Abstract
Background
The detection of a sexually transmitted infection (STI) agent in a urine specimen from a young child is regarded as an indicator of sexual contact. False positives may conceivably arise from the transfer of environmental contaminants in clinic toilet or bathroom facilities into urine specimens.
Methods
The potential for contamination of urine specimens with environmental STI nucleic acid was tested empirically in the male and female toilets or bathrooms at 10 Northern Territory (Australia) clinics, on 7 separate occasions at each. At each of the 140 experiments, environmental contamination with Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis nucleic acid contamination was determined by swabbing 10 locations, and urine collection was simulated 5 times, using a (1) synthetic urine surrogate and (2) a standardized finger contamination procedure.
Results
The most contaminated toilets and bathrooms were in remote Indigenous communities. No contamination was found in the Northern Territory Government Sexual Assault Referral Centre clinics, and intermediate levels of contamination were found in sexual health clinics and in clinics in regional urban centres. The frequency of surrogate urine sample contamination was low but non-zero. For example, 4 of 558 of the urine surrogate specimens from remote clinics were STI positive.
Conclusions
This is by far the largest study addressing the potential environmental contamination of urine samples with STI agents. Positive STI tests arising from environmental contamination of urine specimens cannot be ruled out. The results emphasize that urine specimens from young children taken for STI testing should be obtained by trained staff in clean environments, and duplicate specimens should be obtained if possible.
Keywords: contamination, diagnosis, false positive, sexually transmitted infection, urine
INTRODUCTION
The interpretation of positive sexually transmitted infection (STI) diagnostic test results from young children poses particular challenges. Nucleic acid amplification tests for STIs are very sensitive and do not discriminate between living and dead material. Therefore, false positives are always a concern. Although such false positives will always have potentially serious consequences, they are of particular forensic and medico-legal significance in young children where a positive STI diagnostic test may be regarded as a strong indicator that sexual contact has occurred. Clinical guidelines regarding the interpretation of STI diagnoses in young children are imprecise and do not contain numerical information regarding positive predictive values of such diagnoses to indicate sexual contact. In the United States, guidelines from the Centers for Disease Control state that confirmed diagnoses of gonorrhoea, syphilis, human immunodeficiency virus, or chlamydia in a nonneonatal prepubertal child “suggests sexual abuse”, and that the significance “varies by pathogen” [1]. In the United Kingdom, guidelines state only that “results need to be interpreted based on the limitations of the tests used” [2]. In Australia, guidelines are generated at a state or territory level and, in general, specify that STI diagnosis in a young child is insufficient to conclude that sexual abuse has occurred [3, 4]. In the Northern Territory (NT), a positive STI result in a young child prompts a mandatory notification to NT Government child protection authorities and an investigation.
In the NT, the challenges regarding interpretation of positive STI tests in young children are amplified. Approximately 30% of the NT population are Indigenous Australians, with a large proportion living in remote communities. Sexually transmitted infections are highly prevalent in the NT Indigenous population [5]. In 2006, the NT Government commissioned a report titled “Ampe Akelyernemane Meke Mekarle” (Little Children are Sacred). The authors of this document worked with Indigenous communities to collate evidence of sexual abuse of children and make policy recommendations. The impetus for this report was the frequency of anecdotal evidence for abuse [6]. This document was in large part the catalyst for the socially and politically contentious Australian Commonwealth Government “Northern Territory National Emergency Response” and the subsequent “Stronger Futures in the NT” program.
In this context of heightened community awareness regarding sexual abuse, frontline service providers in the NT may on occasion test children for STIs in the absence of specific disclosure of abuse. In this scenario, a positive test may be virtually the only evidence that sexual contact has occurred, and the pretest probability of sexual contact is potentially low. Accordingly, the postpositive test probability of sexual contact may be very sensitive to the frequency of STI positive tests in the absence of sexual contact.
Two broad categories of postulated mechanisms for false-positive STI diagnoses are (1) those that result from the direct transfer of STI agent environmental contaminants into diagnostic samples and (2) those that result from infection or contamination of the urogenital tract in the absence of sexual contact. These categories are amenable to fundamentally different interventions for reducing the impact of false positives. In this study, we address the first category.
The results of recent studies have revealed environmental contamination with nucleic acid from STI agents in clinical settings [7-9]. These studies incorporated experiments that were designed to indicate the potential for contaminants to be transferred to diagnostic specimens. However, the experiments were small scale or made use of artificially contaminated surfaces.
Here, we report a multisite study (1) to determine the extent of STI nucleic acid contamination of toilets and bathrooms in different types of clinical facilities throughout the NT and (2) to directly test the potential of contaminating material to be transferred to sterile urine specimens from the environment by means of finger contact.
METHODS
Sampling Sites, Locations, and Schedule
Ten clinics located in the NT were included in the study: the 2 Sexual Assault Referral Centre (SARC) clinical facilities in Darwin and Alice Springs, 2 sexual health clinics in Darwin and Alice Springs, 2 primary health clinics in other regional urban centres, and 4 primary health clinics in 4 widely separated remote Indigenous communities. The remote community locations included 2 in Arnhem Land in the far north of the NT and 2 in the south of the NT in central Australia. SARC is an NT Government medical and forensic specialist service responsible for the management of child and adult victims of sexual assault.
At each clinic visit, sampling was performed in 1 male and 1 female toilet or bathroom regularly used by clients to supply urine specimens for diagnostic purposes. Each of the 10 clinics was visited for sampling 7 times, at intervals of 1–2 months. The clinical and managerial staff at each clinic were made aware of impending visits by the investigators, but they agreed not to alter their normal toilet and bathroom cleaning procedures.
Assessment of Surface Contamination
At each clinic visit, 10 surfaces within each of the male and female toilet or bathrooms were sampled by swabbing. These surfaces were as follows: door handle and lock on the side of the door facing into the room or cubicle being assessed; the inner surface of the door to the room or cubicle being tested; the left and right walls of the room or cubicle; the floor close to toilet pedestal; toilet flush buttons; toilet cistern or wall behind toilet; upper surface of toilet seat; toilet bowl and rim; shelf or wash basin edge closest to toilet; and wash basin taps. The surfaces were sampled by swabbing a ∼20 × 20 cm area where possible. For small areas such as taps, the entire surface was swabbed. Either sterile cotton swabs wetted with sterile water or APTIMA Vaginal Swab Specimen Collection kits (Gen-Probe) were used, depending on the analysis procedure (see below).
Simulation of Contamination Procedure of Sterile Urine Surrogate
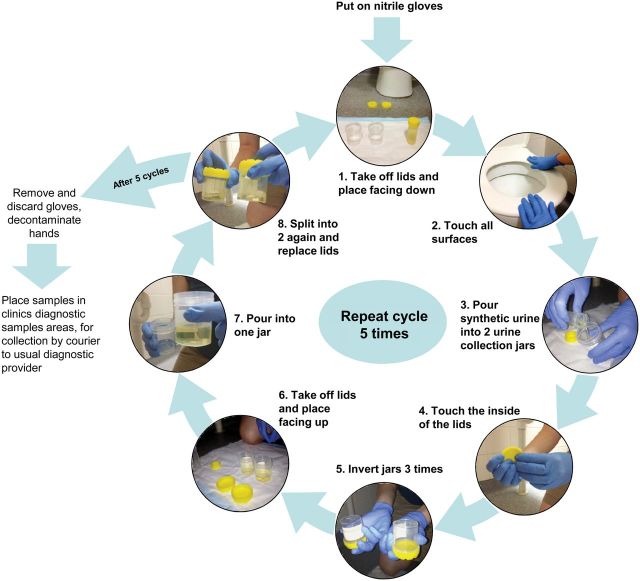
We endeavoured to replicate collection of urine samples by a patient or guardian. This procedure was performed in the same toilet or bathrooms as the swab sampling. The essential elements of the procedure were contact between the experimenter's gloved hands and all the surfaces that were subjected to swab sampling, and also with the insides of lids of urine collection jars, followed by transfer of 20 mL of a urine surrogate solution from a test tube into 2 urine collection jars (approximately 10 mL into each). To maximize the probability of contamination, all 10 surfaces were touched by the experimenter before each transfer of 20 mL of urine surrogate, and the transfer was performed 5 times at each visit to toilet or bathroom, without changing gloves. In this way, each instance of touching of all the clinic and bathroom surfaces resulted in two 10 mL urine surrogate samples. The two 10 mL aliquots arising from each transfer event were combined and then split into two 10 mL aliquots, because it was posited that these would yield duplicate samples that would assist subsequent statistical analysis. The detailed procedure is shown in Figure 1. The urine surrogate used was described by Martino et al [10] and contained the following: 0.65 g/L CaCl2; 0.65 g/L MgCl2; 4.6 g/L NaCl; 2.3 g/L Na2SO4; 0.65 g/L Na3-citrate; 0.02 g/L Na2-oxalate; 2.8 g/L KH2PO4; 1.6 g/L KCl; 1.0 g/L NH4Cl; 25.0 g/L urea; 1.1 g/L creatinine; and 5% Luria Bertani broth (v/v). The pH was adjusted to 5.8 with HCl, and the solution was sterilized by filtration through a 0.22 µm filter. The urine surrogate was stored at 4°C and used within 1 month.
Figure 1.
The method for simulation of collection of urine specimens using a urine surrogate solution.
Because gloves were not changed between the 5 replicates of the urine collection simulation performed in each analysis of a toilet or bathroom, it is reasonable to assume that if a glove becomes sufficiently contaminated to transfer material to a urine surrogate specimen, then there is an increased probability that subsequent specimens arising from that set of 5 transfers may be contaminated. To circumvent this potential confounder, all results from urine surrogate samples from a particular visit to toilet or bathroom that were generated subsequent to a sample that gave a positive result were omitted from analysis (eg, if a positive result was obtained from at least 1 of the duplicate samples from the 2nd of 5 replicate urine surrogate transfer, then the results arising from the 3rd, 4th, and 5th transfers would be discarded).
Analysis of Samples
Samples from each clinic were transported to, and analyzed for Chlamydia trachomatis, Neisseria gonorrhoeae, and Trichomonas vaginalis nucleic acid by, the diagnostic service provider used for STI diagnosis services by that clinic. At the time of the study, the SARC and sexual health clinics used Royal Darwin Hospital Pathology, and the regional and remote clinics used Western Diagnostics for STI diagnostic services. Both of these organizations are accredited by the Australian National Association of Testing Authorities. Royal Darwin Hospital Pathology used the Versant CT/GC DNA 1 0 Assay (kPCR) (Siemens), and Western Diagnostics used the APTIMA Combo 2 Assay system (Gen-Probe). The reports were provided in the same format and with the same information as is routinely provided to the clinics for genuine diagnostic samples.
Role of the Funding Source
The study sponsors had no role in the study design, the collection, analysis or interpretation of data, the preparation of the manuscript, or the decision to submit the manuscript for publication.
RESULTS
Each of the 10 sites was visited 7 times, and a total of 1400 swab samples and 1400 urine surrogate samples were collected. All of the swab samples and 1398 of the urine surrogate samples were analyzed for C trachomatis and N gonorrhoeae. The 2 remaining urine surrogate samples were lost as a result of leakage during transit to the diagnostic service provider. One thousand three hundred and sixty of the swab samples and 1358 of the urine surrogate samples were analyzed for T vaginalis. Forty swab samples and 40 urine surrogate samples were inadvertently not analyzed for T vaginalis. An additional 7 test results were not received from service providers. In total, 4156 results were obtained from swabs and 4151 results were obtained from urine surrogate samples.
The results from the swab samples are shown in Table 1. Sexually transmitted infection agents were detected in all classes of clinics apart from the SARC clinics, which yielded no positive results. The clinics in the remote Indigenous communities were more contaminated than the sexual health and regional clinics. T vaginalis was the most common contaminant in the regional and remote Indigenous communities, whereas N gonorrhoeae was most common in the sexual health clinics.
Table 1.
Surface Contamination of Each Measured STI Agent At Each Clinic in Studya
| SARC |
Sexual Health |
Regional |
Remote Indigenous |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | A | B | C | D | |
| Chlamydia trachomatis | 0 (0 of 139) |
0 (0 of 140) |
0 (0 of 140) |
0.7 (1 of 140) |
5.0 (7 of 140) |
0 (0 of 140) |
3.6 (5 of 140) |
6.4 (9 of 140) |
7.1 (10 of 140) |
9.4 (13 of 139) |
| Neisseria gonorrhoeae | 0 (0 of 140) |
0 (0 of 140) |
6.4 (9 of 140) |
5.0 (7 of 140) |
0 (0 of 140) |
0 (0 of 140) |
0 (0 of 140) |
2.1 (3 of 140) |
25.0 (35 of 140) |
36.7 (51 of 139) |
| Trichomonas vaginalis | 0 (0 of 140) |
0 (0 of 140) |
0.7 (1 of 140) |
0.7 (1 of 140) |
7.1 (10 of 140) |
14.3 (20 of 140) |
26.1 (31 of 119) |
44.2 (53 of 120) |
37.9 (53 of 140) |
32.1 (45 of 140) |
| Positive swabs (any STI agent) | 0 (0 of 140) |
0 (0 of 140) |
7.1 (10 of 140) |
6.4 (9 of 140) |
10 (14 of 140) |
14.3 (20 of 140) |
24.3 (34 of 140) |
40.7 (57 of 140) |
50.7 (71 of 140) |
57.1 (80 of 140) |
Abbreviations: SARC, Sexual Assault Referral Centre; STI, sexually transmitted infection.
aClinics are divided into geographical categories. Data are expressed as a percentage. The fraction of the number of positive tests over total number of tests is stated in parentheses.
The frequencies of contamination on the different surfaces are shown in Table 2. Contamination was found on all classes of surface. A comparison of the contamination of female and male toilets and bathrooms is shown in Table 3. The pooled results from the STI agents were not significantly different. However, the female toilet or bathrooms were more heavily contaminated with C trachomatis and T vaginalis, whereas the male toilet or bathrooms were more heavily contaminated with N gonorrhoeae.
Table 2.
Frequencies, Expressed As Percentages, of Contamination of Different Locations Within the Toilet or Bathrooms Included in the Study
| DHL | CD | LRW | TF | TFB | TC | TS | TB | WBE | WBT | |
|---|---|---|---|---|---|---|---|---|---|---|
| Chlamydia trachomatis | 1.4 (2 of 140) |
1.4 (2 of 140) |
0.7 (1 of 140) |
10.8 (15 of 139) |
0.7 (1 of 140) |
0 (0 of 140) |
5.0 (7 of 139) |
7.9 (11 of 140) |
2.9 (4 of 140) |
1.4 (2 of 140) |
| Neisseria gonorrhoeae | 7.9 (11 of 140) |
4.3 (6 of 140) |
11.4 (16 of 140) |
17.1 (24 of 140) |
7.9 (11 of 140) |
3.6 (5 of 140) |
7.2 (10 of 139) |
7.1 (10 of 140) |
7.1 (10 of 140) |
1.4 (2 of 140) |
| Trichomonas vaginalis | 11.0 (15 of 136) |
5.1 (7 of 136) |
15.4 (21 of 136) |
27.9 (38 of 136) |
14.0 (19 of 136) |
5.9 (8 of 136) |
25.0 (34 of 136) |
27.9 (38 of 136) |
14.8 (20/135) |
103 (14 of 136) |
Abbreviations: CD, cubicle door; DHL, door handle and lock; LRW, left and right walls; TB, toilet bowl; TC, toilet cistern; TF, toilet floor;
TFB, toilet flush button; TS, toilet seat; WBE, wash basin edge; WBT, wash basin taps.
Table 3.
Frequencies, Expressed As Percentages, of Surface Contamination in Female and Male Bathroom and Toilets
| Female | Male | P Valuea | |
|---|---|---|---|
| Chlamydia trachomatis | 4.6 (32 of 699) | 1.9 (13 of 699) | .004 |
| Neisseria gonorrhoeae | 3.6 (25 of 700) | 11.4 (80 of 699) | <.0001 |
| Trichomonas vaginalis | 20.9 (142 of 680) | 10.6 (72 of 679) | <.0001 |
aPearson's χ2 test.
The results of the analyses of the urine surrogate samples are shown in Table 4. The frequency of urine surrogate contamination was much less than the frequency of swab contamination. Consistent with the swab results, no contaminated urine surrogate samples were obtained from the SARC clinics. Additional details regarding the contaminated urine surrogate samples are provided in Table S1. It is noteworthy that our attempt to generate duplicate samples by splitting then recombining the 20 mL samples or surrogate urine was unsuccessful, with instances where 1 of each pair of samples was positive (Table S1). As a result, each 10 mL sample was regarded as independent in subsequent analyses. The pooled results in the lowermost row of Table 4 were provided because a critical parameter is the probability of a sample being contaminated with any of the STIs for which it is tested, and it is usual practice to test urine specimens for more than 1 STI.
Table 4.
Numbers and Frequencies, Expressed As Percentages of Urine Surrogate Contaminationa
| SARC |
Sexual Health |
Regional |
Remote Indigenous |
Total (95% CI) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| A | B | A | B | A | B | A | B | C | D | ||
| Chlamydia trachomatis | 0 (0 of 140) |
0 (0 of 138) |
0 (0 of 139) |
0 (0 of 140) |
0.7 (1 of 137) |
0 (0 of 139) |
0 (0 of 140) |
0.7 (1 of 131) |
0 (0 of 140) |
0 (0 of 140) |
0.14 (0.02–0.57) (2 of 1384) |
| Neisseria gonorrhoeae | 0 (0 of 140) |
0 (0 of 139) |
1.4 (2 of 131) |
0 (0 of 140) |
0 (0 of 140) |
0 (0 of 139) |
0 (0 of 140) |
0 (0 of 140) |
0 (0 of 140) |
0 (0 of 140) |
0.14 (0.02–0.57) (2 of 1389) |
| Trichomonas vaginalis | 0 (0 of 140) |
0 (0 of 139) |
0 (0 of 139) |
0 (0 of 140) |
0 (0 of 140) |
0 (0 of 139) |
0 (0 of 120) |
2.1 (3 of 101) |
0 (0 of 140) |
0 (0 of 140) |
0.22 (0.06–0.71) (3 of 1338) |
| Positive for any STI agent (95% CI) | 0 (0–1.3) (0 of 279) |
0.72 (0.1–2.6) (2 of 279) |
0.36 (0–2.0) (1 of 279) |
0.72 (0.2–1.8) (4 of 558) |
|||||||
Abbreviations: CI, confidence interval; SARC, Sexual Assault Referral Centre; STI, sexually transmitted infection.
a The 95% CI figures were calculated using the Exact Binomial method.
The frequencies obtained are our best estimate but are subject to sampling error. Even though the samples are not entirely independent, we contend that confidence intervals (CIs) calculated assuming independence will provide useful guidance regarding extremely conservative limits for the frequencies of false positives by this mechanism. Upper boundaries for the 95% CIs for the proportions of urine surrogate samples that caused positive tests, calculated using the Exact Method, are 2.6%, 2.0%, and 1.8% for the sexual health, regional, and remote clinics, respectively. The remote clinic figure (2.0%) is probably the most useful because it is derived from clinics with the highest proportion of contaminated urine samples and the largest number of samples. Another useful indicator may be the upper boundary of the frequencies of C trachomatis + N gonorrhoeae positive urine surrogate samples, if T vaginalis is regarded as a less strong indicator of sexual contact than C trachomatis or N gonorrhoeae. The upper boundary of the 95% CI for the proportion of remote clinic urine surrogate samples that are positive for either of these 2 agents (1 of 558) is 1.2%.
DISCUSSION
To our knowledge, this is the largest study to address contamination of surfaces within clinic bathroom and toilet facilities with nucleic acid from STI agents, and it is the only study to encompass multiple visits to multiple clinics. Overall, the degree of environmental contamination in our study is slightly lower than that found in other studies. Meader et al [9] detected C trachomatis from 41 of 104 (39%) swabs (range, 0%–88%) from surfaces such as toilets, curtains, and draining boards within an English genitourinary clinic. Lewis et al [8] sampled similar surfaces at a similar clinic and found that 20 of 154 (13%) of surfaces tested positive for either C trachomatis or N gonorrhoeae. In our study, the highest proportion of C trachomatis-positive swabs was 9.4%, which is much lower than the proportions we sometimes saw for N gonorrhoeae and T vaginalis. The lower contamination rates with C trachomatis in the NT remote clinics compared with sexual health clinics in England may reflect that these remote clinics are general healthcare and not sexual health clinics. However, rates of contamination in NT sexual health clinics were substantially lower still. Whereas the proportion of surface contamination by the individual STI agents is largely consistent with published prevalence of the STI agents in the NT [5], the rate of surface contamination with C trachomatis was lower than expected, possibly due to differences in shedding and environmental stability of the different STI agents. The STI environmental survival and nucleic acid environmental persistence may be an interesting area of study in the hot and often humid NT. Gender distributions of the STI agents were consistent with the higher prevalence of trichomoniasis in women than in men in the NT [5], but there is little difference between the prevalence of C trachomatis and N gonorrhoeae in the NT (5). Although the high frequency of contamination of the floor and the toilet bowl was unsurprising, it is noteworthy that surfaces such as the door handle, toilet flush button, and wash basin taps, which are in elevated positions and frequently touched by hands, also showed appreciable contamination.
This study is also the only one to include a large-scale assessment of the potential of environmental contaminants to be transferred to urine specimens obtained within a variety of facilities. The procedure was designed to provide a greater probability of contamination than would be expected in reality. By incorporating extensive contact between gloved hands and the built environment and deliberate contact with between gloved hands and the inside of the urine jar lids with every tested specimen, we have mimicked what may be regarded as a worst-case scenario for specimen contamination. The rationale was that the results would provide a conservative (ie, high) upper boundary for the probability of sample contamination. This result, in combination with the use of the Exact Method for calculating 95% CI for the proportions, has yielded upper boundaries that are conservative in the extreme.
We feel justified in stating that the probability that a urine sample from the 4 remote clinics in this study will be contaminated with C trachomatis, N gonorrhoeae, or T vaginalis of toilet or bathroom environmental origin cannot reasonably exceed 2.0%, and it is probably much less. Likewise, the probability of contamination with C trachomatis or N gonorrhoea cannot reasonably exceed 1.2% and is also probably much less.
If false positives do occur, even at a low rate, this result has considerable implications when the pretest probability of a true positive is low. For example, using the point estimate urine surrogate contamination rate of 0.7% for the remote clinics (Table 4), and assuming the combined STI testing has a sensitivity of 1, the positive likelihood ratio for the test is 143. Hypothetically, if the pretest probability of a true STI is 0.01, the posttest probability of a true STI is just 0.59. This hypothesis emphasizes the potential peril of broad community-based testing of children for STIs as a screen for young children who have been subjected to sexual contact. Our study provides no evidence to suggest that broad screening is advisable.
This study may be used to estimate the relationship between the extent of toilet or bathroom contamination, as measured by swab sampling, and the probability that a urine specimen will be contaminated. In previous studies, researchers had attempted such estimations to a limited extent. Meader et al [9] demonstrated that a hand that had touched an artificially heavily contaminated wet surface could potentially transfer C trachomatis nucleic acid to a swab. Lewis et al [8] tested the outer surfaces of the caps of 46 sample containers and found that none were contaminated. Chan et al [7] exposed to the air 60 samples of distilled water and 10 unused swabs while the toilet in a sexual health clinic was flushed, and they found none of the samples to be positive for C trachomatis. It is difficult to argue that these experiments provided useful numerical data. Using data from the remote clinics, the ratio between the proportion of swabs positive and the proportion of urine specimens positive is 0.017 (95% CI, .006–.045). Thus, urine surrogate specimens were ∼60.3 (95% CI, 22–167) times less likely to be positive compared with environmental swabs from the same toilet or bathroom. Continued surveillance using standardized procedures would result in refinement of this relationship. This finding may provide a widely applicable means to extrapolate from surface contamination to a “worst case” urine sample contamination probability.
A limitation of this study is that gloved hands instead of bare skin were used in the simulation of urine specimen collection. This process was done in the interests of the health and safety of the experimenters. However, there are recent reports that gloves are effective at transferring microorganisms, suggesting that our experiment does provide information relevant to the potential of bare skin to transfer environmental contaminants [11, 12]. Another limitation was that different diagnostic service providers using different STI diagnosis systems were used for different clinics. This result has the potential to confound comparison between clinics. However, the desirability of using the diagnostic service suppliers used for genuine samples from the clinics in this study outweighed other considerations. Large differences between clinics using the same providers were observed, making it very unlikely that differences between clinics were a function of the diagnostic service provider.
We can suggest a number of ways to reduce the probability of contamination of urine samples. First, cleaning procedures affect environmental contamination. The SARC clinics are treated daily with bleach to remove environmental nucleic acid, and we found no evidence of environmental or urine surrogate contamination in these clinics. Second, patients and caregivers can be instructed to minimize touching of environmental surfaces when obtaining samples. Gloves could be worn to circumvent the effects of preexisting STI agent contamination on the fingers. Third, if it were particularly important to reduce the risk of the contribution of this mode of contamination to false positives, such as when there was an indication to test a child for STIs, trained clinic staff should obtain the sample and duplicate samples could be taken, with different gloves worn for each collection. This process would also eliminate any consequences of preexisting contamination of a parent's or guardian's fingers.
In conclusion, we have performed a multisite investigation of the potential of environmental contaminants in NT clinic bathroom or toilets to be transferred to urine specimens and deduced very conservative upper boundaries for the probabilities of such events. Although the impetus for this investigation was child protection related, the results are of general relevance to STI diagnosis.
Acknowledgments
We thank Barbara Kelly (Northern Territory Government Sexual Assault Referral Centre [Darwin]), and Michael Leung (Western Diagnostics, Western Australia); and Pathwest, (West Australia Government) for facilitating the early stages of this project. We also thank Nathan Ryder (Northern Territory Government Centre for Disease Control) and Terry Donald (Women's and Children's Hospital, Adelaide, South Australia) for critical review of the manuscript prior to submission. Finally, we thank the management and staff of the participating clinics for their participation and assistance. The authors thank Mark Chatfield (Menzies School of Health Research) for statistics advice.
Financial support. This work was supported by the National Health and Medical Research Council (project grant 1004123; and postdoctoral training fellowship 508829 to S. Y. C. T.); and the Northern Territory (Australia) Research and Innovation Board (Research and Innovation Grant “Urine surrogates in the quality control of Chlamydia diagnosis”).
Potential conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Footnotes
The standard license has been updated to Open Access since the original version.
References
- 1.Centers for Disease Control and Prevention. Sexually Transmitted Disease Treatment Guidelines. Available at: http://www.cdc.gov/std/treatment/2010/sexual-assault.htm . Accessed 5 August 2013. [Google Scholar]
- 2.Rogstad K, Thomas A, Williams O, et al. United Kingdom National Guideline on the Management of Sexually Transmitted Infections and Related Conditions in Children and Young People. doi: 10.1258/ijsa.2009.009353. Available at: http://www.bashh.org/documents/2674.pdf . Accessed 5 August 2013. [DOI] [PubMed] [Google Scholar]
- 3.Guidelines for Managing Sexually Transmitted Infections - WA. Available at: http://silverbook.health.wa.gov.au/Default.asp?PublicationID=1§ionID=42 . Accessed 5 August 2013. [Google Scholar]
- 4.Queensland Government. Sexually Transmissible Infections in Children: Queensland Health Guidelines for Public Health Units. Available at: http://www.health.qld.gov.au/cdcg/index/sti_child.asp . Accessed 5 August 2013. [Google Scholar]
- 5.Northern Territory Government. Sexual Health and Blood Borne Viruses Unit Surveillance Updates. Available at: http://www.health.nt.gov.au/Centre_for_Disease_Control/Publications/Sexual_Health_Surveillance_Updates/index.aspx . Accessed 5 August 2013. [Google Scholar]
- 6.Wild RSL, Anderson P. Darwin: Northern Territory Board of Inquiry into the Protection of Aboriginal Children from Sexual Abuse, Northern Territory Government; 2013. Ampe Akelyernemane Meke Mekarle "Little Children are Sacred". Available at: http://www.inquirysaac.nt.gov.au/pdf/bipacsa_final_report.pdf . Accessed 5 August. [Google Scholar]
- 7.Chan SY, Jose S, King R, et al. How likely is environmental or patient cross-contamination of Chlamydia trachomatis DNA to lead to false positive results in patients attending our clinic? Sex Transm Infect. 2013;89:105–7. doi: 10.1136/sextrans-2012-050667. [DOI] [PubMed] [Google Scholar]
- 8.Lewis N, Dube G, Carter C, et al. Chlamydia and gonorrhoea contamination of clinic surfaces. Sex Transm Infect. 2012;88:418–21. doi: 10.1136/sextrans-2012-050543. [DOI] [PubMed] [Google Scholar]
- 9.Meader E, Waters J, Sillis M. Chlamydia trachomatis RNA in the environment: is there potential for false-positive nucleic acid amplification test results? Sex Transm Infect. 2008;84:107–10. doi: 10.1136/sti.2007.027862. [DOI] [PubMed] [Google Scholar]
- 10.Martino PD, Fursy R, Bret L, et al. Indole can act as an extracellular signal to regulate biofilm formation of Escherichia coli and other indole-producing bacteria. Can J Microbiol. 2003;49:443–9. doi: 10.1139/w03-056. [DOI] [PubMed] [Google Scholar]
- 11.Moore G, Dunnill CW, Wilson AP. The effect of glove material upon the transfer of methicillin-resistant Staphylococcus aureus to and from a gloved hand. Am J Infect Control. 2013;41:19–23. doi: 10.1016/j.ajic.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 12.Morgan DJ, Rogawski E, Thom KA, et al. Transfer of multidrug-resistant bacteria to healthcare workers' gloves and gowns after patient contact increases with environmental contamination. Crit Care Med. 2012;40:1045–51. doi: 10.1097/CCM.0b013e31823bc7c8. [DOI] [PMC free article] [PubMed] [Google Scholar]