Abstract
Aims
Several lines of evidence support a functional interaction between the cardiovascular and pain regulatory systems. Elevated resting blood pressure is consistently associated with reduced responsiveness to experimental pain stimuli in healthy normotensive subjects. This prospective observational study was designed to evaluate the relationship between preoperative resting arterial blood pressure and postoperative pain in patients undergoing non-surgical endodontic treatment (also known as root canal therapy).
Methods
Written informed consent was obtained from normotensive patients seeking treatment for teeth with a preoperative diagnosis of pulpal necrosis and periradicular periodontitis. Preoperative resting blood pressure was recorded and non-surgical root canal therapy was initiated using a standardized protocol. Patients recorded their preoperative and postoperative pain intensity on a 100mm visual analog scale for up to 7 days after the procedure. A linear regression model was used to predict postoperative VAS intensity using preoperative pain and blood pressure values as covariates. Pearson correlations were calculated to assess the relationship between the measures of preoperative blood pressure and both preoperative and postoperative pain.
Results
After controlling for preoperative pain, significant correlations were observed between preoperative systolic blood pressure and postoperative pain (p < .05) as well as between preoperative pulse pressure and postoperative pain (p< .005) on day 1.
Conclusion
This study provides further evidence of a functional interaction existing between the cardiovascular and trigeminal pain regulatory systems. Understanding this complex relationship may lead to enhanced pain management strategies.
Keywords: Pain, Cardiovascular system, Orofacial, Endodontics, Blood pressure
Introduction
In vivo studies report an association between high resting blood pressure and a reduced sensitivity to acute pain.1-4 This relationship between blood pressure and pain is consistent not only when comparing hypertensive individuals (>140/90mm Hg) to normotensive individuals 5-8 but also on comparing higher versus lower blood pressure within the normotensive range.9-11 Correlation analysis reveals that the relationship between blood pressure and pain is linear across the normotensive blood pressure range.10,11 Interventions which raise blood pressure, such as exercise and high sodium diets, reduce sensitivity to experimental pain stimuli.12,13 Furthermore, even in the absence of hypertension, the familial risk for hypertension is associated with a diminished responsiveness to acute pain suggesting genetic contributions to the observed relationship.14,15
The functional interaction between blood pressure and pain has been replicated in studies on odontogenic pain as well. For example, studies evaluating pulpal response to electrical stimulation show that both pain threshold and pain tolerance are higher in hypertensive patients than in normotensive individuals.5,16 In a randomized controlled clinical trial untreated hypertensive patients were assigned to receive enalapril (an angiotensin converting enzyme-inhibitor) or losartan (an angiotensin receptor II antagonist) and their response to electrical pulpal stimulation was measured prior to and after the drug treatment. Administration of both of these antihypertensive drugs resulted in a significant reduction in pain threshold and tolerance.17
Most studies evaluating hypertension associated hypoalgesia in humans have used experimental stimuli to elicit pain.18, 10,11 Relatively fewer studies have evaluated the relationship between the cardiovascular system and clinical pain conditions.19-22 These studies report that chronic pain conditions such as temporomandibular disorders and chronic musculoskeletal complaints are less prevalent in individuals with higher resting blood pressure values as compared to those with low blood pressure values.19-21 Individuals with high resting systolic and diastolic blood pressure are ten times less likely to develop temporomandibular disorders as compared to those with low blood pressure.22 To date, no study has evaluated the relationship between resting arterial blood pressure and acute postoperative orofacial pain. A prospective observational study was conducted to evaluate the relationship between resting arterial blood pressure and postoperative pain in patients undergoing non-surgical root canal therapy We hypothesized that elevated preoperative resting arterial blood pressure readings are associated with diminished postoperative pain experiences.
Materials and Methods
This prospective observational study was conducted in accordance with the Declaration of Helsinki and was approved by the Institutional Review Board at the University of North Carolina, Chapel Hill. Patients were recruited from the endodontic clinic in the School of Dentistry, University of North Carolina, Chapel Hill. Inclusion criteria were (1) teeth with a clinical diagnosis of pulpal necrosis as shown by a negative response to both cold (dichlorodifluoromethane Endo Ice; Hygienic®, Akron, OH) and to electric sensibility testing (Sybron Endo Vitality Scanner Model 2006, Glendale, CA), (2) teeth with a periradicular diagnosis of apical periodontitis as shown by the presence of a periradicular radiolucency more than twice the width of the periodontal ligament. Exclusion criteria included patients with an American Society of Anesthesiologists' physical status of 3 or greater, a periodontal pocket site of 6 mm or deeper around the tooth to be treated, the presence of an abscess or swelling around the affected tooth, persistent (>7 days) use of medication that might alter their pain experience (such as steroids and antidepressants) and any use of analgesics up to 6 hours prior to clinical assessment.
After explaining the study and obtaining written informed consent, patients were asked to rate their spontaneous pain related to the affected tooth on a 100mm horizontal visual analog scale (VAS) with the anchors of “No pain” and “Worst pain imaginable”. They reported how long they had had their tooth pain and as well as mechanical allodynia, which was described as the presence or absence of pain on to gentle digital percussion. Patients were asked about the presence of facial or body pain conditions. Facial pain was described as pain in the face, jaw, temple, in front of the ear, or in the ear, not including toothache or ear infection. Patients who reported having facial pain were asked to indicate the mean pain intensity on a 100mm VAS. Bodily pain was described as pains in the body that lasted a day or more, or pain in the body that has occurred several times a year due to any cause. Patients were instructed not to report aches and pains that were fleeting or attributed to known factors such as sore muscles after exercise. Those with bodily pain were asked to indicate the affected area by drawing on a manikin and to report the pain intensity on a 100mm VAS.
After completion of the preoperative questionnaires, the patients' resting arterial blood pressure and heart rate were measured once using a wrist cuff blood pressure monitor. These measurements were taken a minimum of 15 minutes after seating the patient in an upright position.. The monitors used included OMRON Models HEM 605 and HEM 741 (OMRON Vernon Hills, Illinois). Endodontic therapy was initiated using a standardized protocol. Briefly, this protocol consisted of first administering local anesthesia and then isolating the tooth with a rubber dam. The root canal system was then accessed and the canals instrumented using nickel-titanium rotary and stainless steel hand instruments to International Organization for Standardization apical size ≥35 with 0.04 taper. Calcium hydroxide (Henry Schein, Melville, NY, USA) was placed as an intracanal medicament and a temporary restoration (IRM®, Dentsply International Inc. Milford, DE, USA) was placed.
Prior to dismissal patients were given a postoperative pain diary in which they recorded their average daily pain as well as the most intense daily pain experienced on a 100mm VAS. They also recorded the presence or absence of tooth pain when they gently tapped on their tooth with a finger. The name, dose and frequency of any analgesics taken were also recorded in the pain diary. Patients were instructed to complete the questionnaire at a consistent time daily for 1 week and were given an addressed, stamped envelope in which to mail the pain diary back to the investigators after completion.
Data from questionnaires and pain diaries were merged to produce an analytic data file in which each person was represented as one unit record. A linear regression model was used to predict postoperative VAS intensity using preoperative pain and blood pressure values as covariates. Pearson correlations were calculated to assess the relationship between the measures of preoperative blood pressure and both postoperative and preoperative pain. T-tests were used to test the null hypothesis of no association between pre-existing facial pain/bodily pain and the intensity/duration of postoperative pain. A significance level of p<.05 was used. All data analysis was done using R version 2.13 (Vienna, Austria). Values are reported as mean ± standard error of the mean.
Results
A total of 93 patients were enrolled in the study. Of that number, 20 patients did not return their postoperative pain diaries and their data was not included in the statistical analysis. The mean age of patients was 46 ±1.8 years. Just over one third of the study population was male (36%). The majority of the population described themselves as Caucasian (63%). There were over twice as many maxillary teeth (51) treated during the study as mandibular teeth (22).
Fifty-two percent of patients had pain prior to initiation of treatment. All of these patients reported that they had both spontaneous tooth pain and mechanical allodynia. The mean intensity of preoperative pain was reported as 27 ± 4.0 mm on the VAS. Seventy-five percent of patients had pain on postoperative day one with the mean pain intensity reported as 24±3.1 mm on the VAS. Mechanical allodynia was reported by 47% of patients on postoperative day 1. Both the number of patients who reported pain and the mean pain intensity decreased steadily during the postoperative period. Forty-three percent of patients continued to have pain on postoperative day 7 with a mean pain intensity of 3 ± 0.97 mm on the VAS. Sixteen percent of patients continued to have mechanical allodynia on postoperative day 7.
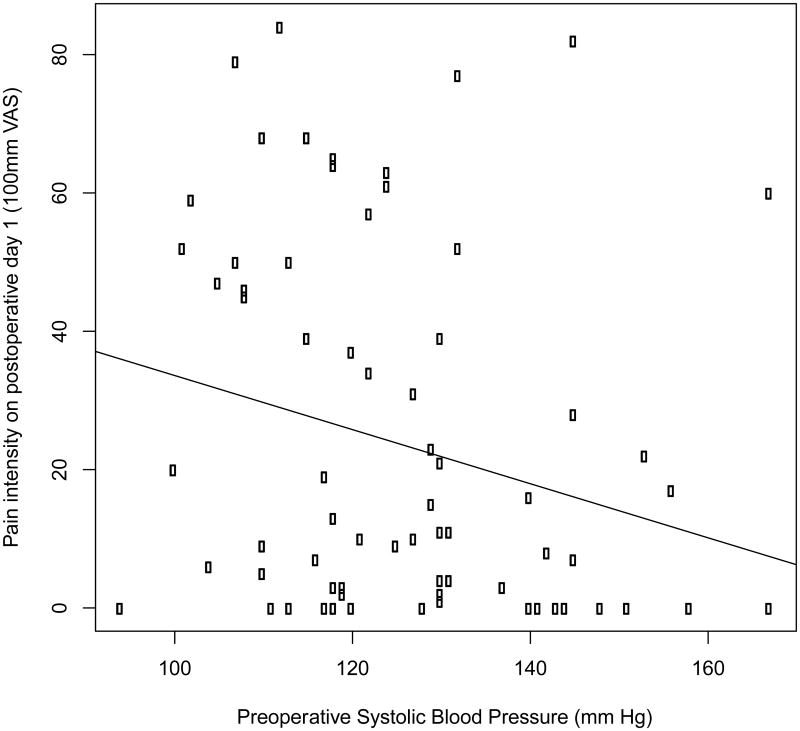
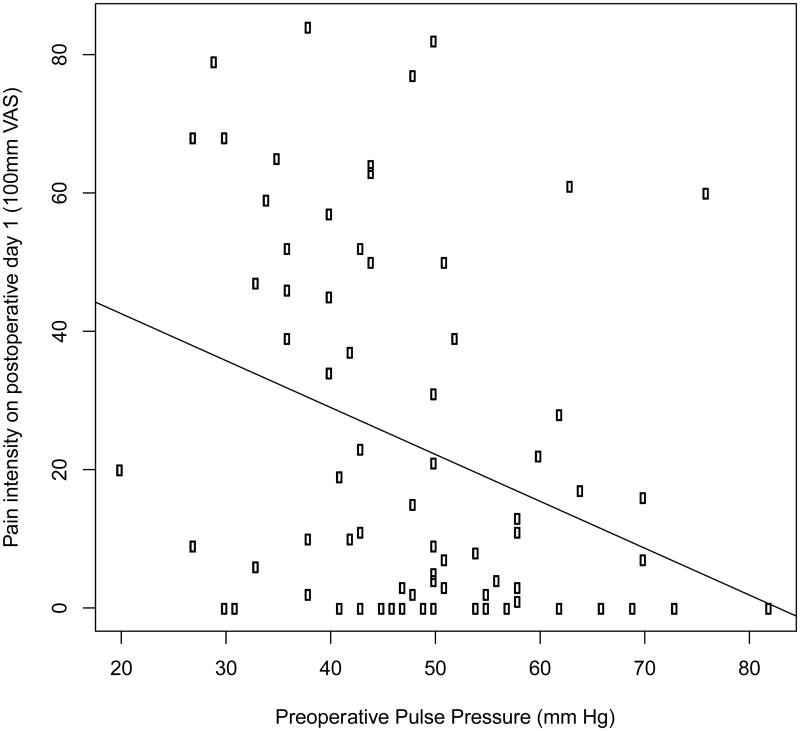
The preoperative systolic blood pressure was 126±1.8 mm of mercury and the preoperative diastolic blood pressure was 78±1.1 mm of mercury. The preoperative pulse pressure was 48±1.5 mm of mercury and the heart rate was 72±1.1 beats per minute. Table 1 shows the preoperative blood pressure variables. A negative correlation was noted between preoperative systolic blood pressure and pain on the first postoperative day (r=-0.24, p=.03) (Figure 1). Additionally we found a negative correlation between preoperative pulse pressure and pain intensity on the first postoperative day (r=-0.32, p =.005) (Figure 2). No significant correlation was observed between diastolic blood pressure (r=0.03; p=.9) or heart rate (r=-0.06; p=.8) and pain on the first postoperative day. Also, no significant correlations were observed between preoperative pain and any of the cardiovascular measures (|r|<0.1 and p>.5 for all four measures).
Table 1.
Preoperative blood pressure variables in patients undergoing endodontic therapy.
| Minimum | Maximum | Mean±SE | |
|---|---|---|---|
| Systolic blood pressue (mm Hg) | 94 | 164 | 126±1.8 |
| Diastolic blood pressure (mm Hg) | 60 | 95 | 78±1.1 |
| Heart Rate (beats/minute) | 47 | 103 | 72±1.1 |
| Pulse Pressure (mm Hg) | 20 | 82 | 48±1.5 |
| Mean Arterial Pressure | 73 | 118 | 94±1.2 |
Figure 1.
Scatter plot showing the negative correlation between preoperative systolic blood pressure and pain on the first postoperative day in patients undergoing endodontic therapy. Patients (N=73) rated their pain intensity on a 100mm visual analog scale. Linear regression was performed to assess the relationship between preoperative systolic blood pressure and postoperative pain. The regression line is shown on the graph. r=-0.24, p=.03
Figure 2.
Scatter plot showing the negative correlation between preoperative pulse pressure and pain on the first postoperative day in patients undergoing endodontic therapy. Patients (N=73) rated their pain intensity on a 100mm visual analog scale. Linear regression was performed to assess the relationship between preoperative pulse pressure and postoperative pain. The regression line is shown on the graph. r=-0.32, p=.005
Thirty-five percent of patients reported having facial pain, which was described as pain in the face, jaw, temple, in front of the ear, or in the ear (not including toothache or ear infection). The mean intensity of the facial pain reported was 48±6.6 mm on the VAS. Twenty-five percent of patients reported having persistent or chronic body pain, and the mean intensity of body pain was 48±4.4 mm on the VAS. The presence of facial and /or body pain was not associated with the intensity or duration of postoperative pain after endodontic therapy (p>.05 for all t-tests performed).
Discussion
Endogenous pain regulatory systems composed of both inhibitory and facilitatory pathways modulate our ability to adapt to acute and persistent pain. The immediate response to a noxious stimulus engages the inhibitory pathways allowing us to escape from the source of injury (or noxious stimulus) without experiencing intense pain.23, 24 Once the acute danger has passed, then the facilitatory pathways amplify the pain which helps to avoid additional injury, encourages rest and facilitates healing. Under normal conditions, when pain persists beyond the initial healing period, inhibitory pathways are once again engaged which enables the resumption of the normal activities needed for survival.23
An important component of the endogenous pain regulatory system is the functional interaction between the cardiovascular and pain regulatory systems which results in an association between elevated resting blood pressure and diminished acute pain sensitivity. There is substantial overlap between the brain regions underlying control of the cardiovascular system and those which contribute to antinociception. The mechanisms that underlie the interaction between blood pressure and pain sensitivity include baroreceptor-, endogenous opioid- and noradrenergic- related mechanisms. It has been proposed that (a) pain increases sympathetic arousal resulting in increased blood pressure, (b) this increased blood pressure results in increased baroreceptor stimulation, which in turn (c) activates the inhibitory pathways resulting in reduced pain sensitivity and thus facilitating the return to a normal homeostatic state.25, 26 Several lines of evidence support the role of baroreceptors in the blood pressure/pain sensitivity relationship. Electrical or pharmacological stimulation of baroreceptors afferents induce antinociception.27-29 Surgical denervation of these afferents eliminates the hypoalgesia displayed in hypertensive animals and produces hyperalgesia in normotensive animals.27, 30 It is important to note that baroreceptor stimulation modulates both the sensory and affective components of pain.
Endogenous opioids, an important component of the pain regulatory system, are proposed to mediate the interaction between the cardiovascular and pain regulatory systems. Elevated blood pressure is associated with increased plasma levels of β endorphin in experimental animals as well as in humans.31 Naloxone, an opioid antagonist, reverses the hypoalgesia seen in hypertensive animals.1,32 Some but not allclinical studies report that opioid blockade reverses the relationship between blood pressure and pain sensitivity.11,33-35 This suggests that in humans, the interaction between blood pressure and pain sensitivity is mediated at least in part by opioid mechanisms.
Central noradrenergic pathways are important players in the descending pain inhibitory pathways and regulation of the cardiovascular system. Structures in the central nervous system which are involved in the cardiovascular and pain regulatory relationship, such as the nucleus tractus solitarius, nucleus raphe magnus, periaqueductal gray, rostral ventrolateral medulla and locus coeruleus, are all potential sources of noradrenergic influences on descending pain modulation. 24, 26, 36 Animal studies provide substantial evidence of the role of supraspinal alpha-2-adrenergic descending pathways in mediating the relationship between blood pressure and pain sensitivity. For example, pharmacological blockade of the adrenergic receptors or lesioning of the brainstem adrenergic system reverses or eliminates hypertension associated antinociception.36-38 In humans, higher blood pressure is associated with both elevated pain tolerance and increased blood levels of norepinephrine.37-38 However there is no direct evidence of the α-2-adrenergic system in the blood pressure /pain sensitivity interaction in humans.6
To our knowledge this is the first prospective study demonstrating a functional interaction between the cardiovascular system and postoperative pain sensitivity following endodontic therapy. The frequency of postoperative pain observed in this study is similar to that reported in other studies on endodontic patients.39-43 The factors known to be correlated with postoperative pain include preoperative swelling, tenderness to percussion prior to initiation of treatment, previous chronic pain problems, and the presence of preoperative pain in the tooth.44-46 We did not recruit patients who presented with an abscess or swelling around the affected tooth. While other studies suggest that previous chronic pain conditions are associated with increased postoperative pain, we did not see a significant correlation between the presence of facial or bodily pain and the intensity or duration of postoperative pain in this study. This may due to the fact that the study was underpowered as only twenty-five percent of the subjects in the present study reported chronic or persistent pain. Prior studies have reported that the administration of antihypertensive medications result in decreased pain sensitivity.47 None of the subjects in this study were taking antihypertensive medications.
While most studies evaluating the interaction between the cardiovascular and pain regulatory systems have reported an association between elevated blood pressure and reduced pain sensitivity, fewer studies have reported the opposite48-50. For example fibromyalgia patients have higher resting systolic and diastolic blood pressure as compared to pain free controls48. Hypertension is reported to be risk factor for migraine chronification49,50. It has been proposed that hypertension exacerbates the effects of migraine on the vascular wall and thus enhances the endothelial dysfunction in cerebral vasculature49. Results of a recent systematic review suggest that angiotensin-converting enzyme inhibitors and angiotensin receptor blockers may have a role in migraine prophylaxis50.
This study has several limitations. Preoperative arterial blood pressure was measured only once in this study and was measured using a wrist cuff monitor. An average of at least three readings obtained using an arm cuff monitor would have been a better estimate of resting blood pressure. Comparison of blood pressure values and pain ratings prior to treatment would then have provided valuable information. We did not obtain information about parental history of hypertension and exercise history. Both of these are known to affect the relationship between blood pressure and pain sensitivity13-15 While patients recorded their postoperative pain intensity, they were not asked to record the period of time for which they experienced pain. In the present study mechanical allodynia was defined as pain on gently tapping the tooth with a finger. Quantitative measurement of mechanical allodynia in the pre- and postoperative period would have been a better approach. We did not evaluate the subjects for anxiety and stress, which may have affected elevated blood pressure as well as pain sensitivity51,52. In future studies a consultation appointment will provide an opportunity for measuring blood pressure in a more relaxed environment.
While the results of this study support an interaction between the cardiovascular system and acute postoperative pain, future studies are needed to elucidate the mechanism underlying this interaction. Continuing this line of research will enhance our ability to manage and / or prevent odontogenic pain associated with clinical procedures.
Acknowledgments
This work was supported by the School of Dentistry, University of North Carolina-Chapel Hill.
The authors thank Drs Lauren Kennedy and Stephen Richardson for their help in recruiting subjects.
Footnotes
Note: The results of this study were presented as an oral presentation at the 2011 Annual Session of the American Association of Endodontists held in April 2011 in San Antonio, Texas, USA. An abstract based on this study has been previously published in the Journal of Endodontics (King W, Bair E, Maixner W, Khan AA. J Endod 2011; 37:26).
Contributor Information
James Wayne King, 101 Hearthstone Lane, Chapel Hill, NC 27516.
Eric Bair, 4503 Koury Oral Science Building, UNC, Chapel Hill, NC 27599.
Derek Duggan, 1180 Old Dental Building, UNC, Chapel Hill, NC 27599.
William Maixner, 4503 Koury Oral Science Building, UNC, Chapel Hill, NC 27599.
Asma A. Khan, 1170 Old Dental Building, UNC, Chapel Hill, NC 27599.
References
- 1.Zamir N, Segal M. Hypertension-induced analgesia: Changes in pain sensitivity and experimentally hypertensive rats. Brain Res. 1979;160:170–173. doi: 10.1016/0006-8993(79)90614-0. [DOI] [PubMed] [Google Scholar]
- 2.Zamir N, Simantov T, Segal M. Pain sensitivity and opioid activity in genetically and experimentally hypertensive rats. Brain Res. 1980;184:299–310. doi: 10.1016/0006-8993(80)90800-8. [DOI] [PubMed] [Google Scholar]
- 3.Maixner W, Touw K, Brody M, Gebhart G, Long J. Factors influencing the altered pain perception in the spontaneously hypertensive rat. Brain Res. 1982;237:137–145. doi: 10.1016/0006-8993(82)90562-5. [DOI] [PubMed] [Google Scholar]
- 4.Maixner W, Randich A. Role of the right vagal nerve trunk in antinociception. Brain Research. 1984:374–377. doi: 10.1016/0006-8993(84)91441-0. [DOI] [PubMed] [Google Scholar]
- 5.Zamir N, Shuber E. Altered pain perception in hypertensive humans. Brain Res. 1980;201:471–474. doi: 10.1016/0006-8993(80)91055-0. [DOI] [PubMed] [Google Scholar]
- 6.Rosa C, Ghione S, Panattoni E, Mezzasalma L. Comparison of pain perception in normotensives and borderline hypertensives by means of a tooth pulp stimulation test. J Cardiovasc Pharmacol. 1986;8:S125–S127. doi: 10.1097/00005344-198608005-00026. [DOI] [PubMed] [Google Scholar]
- 7.Ghione S, Rosa C, Mezzasalma L, Panattoni E. Aterial hypertension is associated with hypalgesia in humans. Hypertension. 1988;12:491–497. doi: 10.1161/01.hyp.12.5.491. [DOI] [PubMed] [Google Scholar]
- 8.Sheps D, Bragdon E, Gray T, Ballenger M, Usedom J, Maixner W. Relationship between systemic hypertension and pain perception. Am J Cardiol. 1992;70:3F–5F. doi: 10.1016/0002-9149(92)90181-w. [DOI] [PubMed] [Google Scholar]
- 9.Elbert T, Rockstroh B, Lutzenberger W, Kessler M, Pietrowsky R, Birbaumer N. Baroreceptor stimulation alters pain sensation depending on tonic blood pressure. Psychophysiology. 1988;25:25–29. doi: 10.1111/j.1469-8986.1988.tb00953.x. [DOI] [PubMed] [Google Scholar]
- 10.Bruehl S, Carlson C, McCubbin J. The relationship between pain sensitivity and blood pressure in normotensives. Pain. 1992;48:463–467. doi: 10.1016/0304-3959(92)90099-W. [DOI] [PubMed] [Google Scholar]
- 11.McCubbin JA, Bruehl S. Do endogenous opioids mediate the relationship between blood pressure and pain sensitivity in normotensives? Pain. 1994;57:63–67. doi: 10.1016/0304-3959(94)90108-2. [DOI] [PubMed] [Google Scholar]
- 12.Ditto BE, Edwards MC, Miller S, D'Antono B, Blum S. The effects of sodium loading on blood pressure and pain responses to the cold pressor test. J Psychosom Res. 1993;3:771–780. doi: 10.1016/0022-3999(93)90106-p. [DOI] [PubMed] [Google Scholar]
- 13.Kemppainen P, Pertovaara A, Huopaniemi T, Johansson G, Karonen SL. Modification of dental pain and cutaneous thermal sensitivity by physical exercise in man. Brain Res. 1985;360(1-2):33–40. doi: 10.1016/0006-8993(85)91217-x. [DOI] [PubMed] [Google Scholar]
- 14.al'Absi M, Buchanan TW, Lovallo WR. Pain perception and cardiovascular responses in men with positive parental history for hypertension. Psychophysiology. 1996;33:655–661. doi: 10.1111/j.1469-8986.1996.tb02361.x. [DOI] [PubMed] [Google Scholar]
- 15.France CR, Froese SA, Stewart JC. Altered central nervous system processing of noxious stimuli contributes to decreased nociceptive responding in individuals at risk for hypertension. Pain. 2002;98:101–108. doi: 10.1016/s0304-3959(01)00477-8. [DOI] [PubMed] [Google Scholar]
- 16.Guasti L, Cattaneo R, Rinaldi O, Rossi MG, Bianchi L, Gaudio G, Grandi AM, Gorini G, Venco A. Twenty-four-hour noninvasive blood pressure monitoring and pain perception. Hypertension. 1995;25(6):1301–1305. doi: 10.1161/01.hyp.25.6.1301. [DOI] [PubMed] [Google Scholar]
- 17.Guasti L, Zanotta D, Diolisi A, Garganico D, Simoni C, Gaudio G, Grandi AM, Venco A. Changes in pain perception during treatment with angiotensin converting enzyme-inhibitors and angiotensin II type 1 receptor blockade. J Hypertens. 2002;20(3):485–491. doi: 10.1097/00004872-200203000-00024. [DOI] [PubMed] [Google Scholar]
- 18.Maixner W, Fillingim R, Kincaid S, Sigurdsson A, Harris B. Relationship between pain sensitivity and resting arterial blood pressure in patients with painful temporomandibular disorders. Psychosom Med. 1997;59:503–511. doi: 10.1097/00006842-199709000-00007. [DOI] [PubMed] [Google Scholar]
- 19.Hagen K, Stovner LJ, Vatten L, Holmen J, Zwart JA, Bovim G. Blood pressure and risk of headache: a prospective study of 22 685 adults in Norway. J Neurol Neurosurg Psychiatry. 2002;72(4):463–466. doi: 10.1136/jnnp.72.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hagen K, Zwart JA, Holeman J, Svebak S, Bovim G, Stovner LJ. Does hypertension protect against chronic musculoskeletal complaints? The Nord-Trondelag Health Study. Arch Intern Med. 2005;165:916–922. doi: 10.1001/archinte.165.8.916. [DOI] [PubMed] [Google Scholar]
- 21.Stovner LJ, Hagen K. Hypertension-associated hypalgesia; a clue to the comorbidity of headache and other pain disorders. Acta Neurol Scand. 2009;120(Suppl. 189):46–50. doi: 10.1111/j.1600-0404.2009.01215.x. [DOI] [PubMed] [Google Scholar]
- 22.Diatchenko L, Anderson AD, Slade GD, Fillingim RB, Shabalina SA, Higgins T, et al. Three major haplotypes of the ADRB2 adrenergic receptor define psychological profile, blood pressure, and the risk for development of a common musculoskeletal pain disorder. Am J Med Genet B Neuropsychiatr Genet. 2006;141B(5):449–462. doi: 10.1002/ajmg.b.30324. 141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maixner W. Pain Modulatory Systems. In: Sessle BJ, Lavigne GJ, Lund JP, Dubner BJ, editors. Orofacial Pain. Hanover Park: Quintessence; 2008. pp. 61–68. [Google Scholar]
- 24.Millan MJ. Descending control of pain. Prog Neurobiol. 2002;66:355–474. doi: 10.1016/s0301-0082(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 25.Zamir N, Maixner W. The relationship between cardiovascular and pain regulatory systems. Ann NY Acad Sci. 1986;467:371–384. doi: 10.1111/j.1749-6632.1986.tb14641.x. [DOI] [PubMed] [Google Scholar]
- 26.Ghione S. Hypertension-associated hypalgesia: Evidence in experimental animals and humans, pathophysiological mechanisms, and potential clinical consequences. Hypertension. 1996;28:494–504. doi: 10.1161/01.hyp.28.3.494. [DOI] [PubMed] [Google Scholar]
- 27.Dworkin B, Filewich R, Miller N, Craigmyle N, Pickering T. Baroreceptor activation reduces reactivity to noxious stimulation: Implications in hypertension. Science. 1979;205:1299–1301. doi: 10.1126/science.472749. [DOI] [PubMed] [Google Scholar]
- 28.Takeda M, Tanimoto T, Ojima K, Matsumoto S. Suppressive effects of vagal afferents on the activity of the trigeminal spinal neurons related to the jaw-opening reflex in rats: Involvement of the endogenous opioid system. Brain Res Bull. 1998;47:49–56. doi: 10.1016/s0361-9230(98)00059-8. [DOI] [PubMed] [Google Scholar]
- 29.Bossut DF, Maixner W. Effects of cardiac vagal afferent electrostimulation on the responses of trigeminal and trigeminothalamic neurons to noxious orofacial stimulation. Pain. 1996;65:101–109. doi: 10.1016/0304-3959(95)00166-2. [DOI] [PubMed] [Google Scholar]
- 30.Maixner W, Touw K, Brody M, Gebhart G, Long J. Factors influencing the altered pain perception in the spontaneously hypertensive rat. Brain Res. 1982;237:137–145. doi: 10.1016/0006-8993(82)90562-5. [DOI] [PubMed] [Google Scholar]
- 31.Rosa C, Ghione S, Mezzasalma L, Pellegrini M, Fasolo B, Giaconi S, Gazzetti P, Ferdeghini M. Relationship between pain sensitivity, cardiovascular reactivity to cold pressor test, and indexes of activity of the adrenergic and opioid system. Clin Exp Theory. 1988;A10(Suppl 1):383–390. doi: 10.3109/10641968809075994. [DOI] [PubMed] [Google Scholar]
- 32.Maixner W, Touw K, Brody M, Gebhart G, Long J. Factors influencing the altered pain perception in the spontaneously hypertensive rat. Brain Res. 1982;237:137–145. doi: 10.1016/0006-8993(82)90562-5. [DOI] [PubMed] [Google Scholar]
- 33.Schobel HP, Handwerker HO, Schmieder RE, Huesser K, Dominiak P, Luft FC. Effects of naloxone on hemodynamic and sympathetic nerve responses to pain in normotensives vs. borderline hypertensive men. J Auton Nerv Syst. 1998;69:49–55. doi: 10.1016/s0165-1838(98)00005-8. [DOI] [PubMed] [Google Scholar]
- 34.Frew AK, Drummond PD. Negative affect, pain and sex: The role of endogenous opioids. Pain. 2007;132(Suppl 1):S77–S85. doi: 10.1016/j.pain.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 35.Lewkowski MD, Young SN, Ghosh S, Ditto B. Effects of opioid blockade on the modulation of pain and mood by sweet taste and blood pressure in young adults. Pain. 2008;135:75–81. doi: 10.1016/j.pain.2007.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Randich A, Maixner W. Interactions between cardiovascular and pain regulatory systems. Neurosci and Behav Rev. 1984;8:343–367. doi: 10.1016/0149-7634(84)90057-5. [DOI] [PubMed] [Google Scholar]
- 37.Bruehl S, Burns JW, Chung OY, Magid E, Chont M, Gilliam W, Matsuura J, Somar K, Goodlad JK, Stone K, Cairl H. Hypoalgesia associated with elevated resting blood pressure: evidence for endogenous opioid involvement. J Behav Med. 2010;33(2):168–76. doi: 10.1007/s10865-009-9241-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chobanian AV, Gavras H, Melby JC, Gavras I, Jick H. Relationship of basal plasma norepinephrine to blood pressure, plasma renin activity, mineralocorticoids, and plasma volume in essential hypertension. Trans Assoc Am Physicians. 1978;91:368–80. [PubMed] [Google Scholar]
- 39.Seltzer S, Bender IB, Ehrenreich J. Incidence and duration of pain following endodontic therapy. Relationship to treatment with sulfonamides and to other factors. Oral Surg Oral Med Oral Pathol. 1961;14:74–82. doi: 10.1016/0030-4220(61)90476-5. [DOI] [PubMed] [Google Scholar]
- 40.Fox J, Atkinson J, Dinin A, Greenfield E, Hechtman E, Reeman C, Salkind M, Todaro C. Incidence of pain following one-visit endodontic treatment. Oral Surg Oral Med Oral Pathol. 1970;30(1):123–130. doi: 10.1016/0030-4220(70)90021-6. [DOI] [PubMed] [Google Scholar]
- 41.Mulhern JM, Patterson SS, Newton CW, Ringel AM. Incidence of postoperative pain after one-appointment endodontic treatment of asymptomatic pulpal necross in single- rooted teeth. J Endod. 1982;8(8):370–375. doi: 10.1016/s0099-2399(82)80197-0. [DOI] [PubMed] [Google Scholar]
- 42.Georgopoulou M, Anastassiadis P, Sykaras S. Pain after chemomechanical preparation. Int Endod J. 1986;19(6):309–314. doi: 10.1111/j.1365-2591.1986.tb00495.x. [DOI] [PubMed] [Google Scholar]
- 43.Sathorn C, Parashos P, Messer H. The prevalence of postoperative pain and flare-up in single and multiple-visit endodontic treatment: a systematic review. Int Endod J. 2008;41(2):91–99. doi: 10.1111/j.1365-2591.2007.01316.x. [DOI] [PubMed] [Google Scholar]
- 44.Polycarpou N, Ng YL, Canavan D, Moles DR, Gulabivala K. Prevalence of persistent pain after endodontic treatment and factors affecting its occurrence in cases with complete radiographic healing. Int Endod J. 2005;38(3):169–178. doi: 10.1111/j.1365-2591.2004.00923.x. [DOI] [PubMed] [Google Scholar]
- 45.Glennon JP, Ng YL, Setchell DJ, Gulabivala K. Prevalence of and factors affecting postpreparation pain in patients undergoing two-visit root canal treatment. Int Endod J. 2004;37(1):29–37. doi: 10.1111/j.1365-2591.2004.00748.x. [DOI] [PubMed] [Google Scholar]
- 46.El Mubarak AH, Abu-bakr NH, Ibrahim YE. Postoperative pain in multiple-visit and single-visit root canal treatment. J Endod. 2010;36(1):36–39. doi: 10.1016/j.joen.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Guasti L, Cattaneo R, Rinaldi O, Rossi MG, Bianchi L, Gaudio G, Grandi AM, Gorini G, Venco A. Twenty-four-hour noninvasive blood pressure monitoring and pain perception. Hypertension. 1995;25(6):1301–1305. doi: 10.1161/01.hyp.25.6.1301. [DOI] [PubMed] [Google Scholar]
- 48.Kulshreshtha P, Gupta R, Yadav RK, Bijlani RL, Deepak KK. A comprehensive study of autonomic dysfunction in the fibromyalgia patients. Clin Auton Res. 2011;22(3):117–22. doi: 10.1007/s10286-011-0150-6. [DOI] [PubMed] [Google Scholar]
- 49.Barbanti P, Aurilia C, Egeo G, Fofi L. Hypertension as a risk factor for migraine chronification. Neurol Sci. 2010;31(Suppl 1):S41–3. doi: 10.1007/s10072-010-0269-6. [DOI] [PubMed] [Google Scholar]
- 50.Gales BJ, Bailey EK, Reed AN, Gales MA. Angiotensin-converting enzyme inhibitors and angiotensin receptor blockers for the prevention of migraines. Ann Pharmacother. 2010;44(2):360–6. doi: 10.1345/aph.1M312. [DOI] [PubMed] [Google Scholar]
- 51.Jeter CR, Bush JP, Porter JH. Situational anxiety and blood pressure lability in the physician's office. Clin Exp Hypertens A. 1988;10(1):169–85. doi: 10.3109/10641968809046806. [DOI] [PubMed] [Google Scholar]
- 52.Clark WC, Yang JC, Janal MN. Altered pain and visual sensitivity in humans: the effects of acute and chronic stress. Ann N Y Acad Sci. 1986;467:116–29. doi: 10.1111/j.1749-6632.1986.tb14623.x. [DOI] [PubMed] [Google Scholar]