. M + B.
Role of noggin in cell proliferation
Noggin increased cell proliferation rate as determined by FACS analysis of cells pulsed with CFSE (25) (Fig. 7; shift to the left in CFSE intensity of the noggin‐treated cells). Noggin‐treated cells showed approximately 25% increase (P < 0.01) in proliferation index over control cells.
Figure 7.
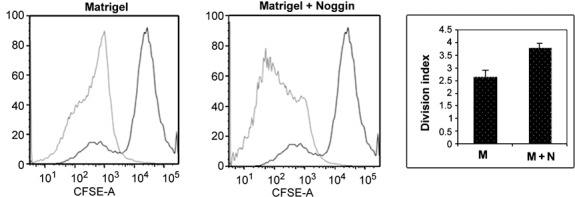
Effect of noggin on cell proliferation. Carboxyfluorescein succinimidyl ester (CFSE) was used to compare the degree of cell proliferation between cells exposed to noggin versus control cells. Cells were cultured on Matrigel or Matrigel supplemented with noggin. CFSE was added on day 4 post‐plating as described in the Materials and methods section and cells were cultured for an additional 7 days. Cells were then washed and analysed by FACS for CFSE intensity. Cells cultured with noggin had reduced levels of CFSE (shift to the left in the graph) indicative of an increased rate of proliferation. Data shown are representative of a set of four separate experiments. Treatment with noggin significantly (P < 0.01) increased the rate of cell proliferation (division index) as shown in the bar graph.
Discussion
Noggin, an antagonist of the BMP family of proteins, binds to BMPs and prevents the binding of BMPs to their receptor, thereby inhibiting any downstream signalling (15). Noggin has also been shown to regulate BMP signalling pathways and helps to maintain stem cells in a pluripotent state (10).
We have found that hESC cultured with noggin (in medium or incorporated into Matrigel matrix) form denser colonies compared to normal hESC cultures, and these findings are suggestive of better growth in the presence of noggin (data not shown). Comparison between conditioned and unconditioned medium was made to explore the possibility of eliminating use of iMEF conditioned medium. It appears from the RT‐PCR analysis that stem cells retain pluripotency better in conditioned medium and, thus, use of noggin by itself is not a substitute for all the factors contributed by iMEF conditioned medium.
Spontaneous differentiation was evident by appearance of colonies negative for Oct4 and positive for hCGβ. These colonies typically developed a doughnut‐like shape with presence of much larger differentiated cells being visible in the case of spontaneously differentiating cultures. These morphological patterns were absent in cultures supplemented with noggin. Flow cytometry data indicated increase in abundance of pluripotency markers SSEA‐4 and Oct4 in stem cells exposed to noggin as compared to control cells. Furthermore, noggin also appeared to support cell proliferation as shown by CFSE experiments.
Based on our present findings, we conclude that noggin can be incorporated as a medium supplement for maintaining stem cells in a pluripotent state, for short‐term culture experiments. Noggin did not trigger differentiation towards a neuronal lineage. Furthermore, when incorporated into the Matrigel, noggin prevented spontaneous differentiation during the time period examined. Thus, it appears that noggin was being slowly released from Matrigel into the medium and constantly neutralized any endogenous BMP or exogenous BMP originating from iMEF conditioned medium. These findings suggest that most of the spontaneous differentiation is BMP‐dependent and predominantly towards the trophoblast lineage.
Our findings indicate that noggin stimulates Oct4 protein levels in hESC cultures. Following removal of noggin and addition of BMP4, Oct4 protein disappeared indicating that noggin did not disrupt the capacity of hESC to differentiate. Additionally, there appeared to be involvement of post‐transcriptional Oct4 modifications upon differentiation, as no change was observed in Oct4 mRNA levels.
Beattie et al. have shown that activin A is able to maintain stem cell pluripotency in the absence of feeder layers (11). They also reported changes in cell and colony morphology when the cells were grown without the feeders, and reduced rate of cell proliferation. In contrast, our system with noggin is based on a desire to grow the embryonic stem cells in the absence of feeder cells and showed no signs of cell or colony morphology changes. In contrast to the findings of Beattie et al. (11), we report an increase in rate of cell proliferation.
Despite favourable reports concerning the use of noggin as a medium supplement for maintenance of pluripotency of hESCs, contradictory results exist in the literature. Pera et al. reported that noggin within the concentration range of 100–500 ng/ml was unsuitable for use, due to changes in colony size, lack of expression of stem cell marker GCTM2, and induction of expression of ectodermal marker Pax6 (3). We believe that these contrasting observations may be due to use of different lines of stem cells (HES‐2 and HES‐3). Noggin has been used in a number of experimental paradigms to aid in stem cell differentiation or to direct towards a specific lineage (26, 27, 28, 29). In most of these cases, the cells were being cultured in conditions that do not favour pluripotency. Xu et al. reported that inclusion of noggin and high concentrations of bFGF (40 ng/ml) in unconditioned medium was sufficient to maintain hESC in a pluripotent state and that lower concentrations of noggin could be used if present with this level of bFGF (15). We avoided use of elevated levels of bFGF due to its possible anti‐apoptotic role (30, 31). Although bFGF may support population growth of stem cells, it may also lead to unwarranted retention/selection of aneuploid cells and possibly neoplastic cells, which are generated as a result of erratic mitosis within the stem cell colony.
In light of our findings, it appears that there was a tight balance between concentrations of BMPs and BMP antagonists present in the culture, as has also been previously suggested (3). Over time this balance favours higher BMP levels, triggering spontaneous differentiation. Our data indicate that exogenous addition of noggin prevents or delays this spontaneous differentiation.
Conclusion
The occurrence of spontaneous differentiation in hESC cultures is a common problem. Several studies have shown a range of factors including noggin that can be included in hESC culture medium to maintain stem cells in a pluripotent state for extended periods of time. However, little is known regarding the composition of fibroblast conditioned medium, which is used for propagation of hESCs. Our findings indicate that noggin alone, when combined with the unknown factors present in conditioned medium, is sufficient to maintain hESC in a pluripotent state for a period of at least 2 weeks. This occurred whether noggin was added to culture medium or was included with Matrigel at the time of its polymerization and coating of the culture plates. Inclusion of noggin into Matrigel matrix has several advantages over its addition to the medium. Cells at the base of a colony are continuously exposed to noggin via the Matrigel matrix. Considerably less noggin is required over the course of an experiment and pluripotency is maintained with lower levels of noggin. Finally, noggin appeared to improve stem cell proliferation. It is currently unclear as to the mechanism of this effect, but may simply be due to suppression of cell differentiation and associated change in cell proliferation.
Acknowledgements
This work was supported by R01 HD20676 – Supplement to Michael J. Soares and a grant from the KUMC‐RI to Michael W. Wolfe. We thank Dr Joyce Slusser for help with FACS scan and analysis.
References
- 1. Xu R‐H, Chen X, Li DS, Li R, Addicks GC, Glennon C, Zwaka TP, Thomson JA (2002) BMP4 initiates human embryonic stem cell differentiation to trophoblast. Stem Cells 20, 1261–1264. [DOI] [PubMed] [Google Scholar]
- 2. Thomson JA, Itskovitz‐Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM (1998) Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147. [DOI] [PubMed] [Google Scholar]
- 3. Pera MF, Andrade J, Houssami S, Reubinoff B, Trounson A, Stanley EG, Ward‐van Oostwaard D, Mummery C (2004) Regulation of human embryonic stem cell differentiation by BMP‐2 and its antagonist noggin. J. Cell Sci. 117, 1269–1280. [DOI] [PubMed] [Google Scholar]
- 4. Klimanskaya I, Chung Y, Meisner L, Johnson J, West MD, Lanza R (2005) Human embryonic stem cells derived without feeder cells. Lancet 365, 1636–1641. [DOI] [PubMed] [Google Scholar]
- 5. Xu C, Inokuma MS, Denham J, Golds K, Kundu P, Gold JD, Carpenter MK (2001) Feeder‐free growth of undifferentiated human embryonic stem cells. Nat. Biotech. 19, 971–974. [DOI] [PubMed] [Google Scholar]
- 6. Richards M, Fong C‐Y, Chan W‐K, Wong P‐C, Bongso A (2002) Human feeders support prolonged undifferentiated growth of human inner cell masses and embryonic stem cells. Nat. Biotech. 20, 933–936. [DOI] [PubMed] [Google Scholar]
- 7. Humphrey RK, Beattie GM, Lopez AD, Bucay N, King CC, Firpo MT, Rose‐John S, Hayek A (2004) Maintenance of pluripotency in human embryonic stem cells is STAT3 independent. Stem Cells 22, 522–530. [DOI] [PubMed] [Google Scholar]
- 8. Sato N, Meijer L, Skaltsounis L, Greengard P, Brivanlou AH (2004) Maintenance of pluripotency in human and mouse embryonic stem cells through activation of Wnt signaling by a pharmacological GSK‐3‐specific inhibitor. Nat. Med. 10, 55–63. [DOI] [PubMed] [Google Scholar]
- 9. Prowse AB, McQuade LR, Bryant KJ, Van Dyk DD, Tuch BE, Gray PP (2005) A proteome analysis of conditioned media from human neonatal fibroblasts used in the maintenance of human embryonic stem cells. Proteomics 5, 978–989. [DOI] [PubMed] [Google Scholar]
- 10. Wang G, Zhang H, Zhao Y, Li J, Cai J, Wang P, Meng S, Feng J, Miao C, Ding M, Li D, Deng H (2005) Noggin and bFGF cooperate to maintain the pluripotency of human embryonic stem cells in the absence of feeder layers. Biochem. Biophys. Res. Commun. 330, 934–942. [DOI] [PubMed] [Google Scholar]
- 11. Beattie GM, Lopez AD, Bucay N, Hinton A, Firpo MT, King CC, Hayek A (2005) Activin A maintains pluripotency of human embryonic stem cells in the absence of feeder layers. Stem Cells 23, 489–495. [DOI] [PubMed] [Google Scholar]
- 12. Ezashi T, Das P, Roberts RM (2005) Low O2 tensions and the prevention of differentiation of hES cells. Proc. Natl. Acad. Sci. USA 102, 4783–4788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Groppe J, Greenwald J, Wiater E, Rodriguez‐Leon J, Economides AN, Kwiatkowski W, Affolter M, Vale WW, Belmonte JC, Choe S (2002) Structural basis of BMP signalling inhibition by the cystine knot protein Noggin. Nature 420, 636–642. [DOI] [PubMed] [Google Scholar]
- 14. Zimmerman LB, De Jesus‐Escobar JM, Harland RM (1996) The spemann organizer signal noggin binds and inactivates bone morphogenetic protein 4. Cell 86, 599–606. [DOI] [PubMed] [Google Scholar]
- 15. Xu R‐H, Peck RM, Li DS, Feng X, Ludwig T, Thomson JA (2005) Basic FGF and suppression of BMP signaling sustain undifferentiated proliferation of human ES cells. Nat. Methods 2, 185–190. [DOI] [PubMed] [Google Scholar]
- 16. Ganusov VV, Pilyugin SS, De Boer RJ, Murali‐Krishna K, Ahmed R, Antia R (2005) Quantifying cell turnover using CFSE data. J. Immunol. Methods 298, 183–200. [DOI] [PubMed] [Google Scholar]
- 17. Ahn J, Serrano de la Pena L, Shore EM, Kaplan FS (2003) Paresis of a bone morphogenetic protein‐antagonist response in a genetic disorder of heterotopic skeletogenesis. J. Bone Joint. Surg. Am. 85, 667–674. [DOI] [PubMed] [Google Scholar]
- 18. D’Amour KA, Agulnick AD, Eliazer S, Kelly OG, Kroon E, Baetge EE (2005) Efficient differentiation of human embryonic stem cells to definitive endoderm. Nat. Biotech. 23, 1534–1541. [DOI] [PubMed] [Google Scholar]
- 19. Iacovitti L, Donaldson AE, Marshall CE, Suon S, Yang M (2007) A protocol for the differentiation of human embryonic stem cells into dopaminergic neurons using only chemically defined human additives: studies in vitro and in vivo . Brain Res. 1127, 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Peiffer I, Belhomme D, Barbet R, Haydont V, Zhou Y‐P, Fortunel NO, Li M, Hatzfeld A, Fabiani JN, Hatzfeld JA (2007) Simultaneous differentiation of endothelial and trophoblastic cells derived from human embryonic stem cells. Stem Cells Dev. 16, 2393–2402. [DOI] [PubMed] [Google Scholar]
- 21. Iida M, Heike T, Yoshimoto M, Baba S, Doi H, Nakahata T (2005) Identification of cardiac stem cells with FLK1, CD31, and VE‐cadherin expression during embryonic stem cell differentiation. FASEB J. 19, 371–378. [DOI] [PubMed] [Google Scholar]
- 22. Reissmann E, Jornvall H, Blokzijl A, Andersson O, Chang C, Minchiotti G, Persico MG, Ibáñez CF, Brivanlou AH (2001) The orphan receptor ALK7 and the Activin receptor ALK4 mediate signaling by Nodal proteins during vertebrate development. Genes Dev. 15, 2010–2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Li W, LoTurco JJ (2000) Noggin is a negative regulator of neuronal differentiation in developing neocortex. Dev. Neurosci. 22, 68–73. [DOI] [PubMed] [Google Scholar]
- 24. Bibikova M, Chudin E, Wu B, Zhou L, Garcia EW, Liu Y, Shin S, Plaia TW, Auerbach JM, Arking DE, Gonzalez R, Crook J, Davidson B, Schulz TC, Robins A, Khanna A, Sartipy P, Hyllner J, Vanguri P, Savant‐Bhonsale S, Smith AK, Chakravarti A, Maitra A, Rao M, Barker DL, Loring JF, Fan JB (2006) Human embryonic stem cells have a unique epigenetic signature. Genome Res. 16, 1075–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Zhang Y, Stastny P (2006) MICA antigens stimulate T cell proliferation and cell‐mediated cytotoxicity. Hum. Immunol. 67, 215–222. [DOI] [PubMed] [Google Scholar]
- 26. Gerrard L, Rodgers L, Cui W (2005) Differentiation of human embryonic stem cells to neural lineages in adherent culture by blocking bone morphogenetic protein signaling. Stem Cells 23, 1234–1241. [DOI] [PubMed] [Google Scholar]
- 27. Davidson KC, Jamshidi P, Daly R, Hearn MTW, Pera MF, Dottori M (2007) Wnt3a regulates survival, expansion, and maintenance of neural progenitors derived from human embryonic stem cells. Mol. Cell. Neurosci. 36, 408–415. [DOI] [PubMed] [Google Scholar]
- 28. Jiang J, Au M, Eshpeter A, Korbutt G, Fisk G, Majumdar AS (2007) Generation of insulin‐producing islet‐like clusters from human embryonic stem cells. Stem Cells 25, 1940–1953. [DOI] [PubMed] [Google Scholar]
- 29. Sonntag KC, Pruszak J, Yoshizaki T, Van Arensbergen J, Sanchez‐Pernaute R, Isacson O (2007) Enhanced yield of neuroepithelial precursors and midbrain‐like dopaminergic neurons from human embryonic stem cells using the bone morphogenic protein antagonist noggin. Stem Cells 25, 411–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Vandermoere F, El Yazidi‐Belkoura I, Adriaenssens E, Lemoine J, Hondermarck H (2005) The antiapoptotic effect of fibroblast growth factor‐2 is mediated through nuclear factor‐κB activation induced via interaction between Akt and IκB kinase‐β in breast cancer cells. Oncogene 24, 5482–5491. [DOI] [PubMed] [Google Scholar]
- 31. Pardo O, Wellbrock C, Khanzada UK, Aubert M, Arozarena I, Davidson S, Bowen F, Parker PJ, Filonenko VV, Gout IT, Sebire N, Marais R, Downward J, Seckl MJ (2006) FGF‐2 protects small cell lung cancer cells from apoptosis through a complex involving PKCɛ, B‐Raf and S6K2. EMBO J. 25, 3078–3088. [DOI] [PMC free article] [PubMed] [Google Scholar]


