Abstract
Catecholamines including dopamine (DA) and norepinephrine (NE) are widely distributed in the body and exert extensive physiological functions by serving as neurotransmitters or neuromodulators. Alterations in the level of these two catecholamines underlie many neurological and psychiatric disorders. The pharmacology of both DA and NE including their individual receptors, signaling mechanisms, agonists and antagonists has been extensively studied. Whereas the traditional idea is that neurotransmitters specifically interact with their receptors, there is compelling evidence indicating that DA and NE exert biological actions by activating the receptors of other family in a variety of regions. Here, I review the experimental evidence showing cross activation of each other’s receptors by these two catecholamines. This promiscuous interaction may represent a novel way for catecholamines to exert their functions.
Keywords: Catecholamine, transmitter, receptor, dopamine, norepinephrine, G protein-coupled receptor
Introduction
Catecholamines including dopamine (DA) and norepinephrine (NE) are classical neurotransmitters or neuromodulators involved in modulation of a variety of physiological functions including working memory, locomotion and reproduction [1,2]. Alterations in DA neurotransmission result in major neurological and psychiatric disorders including Parkinson’s disease,addiction, schizophrenia, bipolar disorder,Huntington’s disease, attention deficit hyperactivity disorder and Tourette’s syndrome [3,4]. In the DA- or NE-containing cells, the initial substrate, tyrosine, is hydroxylized by tyrosine hydroxylase to produce dihydroxyp-henylalanine (DOPA). The formed DOPA is further carboxylized by amino acid decarboxylase to generate DA which serves as a neurotransmitter in the DA-containing neurons. In the NE-containing cells, DA is further hydroxylized by dopamine-β-hydroxylase to synthesize NE which is the neurotransmitter for these neurons.In the adrenal medulla, the unique enzyme, phenylethanolamine-N-methyltransferase further converts NE to epinephrine which serves as a hormone when released. After release, DA is removed from the synaptic cleft by DA transporters whereas NE is either re-uptaken into neurons by NE transporters or metabolized by two enzymes, the monoamine oxidase (MAO) or the catechol-O-methyltransferase(COMT).
DA acts normally by activating 5 DA receptor subtypes: D1, D2, D3, D4, and D5. The proteins of these 5 receptor subtypes display significant homology in their structure and are the products of 5 genes from different chromosomal loci [5]. The genes for the D1 and D5 receptors are located in chromosome 5 and chromosome 4, respectively and the proteins of these two receptors show 80% homology in their transmembrane domains, thereby being named as D1-like receptors. The D1-like receptors are coupled to Gs proteins to stimulate adenylyl cyclase (AC) activity resulting in increased production of cyclic cAMP (cAMP) and elevated activity of protein kinase A (PKA). The D2, D3, and D4 receptors are classified as D2-like receptors and their genes are located in chromosome 11. The D2 and D3 receptors show 75% homology in their transmembrane domain, whereas D2 and D4 exhibit 54% homology [5]. The D2-like receptors are coupled to Gi/o proteins and exert inhibitory function on AC resulting in inhibition of cAMP/PKA pathway [6].
NE usually interacts with α and β adrenergic receptors. There are two major types of α adrenergic receptors: α1 and α2 adrenoceptors. The α1 adrenoceptors can be further divided into α1A, α1B and α1D adrenoceptors, all of which are coupled to Gq/11 proteins leading to inositol phosphate turnover. The α2 adrenoceptors are classified into three different subtypes, α2A, α2B and α2C. These receptors are coupled to Gi/o proteins leading to inhibition of AC-cAMP-PKA pathway. There are three β adrenoceptors: β1, β2 and β3, although each type of the β receptors can be further divided into different subtypes. For example, β3-adrenoceptors can be further classified as β3a, β3b and β3c. The β adrenoceptors are coupled to Gs proteins to elevate AC-cAMP-PKA signals.
Whereas DA and NE normally interact with their individual receptors, there is compelling evidence indicating that they promiscuously interact with each other’s receptors in some situations.Here, I review evidence regarding their promiscuous interactions in the hope that this kind of promiscuous actions would bring the attention of researchers in the field.
Effects of DA on α adrenoceptors
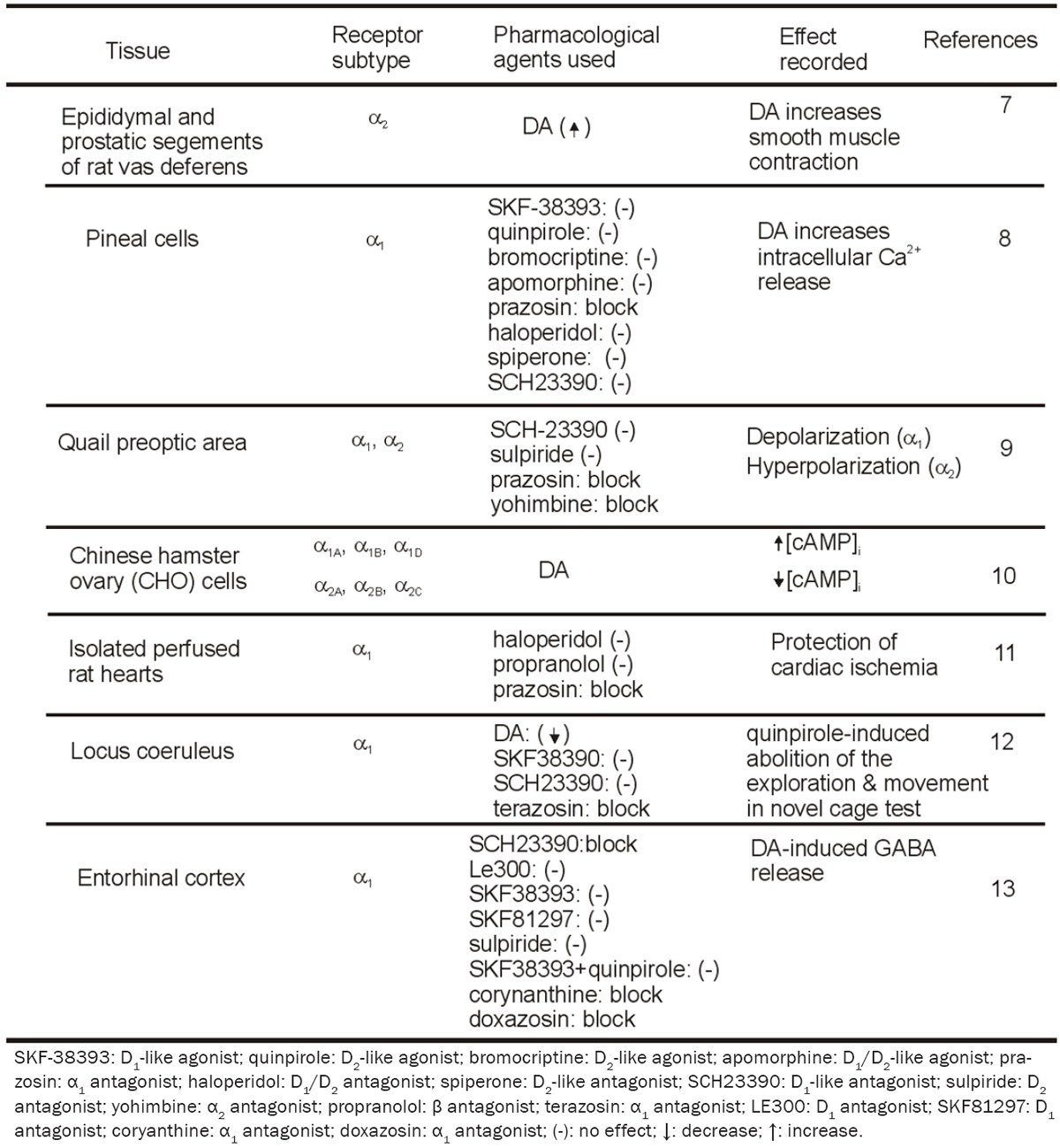
Using epididymal and prostatic segments of the rat vas deferens, Leedham and Pennefather compared the potency of many agonists of catecholamine receptors in modulating smooth muscle contraction and found, initially, that DA may modulate smooth muscle contraction via activation of α adrenergic receptors [7]. In pineal cells, application of DA dose-dependently increases intracellular Ca2+ release [8]. Intracellular Ca2+ is unaltered in response to applications of DA receptor agonists, SKF-38393 (a D1-like agonist), quinpirole (a D2-like agonist), bromocriptine (a D2-like agonist) or apomorphine (a D1/D2-like agonist). The lack of effect of DA receptor agonists on intracellular Ca2+ levels indicates that the Ca2+ responses induced with DA in pineal cells are not mediated through DA receptors. Instead, DA-induced increase in intracellular Ca2+ is blocked by the α1 receptor antagonist, prazosin, but insensitive to haloperidol (D1/D2 antagonist), spiperone (D2-like antagonist) and R(+)-SCH23390 (D1-like antagonist) suggesting that DA-induced increases in intracellular Ca2+ release is mediated by activation of α1 instead of DA receptors. Results from experiments by using different α1 receptor antagonists suggest that DA increases intracellular Ca2+ release by activating α1B receptors.
In the quail preoptic area, DA exerts dual effects, i.e., inhibits the firing of most cells by generating hyperpolarization but depolarizes and excites a few others [9]. The effects of DA are insensitive to D1 or D2 antagonists (SCH-23390 and sulpiride) but blocked by α2 (yohimbine)and α1 (prazosin) adrenoceptor antagonists,suggesting the involvement of adrenergic not dopaminergic receptors. The results that application of two dopamine-β-hydroxylase inhibitors (cysteine and fusaric acid) failed to block the effects of DA indicate that DA is not converted into NE to produce its effects. These results together demonstrate that DA directly interacts with adrenergic receptors to mediate its effects.
DA has also been show to activate adrenergic receptors expressed in Chinese hamster ovary (CHO) cells [10]. By measuring increases in intracellular Ca2+ release in response to the activation of α1A, α1B and α1D, and reduction of cAMP level in response to the activation of α2A, α2B and α2C, these investigators have shown that DA is nearly as efficacious in this system but is from 1 to 4 orders of magnitude less potent in comparison to NE or epinephrine. In CHO cells expressing β1, β2, β3a or β3b, DA concentration-dependently induces an increase in cAMP level although less potent than NE or epinephrine.These results demonstrate that DA is capable of activating adrenergic receptors at least in transfected cells.
The protective effect of DA on cardiac ischemia is also demonstrated to be mediated by activation of α1 adrenoceptors [11]. Pretreatment of isolated perfused rat hearts with DA at the concentration of 5-10 μM significantly improves postischemic functional recovery and reduces infarction size in response to 45 min of sustained ischemia followed by 60 min reperfusion.The protective effects of DA are not attenuated by pretreatment with the mixed D1/D2 dopaminergic receptor antagonist, haloperidol, or the β-adrenoceptor-selective antagonist, propranolol, whereas administration of the selective α1-adrenoceptor antagonist, prazosin, blocks the cardioprotective effect of DA, suggesting that pharmacological preconditioning with DA protects the myocardium against ischemia through activation of α1 instead of DA receptors.
DA-mediated activation of α1 adrenoceptors in the locus coeruleus has also been shown to be involved in the behavioral test, exploratory activity in a novel cage [12]. Application of the D2/D3 agonist, quinpirole, which selectively blocks the release of DA in the brain, dose-dependently or virtually completely abolishes the exploration and all movement in the novel cage test. Co-infusion of DA not the D1 agonist, SKF38390, significantly attenuates the quinpirole-induced inactivity. The DA-induced reduction of quinpirole inactivity is blocked by co-infusion of the α1 adrenergic receptor antagonist,terazosin, but not by the D1 receptor antagonist,SCH23390. Infusion of quinpirole or terazosin into the locus coeruleus of the DA-β-hydroxylase knockout mice that lack NE also induces profound inactivity, indicating that their behavioral effects are not due to an alteration of the release or action of NE in the locus coeruleus.Measurement of endogenous DA, NE, and serotonin and their metabolites in the locus coeruleus during exposure to the novel cage indicates an increase in the turnover of DA and NE but not serotonin. These results together suggest that endogenous DA activate α1 receptors to effect.
In the entorhinal cortex (EC), application of DA concentration-dependently increases GABA release [13]. The DA-induced facilitation of GABA release is sensitive to the selective D1-like receptor antagonist, SCH23390, but insensitive to another D1 antagonist of distinct structure and higher potency, LE300. Application of two selective D1 agonists, SKF38393 and SKF81297, does not increase GABA release. Whereas the result from SCH23390 suggests the involvement of D1-like receptors, the other results do not support the requirement of D1-like receptors. Because SCH23390 is also a blocker for the inwardly rectifier K+ channels [14-17] and the DA-induced increases in GABA release in the EC are mediated by inhibition of the inwardly rectifier K+ channels, these results together indicate that D1-like receptors are not required for DA-mediated facilitation of GABA release. DA-induced increases in GABA release are not blocked by sulpiride, a selective D2-like receptor antagonist and co-application of D1-like and D2-like agonists, SKF38393 and quinpirole, does not mimic the effects of DA. These results together demonstrate that DA-induced increases in GABA release are not mediated by activation of DA receptors. DA-mediated facilitation of GABA release is blocked by application of the selective α1 receptor antagonists, corynanthine or doxazosin, demonstrating that DA enhances GABA release by activation of α1 adrenoceptors.Table 1 is the summary of the evidence demonstrating DA cross-activates α adreno-ceptors.
Table 1.
Cross activation of α adrenoceptors by DA
|
Effects of DA on β adrenoceptors
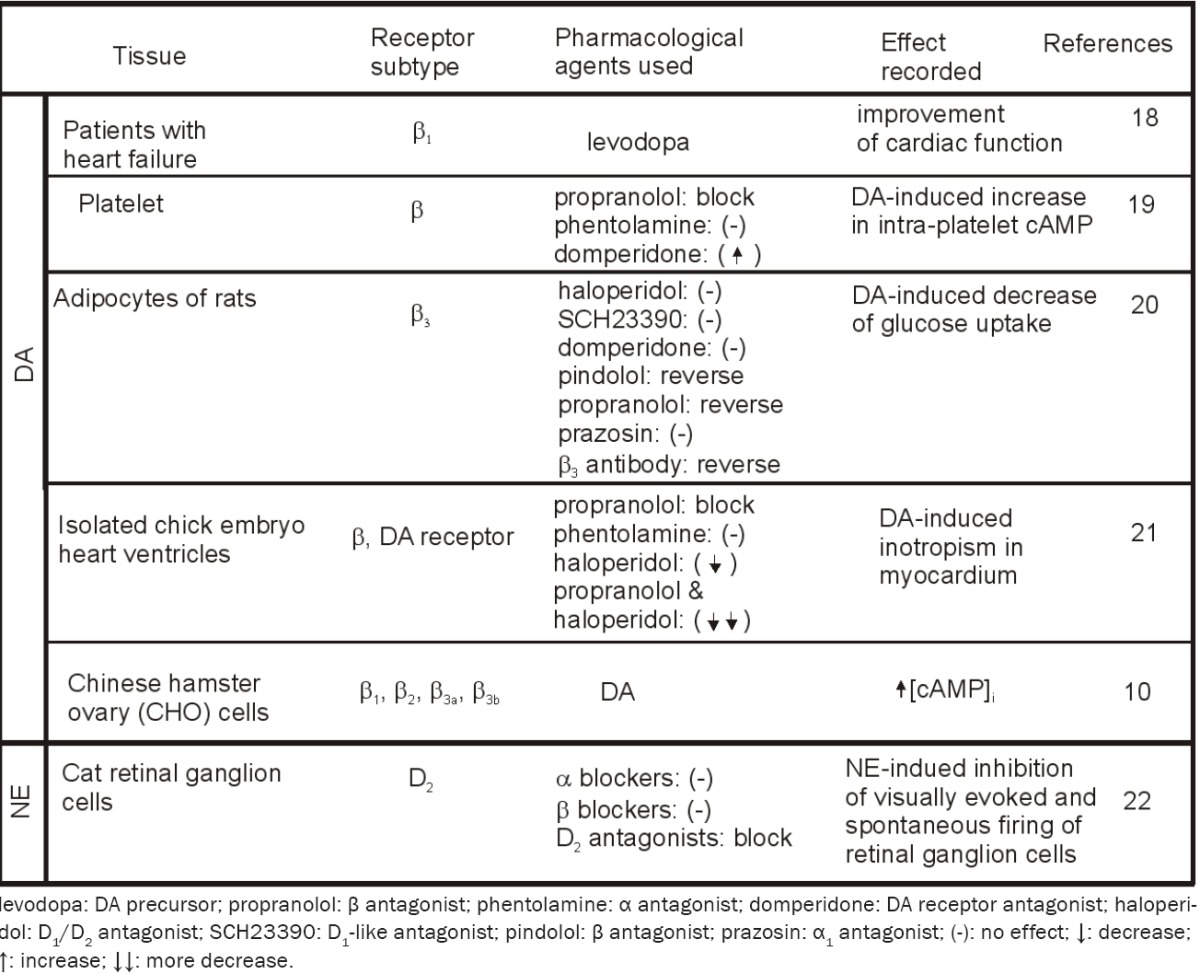
In addition to activating α adrenoceptors, DA also activate β adrenoceptors. The ingestion of levodopa, which is decarboxylated endogenously to DA, is associated with a sustained improvement in cardiac function. The beneficial hemodynamic actions of DA in patients with heart failure have been attributed to a positive inotropic effect that is mediated through activation of β1 adrenoceptors [18].
Application of DA to healthy subjects increases intra-platelet cAMP concentration [19]. The effect of DA is prevented by pre-incubation of platelets with propranolol (β-adrenoceptor antagonist) whereas pre-incubation with phentolamine(α-adrenoceptor antagonist) does not modify the platelet response to DA. Dopamine receptor antagonist, domperidone, directly increases cAMP levels and enhances the effects of DA. These results indicate that DA enhances intra-platelet cAMP concentration by β-adrenoceptor stimulation.
The effects of DA on glucose uptake into adipocytes of the rats have also been found to be mediated by activation of β-adrenoceptors [20]. Application of DA to adipocytes of Wistar rats concentration-dependently decreases glucose uptake. This effect is not modified by haloperidol at concentrations sufficient to block dopaminergic receptors. Pretreatment of the adipocytes with other two dopaminergic antagonists,SCH23390 and domperidone, does not block DA-induced decreases of glucose uptake. Application of dopaminergic agonists has no effects on glucose uptake. Consistent with these pharmacological results, immunoblotting analysis indicates a negative immunoreactive response to DA receptor antibodies further supporting the idea that there is no expression of DA receptors in the adipocytes. DA-induced inhibition of glucose intake is reversed by applications of the β-adrenoceptor blockers, pindolol and propranolol, but unaffected by prazosin at concentrations sufficient to block α-adreno-ceptors, suggesting the involvement of β not α adrenoceptors. The result that application of Rp-cAMPS, a membrane-permeable antagonist of cAMP, reduces the effects of DA suggesting that intracellular cAMP is required for DA-induced inhibition. Application of the antibody to β3-adrenoceptors reverses DA-induced inhibition suggesting that DA activates β3-adrenoc-eptors to lower glucose uptake into rat adipocytes which lack dopaminergic receptors.
DA exerts positive inotropic effects in electrically stimulated isolated chick embryo heart ventricles [21]. The DA-induced inotropism in myocardium is competitively antagonized by propranolol (β-blocker), not by phentolamine (α-blocker), suggesting that DA induces its positive inotropic effect via stimulation of β, not α adrenoceptors. Application of haloperidol alone antagonizes the positive inotropic response to DA. Combined application of both propranolol and haloperidol antagonizes DA-induced positive inotropic response to a greater extent than when these two antagonists are given alone. These results together demonstrate that DA induces its positive inotropism via stimulation of both β-adrenergic and dopaminergic receptors.Cross interactions of DA and β adrenoceptors are summarized in Table 2.
Table 2.
Cross activation of β adrenoceptors by DA and interaction of DA receptors with NE
|
Effects of NE on DA receptors
Whereas all the above lines of evidence indicate that DA effects by promiscuously activating adrenoceptors, evidence also indicates that NE interacts with DA receptors. Iontophoretically applied NE inhibits visually evoked and spontaneous firing of retinal ganglion cells [22]. The NE-induced inhibition is unaffected by all the α- and β-adrenoceptor blockers, but effectively blocked by DA D2-receptor antagonists, demonstrating that NE effects via activation of D2 receptors (Table 2).
Conclusion
DA and NE are classical neurotransmitters and they share the same synthesis pathway. These two neurotransmitters are possibly co-localized in some neurons in the brain. Whereas the traditional notion holds that neurotransmitters interact with their specific receptors and the pharmacology of the dopaminergic and adrenergic receptors has been extensively studied, increasing evidence indicates that there are promiscuous interactions between these two systems. The purpose of this review is therefore to bring the attention of researchers in that DA and NE can activate each other’s receptors to play a physiological function.
Acknowledgements
This work was supported by the National Institutes of Health (R01MH082881).
Disclosure of conflict of interest
None to declare.
References
- 1.Phillips AG, Vacca G, Ahn S. A top-down perspective on dopamine, motivation and memory. Pharmacol Biochem Behav. 2008;90:236–249. doi: 10.1016/j.pbb.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Sara SJ. The locus coeruleus and noradrenergic modulation of cognition. Nat Rev Neurosci. 2009;10:211–223. doi: 10.1038/nrn2573. [DOI] [PubMed] [Google Scholar]
- 3.Beaulieu JM, Gainetdinov RR. The physiology, signaling, and pharmacology of dopamine receptors. Pharmacol Rev. 2011;63:182–217. doi: 10.1124/pr.110.002642. [DOI] [PubMed] [Google Scholar]
- 4.Kurian MA, Gissen P, Smith M, Heales S Jr, Clayton PT. The monoamine neurotransmitter disorders: an expanding range of neurological syndromes. Lancet Neurol. 2011;10:721–733. doi: 10.1016/S1474-4422(11)70141-7. [DOI] [PubMed] [Google Scholar]
- 5.Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- 6.Enjalbert A, Bockaert J. Pharmacological characterization of the D2 dopamine receptor negatively coupled with adenylate cyclase in rat anterior pituitary. Mol Pharmacol. 1983;23:576–584. [PubMed] [Google Scholar]
- 7.Leedham JA, Pennefather JN. Selectivities of some agonists acting at α1- and α2-adrenoreceptors in the rat vas deferens. J Auton Pharmacol. 1986;6:39–46. doi: 10.1111/j.1474-8673.1986.tb00629.x. [DOI] [PubMed] [Google Scholar]
- 8.Rey E, Hernandez-Diaz FJ, Abreu P, Alonso R, Tabares L. Dopamine induces intracellular Ca2+ signals mediated by α1B-adrenoceptors in rat pineal cells. Eur J Pharmacol. 2001;430:9–17. doi: 10.1016/s0014-2999(01)01250-x. [DOI] [PubMed] [Google Scholar]
- 9.Cornil CA, Balthazart J, Motte P, Massotte L, Seutin V. Dopamine activates noradrenergic receptors in the preoptic area. J Neurosci. 2002;22:9320–9330. doi: 10.1523/JNEUROSCI.22-21-09320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang WP, Ouyang M, Thomas SA. Potency of catecholamines and other L-tyrosine derivatives at the cloned mouse adrenergic receptors. Neuropharmacology. 2004;47:438–449. doi: 10.1016/j.neuropharm.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 11.Lazou A, Markou T, Zioga M, Vasara E, Efstathiou A, Gaitanaki C. Dopamine mimics the cardioprotective effect of ischemic preconditioning via activation of α1-adrenoceptors in the isolated rat heart. Physiol Res. 2006;55:1–8. doi: 10.33549/physiolres.930694. [DOI] [PubMed] [Google Scholar]
- 12.Lin Y, Quartermain D, Dunn AJ, Weinshenker D, Stone EA. Possible dopaminergic stimulation of locus coeruleus alpha1-adrenoceptors involved in behavioral activation. Synapse. 2008;62:516–523. doi: 10.1002/syn.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cilz NI, Kurada L, Hu B, Lei S. Dopaminergic Modulation of GABAergic Transmission in the Entorhinal Cortex: Concerted Roles of α1 Adrenoreceptors, Inward Rectifier K+, and T-Type Ca2+ Channels. Cereb Cortex. 2013 doi: 10.1093/cercor/bht177. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kuzhikandathil EV, Oxford GS. Classic D1 dopamine receptor antagonist R-(+)-7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepine hydrochloride (SCH23390) directly inhibits G protein-coupled inwardly rectifying potassium channels. Mol Pharmacol. 2002;62:119–126. doi: 10.1124/mol.62.1.119. [DOI] [PubMed] [Google Scholar]
- 15.Shankar H, Murugappan S, Kim S, Jin J, Ding Z, Wickman V, Kunapuli SP. Role of G protein-gated inwardly rectifying potassium channels in P2Y12 receptor-mediated platelet functional responses. Blood. 2004;104:1335–1343. doi: 10.1182/blood-2004-01-0069. [DOI] [PubMed] [Google Scholar]
- 16.Sosulina L, Schwesig G, Seifert G, Pape HC. Neuropeptide Y activates a G-protein-coupled inwardly rectifying potassium current and dampens excitability in the lateral amygdala. Mol Cell Neurosci. 2008;39:491–498. doi: 10.1016/j.mcn.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Chee MJ, Price CJ, Statnick MA, Colmers WF. Nociceptin/orphanin FQ suppresses the excitability of neurons in the ventromedial nucleus of the hypothalamus. J Physiol. 2011;589:3103–3114. doi: 10.1113/jphysiol.2011.208819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rajfer SI, Borow KM, Lang RM, Neumann A, Carroll JD. Effects of dopamine on left ventricular afterload and contractile state in heart failure:relation to the activation of β1-adrenoceptors and dopamine receptors. J Am Coll Cardiol. 1988;12:498–506. doi: 10.1016/0735-1097(88)90426-3. [DOI] [PubMed] [Google Scholar]
- 19.Anfossi G, Massucco P, Mularoni E, Mattiello L, Cavalot F, Burzacca S, Trovati M. Effect of dopamine on adenosine 3’,5’-cyclic monophosphate levels in human platelets. Gen Pharmacol. 1993;24:435–438. doi: 10.1016/0306-3623(93)90329-v. [DOI] [PubMed] [Google Scholar]
- 20.Lee TL, Hsu CT, Yen ST, Lai CW, Cheng JT. Activation of β3-adrenoceptors by exogenous dopamine to lower glucose uptake into rat adipocytes. J Auton Nerv Syst. 1998;74:86–90. doi: 10.1016/s0165-1838(98)00120-9. [DOI] [PubMed] [Google Scholar]
- 21.Ouedraogo L, Magnon M, Sawadogo L, Tricoche R. Receptors involved in the positive inotropic action induced by dopamine on the ventricle of a 7-day-old chick embryo heart. Fundam Clin Pharmacol. 1998;12:133–142. doi: 10.1111/j.1472-8206.1998.tb00933.x. [DOI] [PubMed] [Google Scholar]
- 22.Robbins J, Wakakuwa K, Ikeda H. Noradrenaline action on cat retinal ganglion cells is mediated by dopamine D2 receptors. Brain Res. 1988;438:52–60. doi: 10.1016/0006-8993(88)91322-4. [DOI] [PubMed] [Google Scholar]


