Abstract
Sirtuin 2 (SIRT2) is a member of sirtuin protein family. Previous studies have suggested that SIRT2 plays differential roles in the survival and cell cycle regulation of various cell types. Because microglia plays critical roles in multiple major neurological disorders, in our current study we investigated the roles of SIRT2 in regulation of the cell cycle and cell survival of BV2 microglia by applying SIRT2 siRNA. We found that SIRT2 reductions by SIRT2 siRNA can produce cell cycle arrest of the cells at G0/G1 phase, by significantly increasing percentage of the cells in G0/G1 phase as well as decreasing percentage of the cells in S phase. The SIRT2 reductions can also increase late-stage apoptosis of the cells. We further found that SIRT2 silencing can lead to a decrease in the number of surviving BV2 cells, which may result from the effects of SIRT2 siRNA on both cell cycle and cell survival of the cells. Collectively, our study has suggested an important role of SIRT2 in regulating both the cell cycle and basal survival of microglia.
Keywords: SIRT2, microglia, cell cycle, apoptosis, neurological disorders
Introduction
Sirtuin family proteins (SIRT1-7), the mammalian homologs of yeast silent information regulator 2 (Sir2), are NAD+-dependent histone deacetylases [1-3]. Cumulative evidence has indicated that sirtuins play crucial regulatory roles in a variety of cellular processes, such as cell survival/death, metabolism, genome stability,and stress resistance [4,5]. Among these sirtuins, there has been no sufficient information regarding the biological functions of SIRT2.
SIRT2 has been shown to play seemingly paradoxical roles in cell survival: SIRT2 has been indicated as a key mediator of programmed necrosis [6]; and SIRT2 inhibition has been shown to produce beneficial effects in models of Parkinson’s disease (PD) and Huntington’s disease (HD) [7,8]. In contrast, some studies have suggested that SIRT2 activity is required for the survival of such cell types as C6 glioma cells and Hela cells [9,10].
There are also conflicting reports regarding the roles of SIRT2 in cell cycle regulation: The study of Dryden et al. suggested that SIRT2 can inhibit the exit from the mitosis of osteoblastic cell line Saos2 [11], which was indicated by the overexpression of wild-type SIRT2, but not the deacetylase activity-deficient mutant of SIRT2, to produce a delay of the exit from mitosis. Similar results were reported by a study using myelomonocytic cell line U937 [12]. However, several studies did not find any significant roles of SIRT2 in the cell cycle regulation of U251MG cells [13], HeLa cells and HEK293 cells [14].
Microglia are the resident macrophages of the central nervous system (CNS) [15], which show a ‘resting’ phenotype characterized by ramified morphology in the healthy adult CNS [16]. Numerous studies have suggested significant roles of microglia in multiple major neurological disorders including stroke and PD [17,18]. Therefore, it is of both theoretical and therapeutic significance to elucidate the mechanisms underlying microglial survival.
Latest studies have suggested that SIRT2 plays a crucial role of microglia activation [19]. However, there has been no sufficient information regarding the roles of SIRT2 in both the biological functions and survival of resting microglia.Considering the studies reporting complex roles of SIRT2 in affecting the survival and cell cycle of different cell types, in our current study we applied the approach of SIRT2 silencing to investigate the roles of SIRT2 in regulating the survival and cell cycle of microglia, using BV2 microglia as a cellular model. Our study has suggested that SIRT2 plays significant roles in regulation of both survival and cell cycle of BV2 microglia.
Materials and methods
Cell culture
BV2 microglia cells were purchased from the Cell Resource Center of Shanghai Institute of Biological Sciences, Chinese Academy of Sciences. The cells were plated onto 24-well or 6-well cell culture plates in Dulbecco’s Modified Eagle Medium (Thermo Scientific, Waltham, MA, USA) containing 1% penicillin and streptomycin(Invitrogen, Carlsbad, CA, USA) and 10% fetal bovine serum (Gibco, Grand Island, NY, USA) in an incubator with 5% CO2 at 37°C.
siRNA transfection
Stealth RNAi oligonucleotides were used for siRNA transfection (Invitrogen,Carlsbad, CA, USA). The sequences targeting mouse SIRT2 were as follows: sense, 5’- AUGAUGAGGAG-GUCCACCUUGGAGA -3’; antisense, 5’- UCUC-CAAGGUGGACCUCCUCAUCAU -3’. A medium GC duplex of Stealth RNAi Negative Control Duplexes (Invitrogen, Carlsbad, CA, USA) was used as a negative control. For each well, 100 nM Stealth RNAi oligonucleotides were transfected into BV2 cells using lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer’s protocols.
Western blot
BV2 cells were harvested and lysed in RIPA buffer(Millipore, Temecula, CA, USA) containing Complete Protease Inhibitor Cocktail (Roche Diagnostics, Mannheim, Germany) plus 1 mM PMSF. Thirty μg of total protein was electrophoresed through a 10% SDS-polyacrylamide gel, and then transferred to 0.45 μm nitrocellulose membranes (Millipore, CA, USA) using an electroblotting apparatus (Bio-Rad Laboratories, CA, USA). Blots were incubated overnight at 4°C with a rabbit polyclonal anti-SIRT2 antibody(Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:500 dilution), then incubated with appropriate HRP-conjugated secondary antibody(EPITOMICS, Hangzhou, Zhejiang Province, China, 1:5000 dilution). Protein signals were detected using an ECL detection system (Pierce Biotechonology, Rockford, IL, USA). An anti-actin antibody (Santa Cruz Biotechnology, Santa Cruz, CA, USA, 1:1000 dilution) was used to normalize sample loading and transfer. The intensities of the bands were quantified by densitometry using Gel-Pro Analyzer.
Intracellular lactate dehydrogenase (LDH) assay
Intracellular LDH assay was conducted to determine cell survival, as described previously [9].
Cell cycle analysis
Forty-eight hours after transfection, BV2 cells were collected by trypsinisation and assessed for cell cycle by flow cytometry as described[20]. Briefly, the cells were fixed with 70% cold ethanol.Then the fixed cells were washed with PBS, treated with 50 μg/ml RNase A, and stained with 50 μg/ml propidium iodide for 30 mins in the dark. Subsequently, the cells were analyzed by flow cytometry (BD FACSCalibur). The cell populations at the G0/G1, S and G2/M phases were quantified using the Modfit software (BD).
FACS-based Annexin V/7-AAD staining
The FACS assay was conducted to measure the degrees of both apoptosis and necrosis using ApoScreen Annexin V kit (SouthernBiotech, Birmingham, AL, USA) according to the manufacturer’s instructions. In brief, BV2 cells were digested by 0.1% trypsin and resuspended in cold binding buffer (10 mM HEPES, pH 7.4, 140 mM NaCl, 2.5 mM CaCl2, 0.1% BSA) at concentrations between 1 × 106 and 1 × 107 cells/ml. Ten μL of labeled Annexin V was added into 100 μL of the cell suspension. After 15-min incubation on ice, 380 μL binding buffer and 10 μL 7-AAD solution were added into the cell suspension.The number of stained cells was assessed by a flow cytometer (BD FACSAriaII).
Statistical analyses
All data are presented as mean mean ± SE. Data were assessed by one-way ANOVA, followed by Student-Newman-Keuls post hoc test. P values less than 0.05 were considered statistically significant.
Results
We applied SIRT2 siRNA to decrease the SIRT2 levels in BV2 cells. At 48 hrs or 72 hrs after the treatment of 100 nM SIRT2 siRNA, SIRT2 levels were assessed by Western blot (Figure 1). Quantifications of the Western blots showed that SIRT2 silencing led to significant decreases in the SIRT2 levels (Figure 1). Intracellular LDH assay was conducted to determine the effects of SIRT2 silencing on the survival of the cells, which showed that treatment of the cells with SIRT2 siRNA for 48 or 72 hrs led to a significant decrease in the number of surviving BV2 cells (Figure 2).
Figure 1.
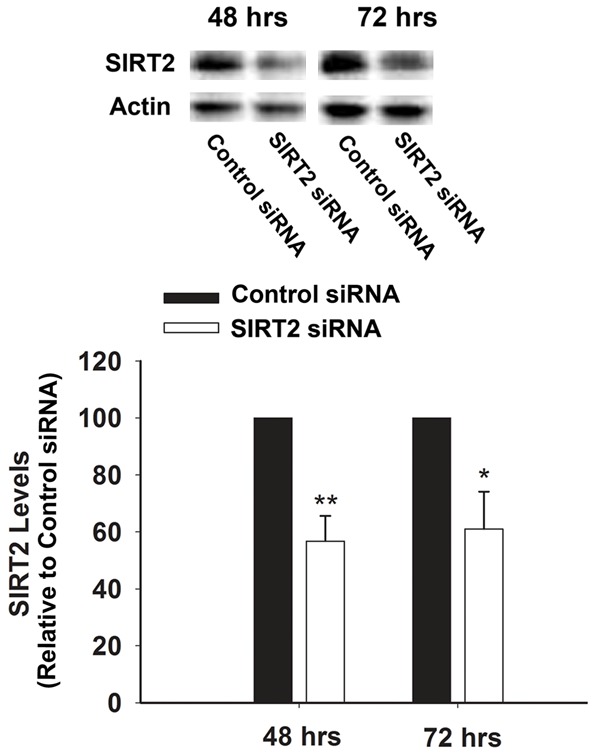
Western blot assay showed that the SIRT2 siRNA treatment led to a significant decrease in the SIRT2 levels of BV2 cells. The cells were transfected with SIRT2 siRNA for either 48 or 72 hrs, and subsequentlythe SIRT2 levels of the cells were determined by Western blot assay. N = 4-5. Data were collected from four independent experiments. *, p< 0.05; **, p< 0.01.
Figure 2.
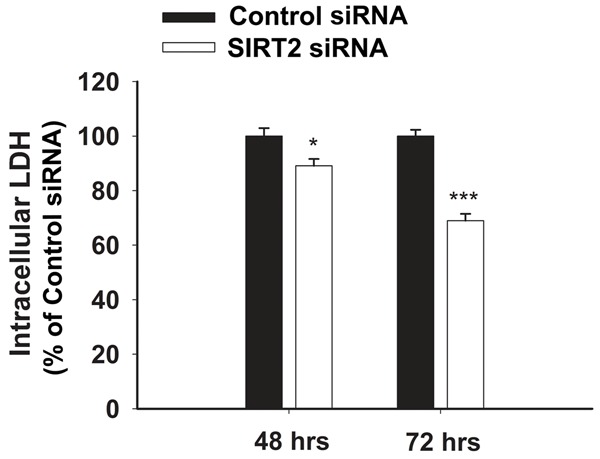
Treatment of BV2 cells with SIRT2 siRNA led to a significant decrease in the number of surviving BV2 cells, as assessed by intracellular LDH assay. The cells were treated with 100 nM SIRT2 siRNA for either 48 or 72 hrs, and subsequently intracellular LDH assay was conducted to determine the effects of SIRT2 silencing on the cell survival. N = 12. Data were collected from three independent experiments. *, p< 0.05; ***, p< 0.001.
Cell cycle analysis was conducted to determine if the SIRT2 reductions led to the decreased in the number of surviving cells by producing inhibition of cell cycle of the cells. Our study has suggested that SIRT2 silencing produced cell cycle arrest of BV2 cells at G0/G1 phase: The SIRT2 silencing led to a significant increase in the percentage of cells in G0/G1 phase from 61.2% to 79.6%, as well as a significant decrease in the percentage of cells in S phase from 31.1% to 14.7% (Figure 3A, 3B).
Figure 3.
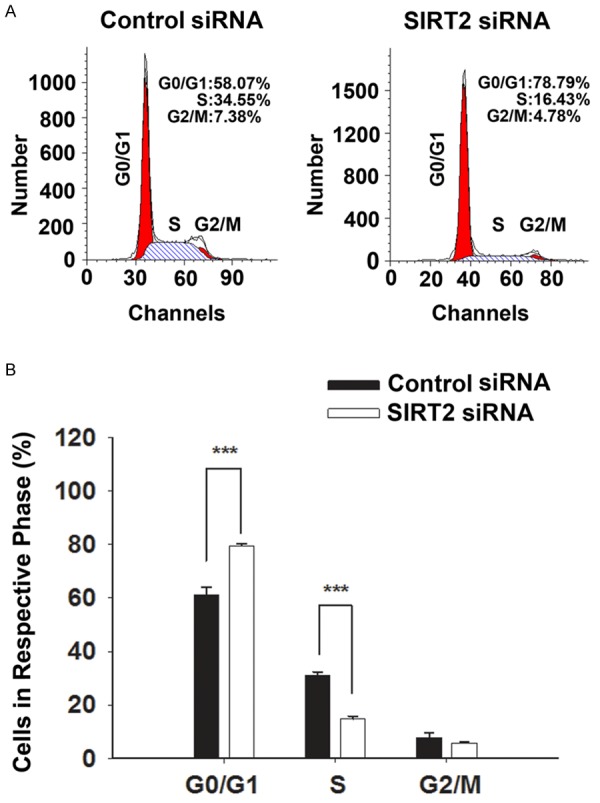
SIRT2 silencing led to significant alterations of the cell cycle of BV2 cells. A. Representative histograms depicting cell cycle profiles of control BV2 cells and the BV2 cells transfected with 100 nM siRNA. B. Quantifications of the histograms suggested that the SIRT2 silencing led to a significant increase in the percentage of cells in G0/G1 phase, as well as a significant decrease in the percentage of cells in S phase. BV2 cells were transfected with SIRT2 siRNA for 48 hrs, and then cell cycle analysis was conducted. N = 5. Data were collected from five independent experiments. ***, p< 0.001.
We further determined if SIRT2 silencing may also affect the apoptosis and necrosis of the cells by conducting FACS-based Annexin V/7-AAD staining assay. The SIRT2 silencing was shown to produce an increase in the late-stage apoptosis cells, as indicated by the increase in Annexin V+/7-AAD+ cells (Figure 4A, 4B). In contrast,the SIRT2 silencing did not affect the number of necrotic cells (Annexin V-/7-AAD+ cells) (Figure 4A, 4B).
Figure 4.
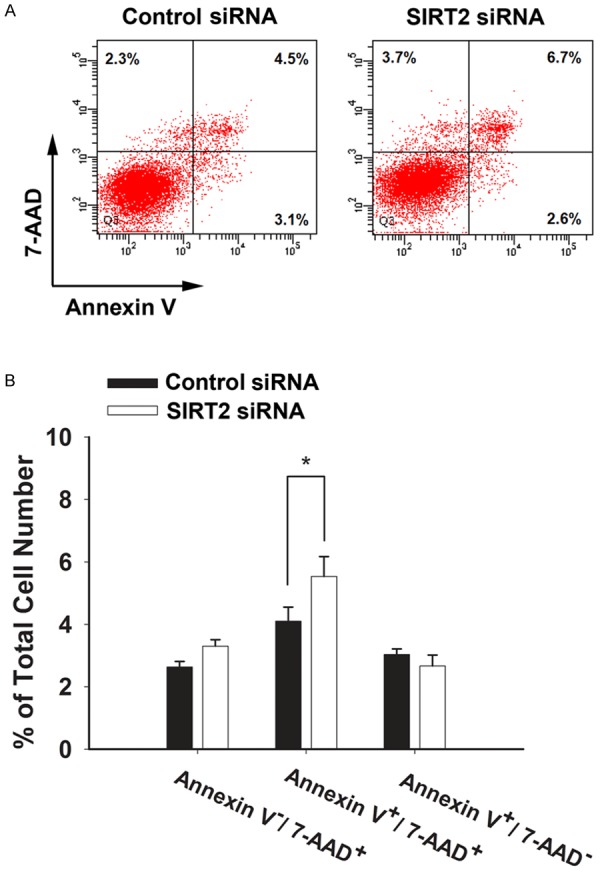
Treatment of microglial BV2 cells with SIRT2 siRNA led to a significant increase in late-stage apoptosis of the cells, as assessed by FACS-based Annexin V/7-AAD staining. A. The FACS diagrams showed that SIRT2 siRNA induced an increase in the number of Annexin V+/7-AAD+ cells, assessed at 72 hrs after siRNA transfection. In the four fields of the original images from the flow cytometry-based study, the number of the dots indicates the number of Annexin V-/7-AAD- (the bottom-left field), Annexin V+/7-AAD- (the bottom-right field), Annexin V-/7-AAD+ (the top-left field), and Annexin V+/7-AAD+ cells (the top-right field), respectively. B. Quantifications of the results from the flow cytometry-based study indicated that SIRT2 siRNA led to a significant increase in the number of Annexin V+/7-AAD+ cells, assessed at 72 hrs after siRNA treatment. N = 3. Data were collected from three independent experiments. *, p< 0.05.
Discussion
The major findings of our current studies include: First, SIRT2 reductions by SIRT2 siRNA can produce cell cycle arrest of BV2 cells at G0/G1 phase, by both significantly increasing percentage of the cells in G0/G1 phase and significantly decreasing percentage of the cells in S phase; second, the SIRT2 reductions can also increase late-stage apoptosis of the cells; and third, the SIRT2 reductions can lead to a decrease in the number of surviving cells,which may result from the effects of SIRT2 reductions on both cell cycle and cell survival of BV2 cells. Collectively, our study has suggested important roles of SIRT2 in regulating both the cell cycle and the basal survival of microglial BV2 cells.
SIRT2 has been shown to play seemingly paradoxical roles in both cell cycle and cell survival: Several studies did not find any significant roles of SIRT2 in the cell cycle regulation of U251MG cells [13], HeLa cells and HEK293 cells [14], while SIRT2 has been shown to inhibit the exit from the mitosis of osteoblastic cell line Saos2 [11] and myelomonocytic cell line U937 [12]. Multiple studies have also suggested contrasting roles of SIRT2 inhibition in cell death under various conditions: SIRT2 inhibition has been shown to produce beneficial effects in models of PD, HD [7,8] and ischemic myocardial damage [6]. However, SIRT2 inhibition has also been shown to produce apoptosis of C6 glioma cells and Hela cells [9,10].
Due to the differential roles of SIRT2 in the cell cycle and cell death in different cell types, it appears to be necessary to conduct studies to determine the roles of SIRT2 in certain type of cells. In our current study, we determined the roles of SIRT2 in both cell cycle and cell survival of microglia. Because microglia play significant roles in multiple major neurological disorders [18], our study on the roles of SIRT2 in both cycle and cell survival of microglia may not only improve our understanding on the fundamental regulatory mechanisms of microglia, but also enhance our understanding regarding the roles of SIRT2 in neurological disorders.
Our current study has provided evidence suggesting that SIRT2 plays important roles in the cell cycle regulation of BV2 cells: The SIRT2 silencing led to a significant increase in the percentage of cells in G0/G1 phase, as well as a significant decrease in the percentage of cells in S phase. This finding has suggested that SIRT2 plays a significant role in cell cycle regulation in microglia, in contrast to the minimal roles of SIRT2 in such cell types as U251MG cells [13], HeLa cells and HEK293 cells [14]. SIRT2 is a NAD+-dependent enzyme. Because such factors as oxidative stress can significantly decrease intracellular NAD+ levels [21], our study has suggested that oxidative stress might alter the cell cycle progression of microglia by affecting NAD+ and SIRT2.
Our current study has also shown that SIRT2 silencing can produce an increase in late-stage apoptosis of the microglia, without affecting the number of necrotic cells. This observation is consistent with previous reports that SIRT2 inhibition can lead to apoptosis of C6 glioma cells, Hela cells [9,10] and PC12 cells (unpublished observation). Our finding that SIRT2 silencing can induce BV2 cell death is also consistent with our previous study showing that treatment of BV2 cells with AGK2 – a widely used SIRT2 inhibitor – can lead to cell death of BV2 cells [22]. Combined our studies using either molecular approach or pharmacological approaches to inhibit SIRT2, our observations from these two studies have collectively suggested that SIRT2 is required for the basal survival of BV2 microglia.
Our intracellular LDH assay has suggested that the SIRT2 reductions can lead to a decrease in the number of surviving cells. Both inhibition of cell cycle progression and cell death can lead to decreased number of surviving cells. Therefore, our observations that SIRT2 reductions can lead to both cell cycle arrest and late-stage apoptosis have suggested that both of these two effects of the SIRT2 reductions may contribute to the SIRT2 reductions-induced decrease in the number of surviving cells.
In summary, our study has suggested important roles of SIRT2 in regulating both the cell cycle and the basal survival of microglial BV2 cells. Because microglia play critical roles in the pathologies of such neurological disorders as stroke and PD [17,18], our study has suggested that SIRT2 may become a therapeutic target for the disorders due to the major effects of SIRT2 on the cell cycle and survival of microglia.
Acknowledgements
This study was supported by Chinese National Science Foun-dation Grants #81171098 and #81271305 (to W. Y.), a National Key Basic Research ‘973 Program’ Grant #2010CB834306 (to W.Y.), and Shanghai Jiao Tong University Grants for Interdisciplinary Research on Medicine and Engineering (to W.Y.).
References
- 1.Frye RA. Characterization of five human cDNAs with homology to the yeast SIR2 gene: Sir2-like proteins (sirtuins) metabolize NAD and may have protein ADP-ribosyltransferase activity. Biochem Biophys Res Commun. 1999;260:273–279. doi: 10.1006/bbrc.1999.0897. [DOI] [PubMed] [Google Scholar]
- 2.Nakagawa T, Guarente L. Sirtuins at a glance. J Cell Sci. 2011;124:833–838. doi: 10.1242/jcs.081067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feldman JL, Dittenhafer-Reed KE, Denu JM. Sirtuin catalysis and regulation. J Biol Chem. 2012;287:42419–42427. doi: 10.1074/jbc.R112.378877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Houtkooper RH, Pirinen E, Auwerx J. Sirtuins as regulators of metabolism and healthspan. Nat Rev Mol Cell Biol. 2012;13:225–238. doi: 10.1038/nrm3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Finkel T, Deng CX, Mostoslavsky R. Recent progress in the biology and physiology of sirtuins. Nature. 2009;460:587–591. doi: 10.1038/nature08197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Narayan N, Lee IH, Borenstein R, Sun J, Wong R, Tong G, Fergusson MM, Liu J, Rovira II, Cheng HL, Wang G, Gucek M, Lombard D, Alt FW, Sack MN, Murphy E, Cao L, Finkel T. The NAD-dependent deacetylase SIRT2 is required for programmed necrosis. Nature. 2012;492:199–204. doi: 10.1038/nature11700. [DOI] [PubMed] [Google Scholar]
- 7.Outeiro TF, Kontopoulos E, Altmann SM, Kufareva I, Strathearn KE, Amore AM, Volk CB, Maxwell MM, Rochet JC, McLean PJ. Sirtuin 2 inhibitors rescue α-synuclein-mediated toxicity in models of Parkinson’s disease. Science. 2007;317:516–519. doi: 10.1126/science.1143780. [DOI] [PubMed] [Google Scholar]
- 8.Luthi-Carter R, Taylor DM, Pallos J, Lambert E, Amore A, Parker A, Moffitt H, Smith DL, Runne H, Gokce O. SIRT2 inhibition achieves neuroprotection by decreasing sterol biosynthesis. Proc Natl Acad Sci U S A. 2010;107:7927–7932. doi: 10.1073/pnas.1002924107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.He X, Nie H, Hong Y, Sheng C, Xia W, Ying W. SIRT2 activity is required for the survival of C6 glioma cells. Biochem Biophys Res Commun. 2012;417:468–472. doi: 10.1016/j.bbrc.2011.11.141. [DOI] [PubMed] [Google Scholar]
- 10.Li Y, Matsumori H, Nakayama Y, Osaki M, Kojima H, Kurimasa A, Ito H, Mori S, Katoh M, Oshimura M. SIRT2 down-regulation in HeLa can induce p53 accumulation via p38 MAPK activation-dependent p300 decrease, eventually leading to apoptosis. Genes Cells. 2011;16:34–45. doi: 10.1111/j.1365-2443.2010.01460.x. [DOI] [PubMed] [Google Scholar]
- 11.Dryden SC, Nahhas FA, Nowak JE, Goustin AS, Tainsky MA. Role for human SIRT2 NAD-dependent deacetylase activity in control of mitotic exit in the cell cycle. Mol Cell Biol. 2003;23:3173–3185. doi: 10.1128/MCB.23.9.3173-3185.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bae NS, Swanson MJ, Vassilev A, Howard BH. Human histone deacetylase SIRT2 interacts with the homeobox transcription factor HOXA10. J Biochem. 2004;135:695–700. doi: 10.1093/jb/mvh084. [DOI] [PubMed] [Google Scholar]
- 13.Inoue T, Hiratsuka M, Osaki M, Yamada H, Kishimoto I, Yamaguchi S, Nakano S, Katoh M, Ito H, Oshimura M. SIRT2, a tubulin deacetylase,acts to block the entry to chromosome condensation in response to mitotic stress. Oncogene. 2007;26:945–957. doi: 10.1038/sj.onc.1209857. [DOI] [PubMed] [Google Scholar]
- 14.Pandithage R, Lilischkis R, Harting K, Wolf A, Jedamzik B, Lüscher-Firzlaff J, Vervoorts J, Lasonder E, Kremmer E, Knöll B. The regulation of SIRT2 function by cyclin-dependent kinases affects cell motility. J Cell biol. 2008;180:915–929. doi: 10.1083/jcb.200707126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Perry VH, Nicoll JAR, Holmes C. Microglia in neurodegenerative disease. Nat Rev Neurol. 2010;6:193–201. doi: 10.1038/nrneurol.2010.17. [DOI] [PubMed] [Google Scholar]
- 16.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308:1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 17.Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol. 2007;184:53–68. doi: 10.1016/j.jneuroim.2006.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gomes-Leal W. Microglial physiopathology: how to explain the dual role of microglia after acute neural disorders? Brain Behav. 2012;2:345–356. doi: 10.1002/brb3.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pais TF, Szego EM, Marques O, Miller-Fleming L, Antas P, Guerreiro P, de Oliveira RM, Kasapoglu B, Outeiro TF. The NAD-dependent deacetylase sirtuin 2 is a suppressor of microglial activation and brain inflammation. EMBO J. 2013;32:2603–16. doi: 10.1038/emboj.2013.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Feng R, Chen X, Yu Y, Su L, Yu B, Li J, Cai Q, Yan M, Liu B, Zhu Z. miR-126 functions as a tumour suppressor in human gastric cancer. Cancer Lett. 2010;298:50–63. doi: 10.1016/j.canlet.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 21.Alano CC, Ying W, Swanson RA. Poly(ADP-ribose) polymerase-1-mediated cell death in astrocytes requires NAD+ depletion and mitochondrial permeability transition. J Biol Chem. 2004;279:18895–18902. doi: 10.1074/jbc.M313329200. [DOI] [PubMed] [Google Scholar]
- 22.Li Y, Nie H, Wu D, Zhang J, Wei X, Ying W. Poly(ADP-ribose) polymerase mediates both cell death and ATP decreases in SIRT2 inhibitor AGK2-treated microglial BV2 cells. Neurosci Lett. 2013;544:36–40. doi: 10.1016/j.neulet.2013.03.032. [DOI] [PubMed] [Google Scholar]


