Abstract
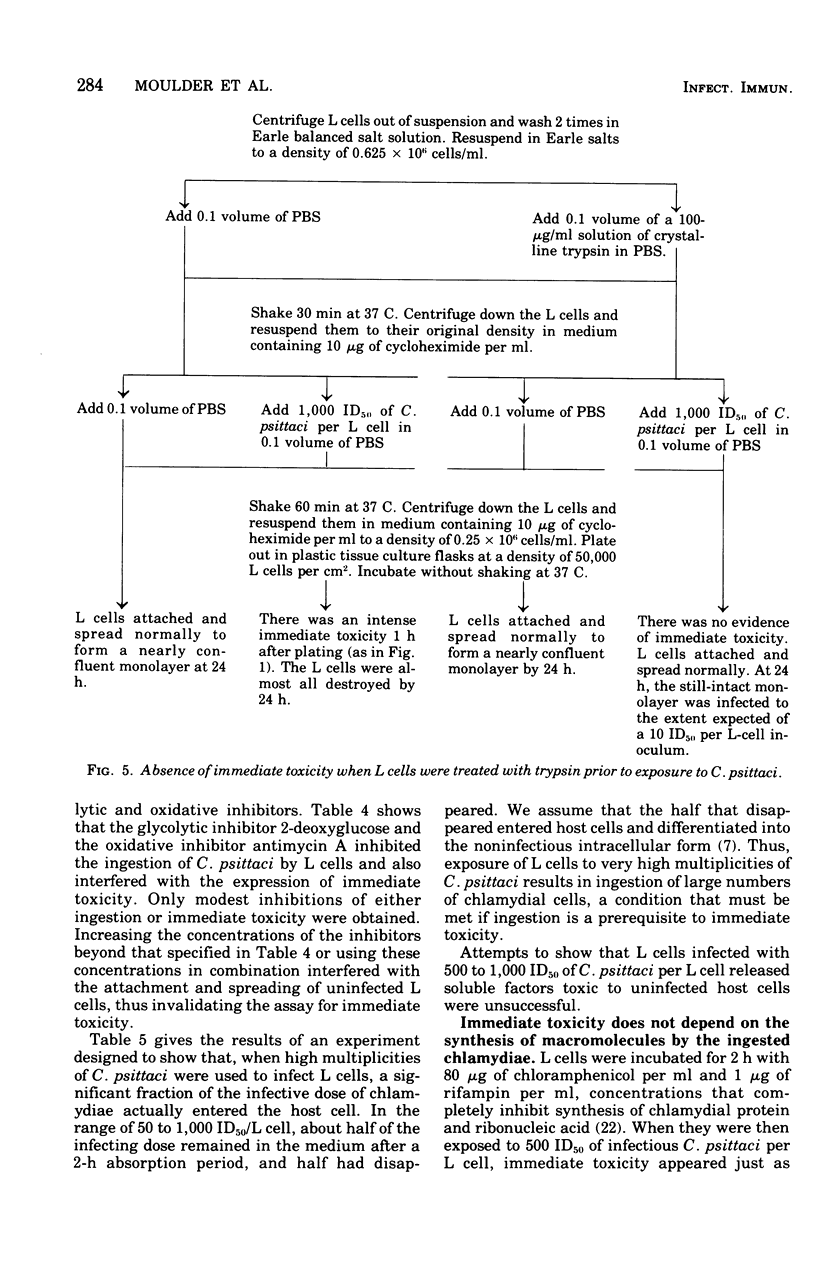
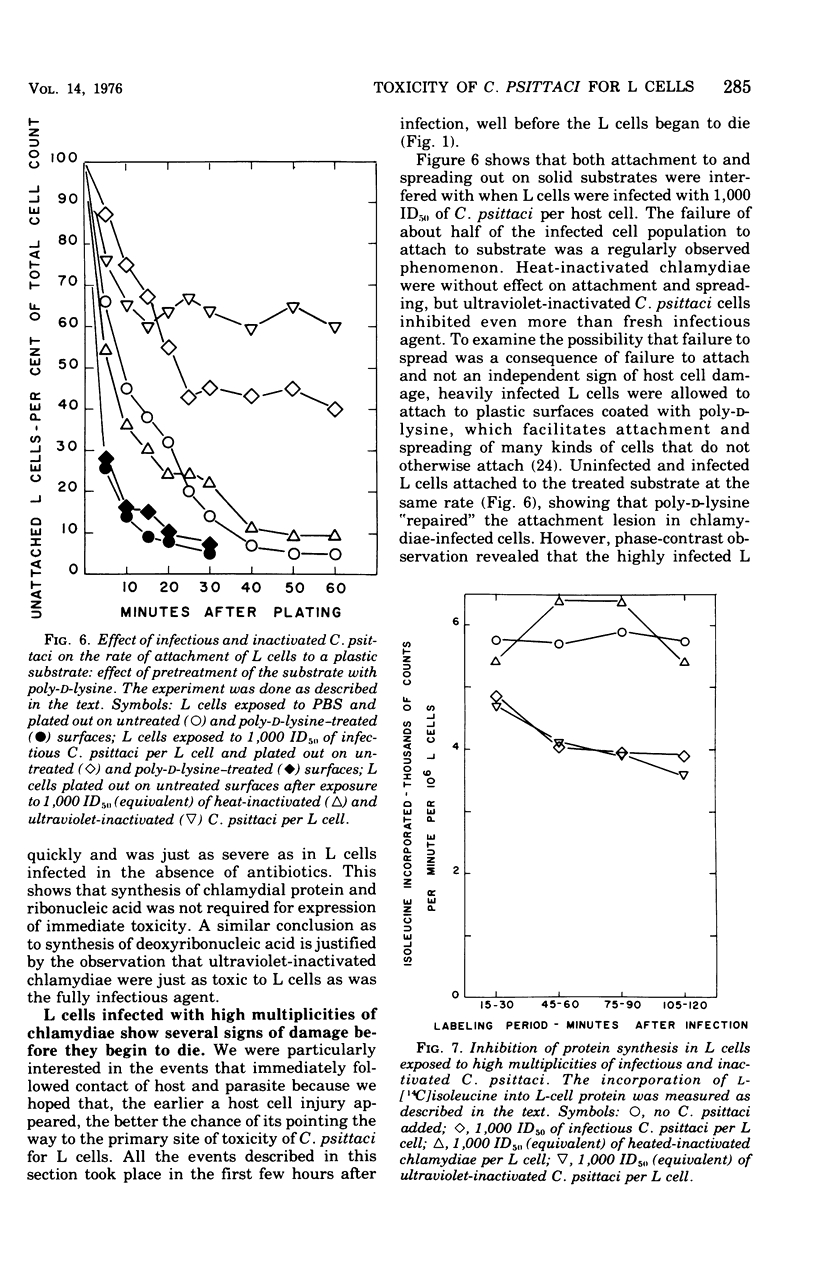
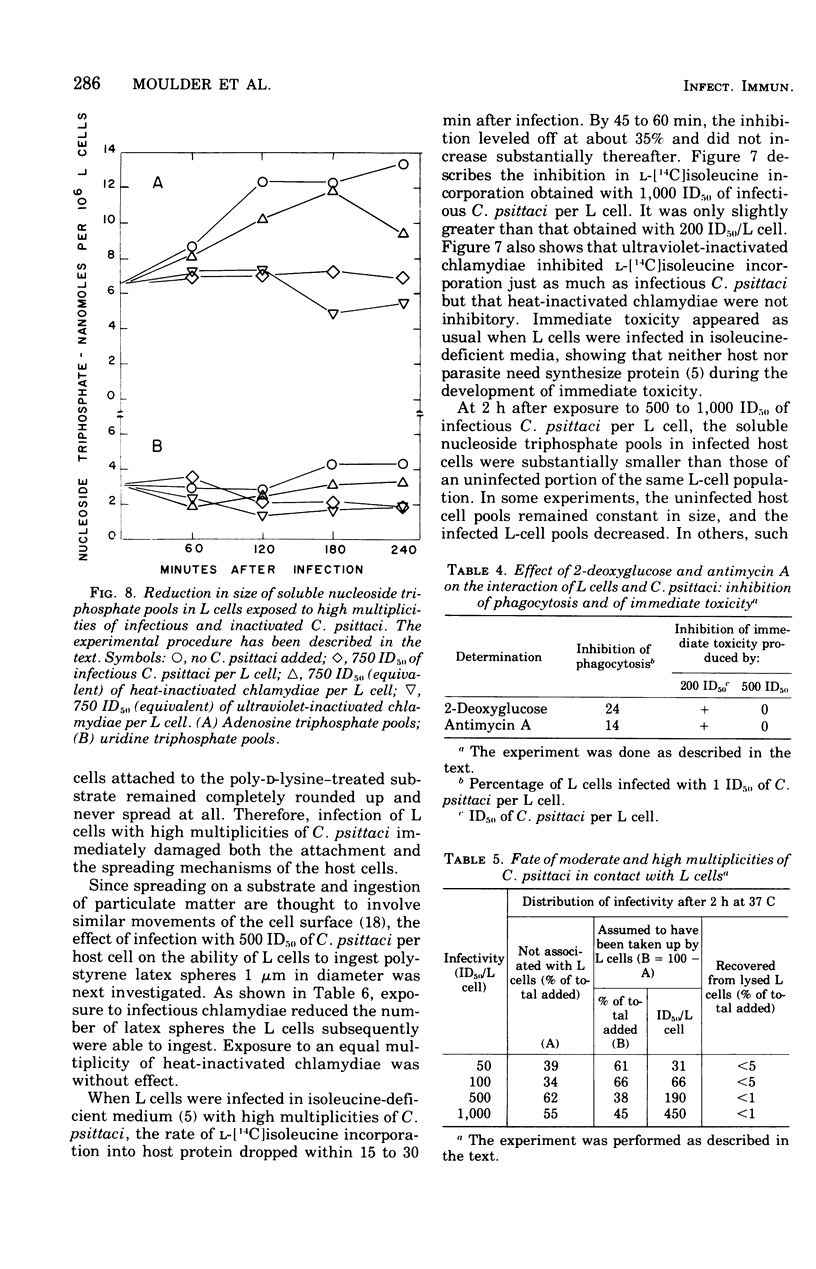
One hour after suspensions of mouse fibroblasts (L cells) were exposed to 500 to 1,000 L-cell 50% infectious doses of Chlamydia psittaci (6BC), the L cells failed to attach to and spread out on solid substrates, phagocytosed polystyrene latex spheres at reduced rates, incorporated less [14C]isoleucine into protein, and had smaller soluble pools of nucleoside triphosphates. The infected L cells began to die at 8 h and were all dead by 20 h. Lower multiplicities of infection took correspondingly longer to kill the L cells. These effects of high multiplicities of C. psittaci on L cells will be referred to collectively as immediate toxicity. Similar effects were obtained with other strains of C. psittaci and C. trachomatis and with other cell lines. Ultraviolet-inactivated C. psittaci retained the ability to cause immediate toxicity, but heat-inactivated chlamydiae did not. C.psittaci cells had to be ingested by L cells before they were immediately toxic but, once they were phagocytosed, they did not need to multiply or to synthesize macromolecules in order to cause immediate injury to their hosts. Immediate toxicity was not the result of depression of energy metabolism, changes in the levels of intracellular cyclic nucleotides, or release of hydrolases from lysosomes. It was suggested that a lesion is produced in the plasma membrane of the L cell every time it ingests a chlamydial cell, that each act of ingestion produces an independent lesion, and that their injurious effects are additive. Thus, the more ingestion lesions there are, the faster the host cell dies. It was also suggested that induced phagocytosis, inhibition of lysosomal fusion, and death of mice and of cells in culture may all depend on a single activity of C. psittaci.
Full text
PDF
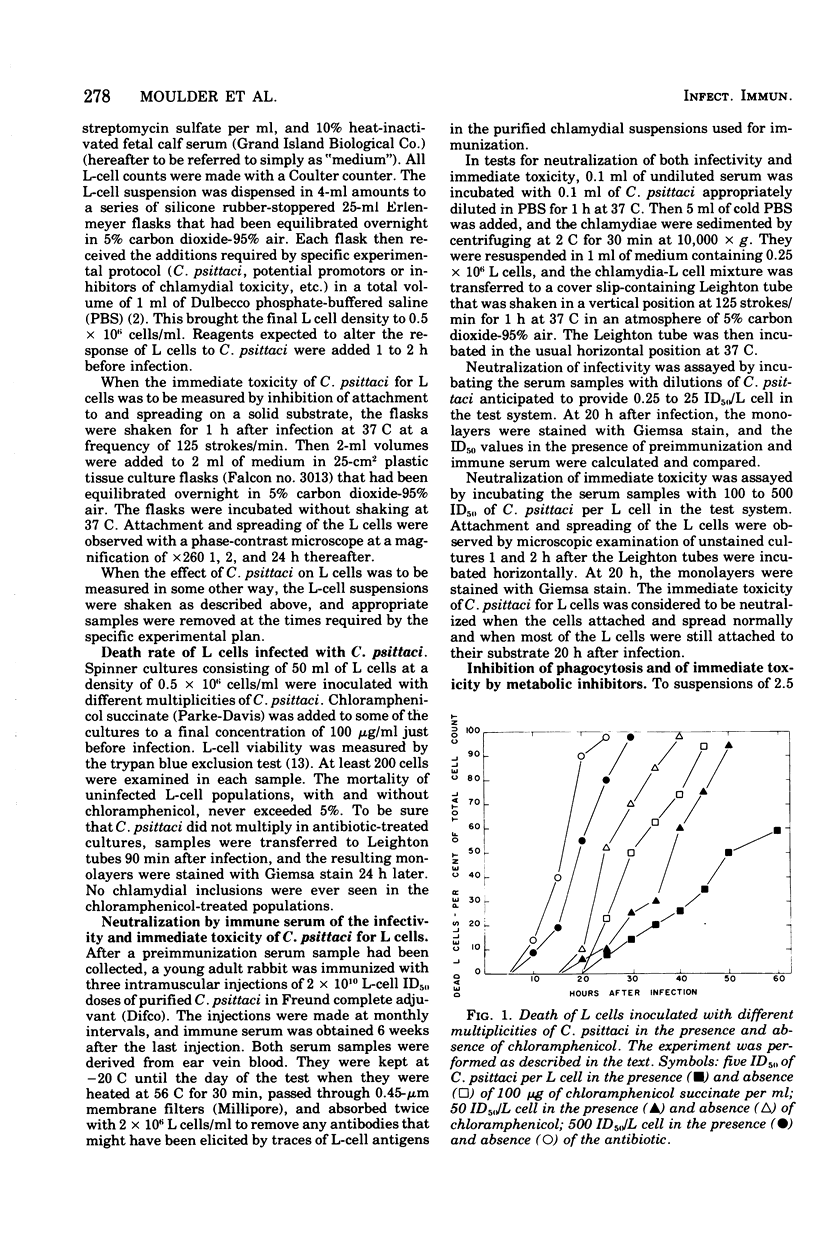

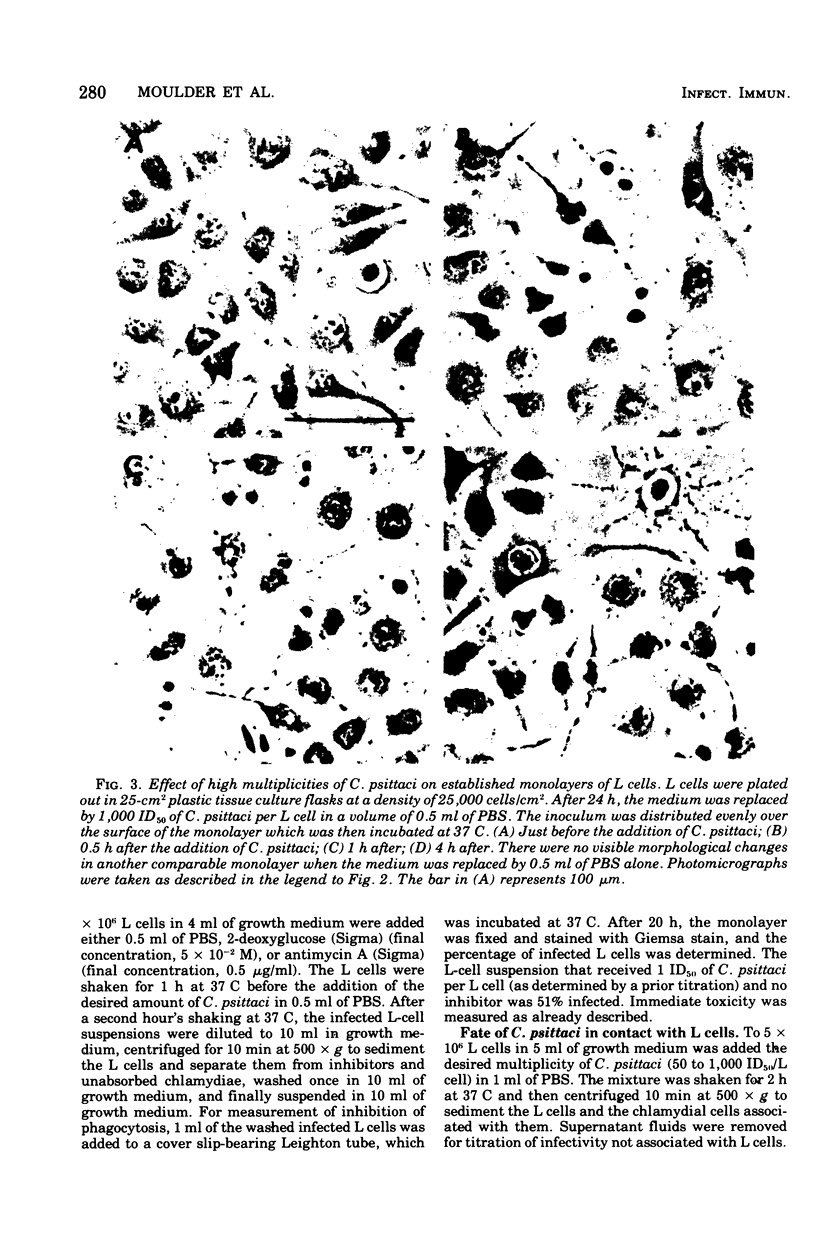





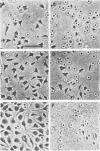
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alexander J. J. Separation of protein synthesis in meningopneumonitisgent from that in L cells by differential susceptibility to cycloheximide. J Bacteriol. 1968 Feb;95(2):327–332. doi: 10.1128/jb.95.2.327-332.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. Plaque formation and isolation of pure lines with poliomyelitis viruses. J Exp Med. 1954 Feb;99(2):167–182. doi: 10.1084/jem.99.2.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friis R. R. Interaction of L cells and Chlamydia psittaci: entry of the parasite and host responses to its development. J Bacteriol. 1972 May;110(2):706–721. doi: 10.1128/jb.110.2.706-721.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HIGASHI N., TAMURA A., IWANAGA M. Developmental cycle and reproductive mechanism of the meningopneumonitis virus in strain L cells. Ann N Y Acad Sci. 1962 Mar 5;98:100–121. doi: 10.1111/j.1749-6632.1962.tb30536.x. [DOI] [PubMed] [Google Scholar]
- Hatch T. P. Competition between Chlamydia psittaci and L cells for host isoleucine pools: a limiting factor in chlamydial multiplication. Infect Immun. 1975 Jul;12(1):211–220. doi: 10.1128/iai.12.1.211-220.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatch T. P. Utilization of L-cell nucleoside triphosphates by Chlamydia psittaci for ribonucleic acid synthesis. J Bacteriol. 1975 May;122(2):393–400. doi: 10.1128/jb.122.2.393-400.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Rothman-Denes L. B., Epstein W. A convenient erythrocyte membrane cyclic AMP binding assay. Anal Biochem. 1975 Sep;68(1):202–208. doi: 10.1016/0003-2697(75)90695-8. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. L. Metabolic basis of phagocytic activity. Physiol Rev. 1962 Jan;42:143–168. doi: 10.1152/physrev.1962.42.1.143. [DOI] [PubMed] [Google Scholar]
- Kordová N., Poffenroth L., Wilt J. C. Lysosomes and the "toxicity" of rickettsiales. 3. Response of L cells infected with egg-attenuated C. psittaci 6BC strain. Can J Microbiol. 1972 Aug;18(8):1343–1348. doi: 10.1139/m72-206. [DOI] [PubMed] [Google Scholar]
- Kordová N., Wilt J. C. Lysosomes and the "toxicity" of Rickettsiales. I. Cytochemical studies of macrophages inoculated in vitro with C. psittaci 6BC. Can J Microbiol. 1972 Apr;18(4):457–464. doi: 10.1139/m72-071. [DOI] [PubMed] [Google Scholar]
- Lawn A. M., Blyth W. A., Taverne J. Interactions of TRIC agents with macrophages and BHK-21 cells observed by electron microscopy. J Hyg (Lond) 1973 Sep;71(3):515–528. doi: 10.1017/s0022172400046507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowrie D. B., Jackett P. S., Ratcliffe N. A. Mycobacterium microti may protect itself from intracellular destruction by releasing cyclic AMP into phagosomes. Nature. 1975 Apr 17;254(5501):600–602. doi: 10.1038/254600a0. [DOI] [PubMed] [Google Scholar]
- MANIRE G. P., MEYER K. F. The toxins of the psittacosis-lymphogranuloma group of agents; effect of aureomycin and penicillin upon the toxins of psittacosis viruses. J Infect Dis. 1950 May-Jun;86(3):233–240. doi: 10.1093/infdis/86.3.233. [DOI] [PubMed] [Google Scholar]
- McLIMANS W. F., DAVIS E. V., GLOVER F. L., RAKE G. W. The submerged culture of mammalian cells; the spinner culture. J Immunol. 1957 Nov;79(5):428–433. [PubMed] [Google Scholar]
- Moulder J. W. Glucose Metabolism of L Cells Before and After Infection with Chlamydia psittaci. J Bacteriol. 1970 Dec;104(3):1189–1196. doi: 10.1128/jb.104.3.1189-1196.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinman R. M., Silver J. M., Cohn Z. A. Pinocytosis in fibroblasts. Quantitative studies in vitro. J Cell Biol. 1974 Dec;63(3):949–969. doi: 10.1083/jcb.63.3.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taverne J., Blyth W. A., Ballard R. C. Interactions of TRIC agents with macrophages: effects on lysosomal enzymes of the cell. J Hyg (Lond) 1974 Apr;72(2):297–309. doi: 10.1017/s0022172400023512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd W. J., Storz J. Ultrastructural cytochemical evidence for the activation of lysosomes in the cytocidal effect of Chlamydia psittaci. Infect Immun. 1975 Sep;12(3):638–646. doi: 10.1128/iai.12.3.638-646.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tribby I. I., Friis R. R., Moulder J. W. Effect of chloramphenicol, rifampicin, and nalidixic acid on Chlamydia psittaci growing in L cells. J Infect Dis. 1973 Feb;127(2):155–163. doi: 10.1093/infdis/127.2.155. [DOI] [PubMed] [Google Scholar]
- Werb Z., Cohn Z. A. Plasma membrane synthesis in the macrophage following phagocytosis of polystyrene latex particles. J Biol Chem. 1972 Apr 25;247(8):2439–2446. [PubMed] [Google Scholar]
- Yavin E., Yavin Z. Attachment and culture of dissociated cells from rat embryo cerebral hemispheres on polylysine-coated surface. J Cell Biol. 1974 Aug;62(2):540–546. doi: 10.1083/jcb.62.2.540. [DOI] [PMC free article] [PubMed] [Google Scholar]