Abstract
Background
A Pediatric Heart Network trial compared outcomes in infants with single right ventricle (RV) anomalies undergoing a Norwood procedure randomized to modified Blalock-Taussig shunt (MBTS) or right ventricle-to-pulmonary artery shunt (RVPAS). Doppler patterns in the neo-aorta and RVPAS may characterize physiologic changes after staged palliations that impact outcomes and RV function.
Methods
Neo-aortic cardiac index (CI), retrograde fraction (RF) in the descending aorta and RVPAS conduit, RVPAS/neo-aortic systolic ejection time (ET) ratio, and systolic/diastolic (S/D) ratio were measured early post-Norwood, prior to stage II palliation and at 14 months. These parameters were compared with transplant-free survival, length of hospital stay, and RV functional indices.
Results
In 529 subjects (mean follow-up of 3.0±2.1 years), neo-aortic CI and descending aortic RF were significantly higher in the MBTS cohort post-Norwood. The RVPAS RF averaged <25% at both interstage intervals. Higher pre-stage II descending aortic RF correlated with lower RV ejection fraction (R=−0.24; p=0.032) at 14 months for the MBTS cohort. Higher post-Norwood CI (5.6 vs. 4.4 L/min/m2; p=0.04) and lower S/D ratio (1.40 vs. 1.68; p=0.01) correlated with better interstage transplant-free survival for the RVPAS cohort. No other Doppler flow patterns correlated with outcomes.
Conclusion
After the Norwood procedure, infants tolerate significant descending aortic RF (MBTS) and conduit RF (RVPAS) with little correlation with clinical outcome or RV function. Neo-aortic CI, ET and S/D ratios also have limited correlation with outcome/RV function, but higher post-Norwood neo-aortic CI and lower S/D ratio correlate with better interstage survival in those with an RVPAS.
Keywords: hypoplastic left heart syndrome, Norwood, echocardiography, single ventricle
INTRODUCTION
Initial surgical palliation for hypoplastic left heart syndrome (HLHS) and other single right ventricular (RV) anatomic variants has evolved to two different strategies that vary based upon the source of pulmonary blood flow—the modified Blalock-Taussig shunt (MBTS)1 or the RV-to-pulmonary artery shunt (RVPAS).2 These surgical strategies result in different physiologic states that impact flow patterns in the reconstructed aorta (neo-aorta).3 In the patient with HLHS and MBTS, all RV cardiac output is exclusively ejected into the neo-aorta before being distributed to the systemic and pulmonary vascular beds; the aortopulmonary shunt allows diastolic “steal” of systemic blood into the pulmonary vascular bed. This is in contrast to the patient with HLHS and an RVPAS, where RV cardiac output is distributed directly to both the systemic vascular bed (through the neo-aorta) and the pulmonary vascular bed (through the RVPAS) during systole. No diastolic steal is present, but an additional volume load is placed on the RV as a result of diastolic retrograde flow from the pulmonary artery back into the RV through the non-valved conduit. Changes in RV systolic and diastolic function, altered systemic and pulmonary vascular resistances, and anatomic resistance to flow into both shunts can impact these neo-aortic and RVPAS flow patterns and are identifiable by Doppler interrogation using echocardiography after initial staged palliation. Specifically, these patterns can estimate neo-aortic cardiac output, antegrade and retrograde flow profiles in the RVPAS and descending aorta (to quantify retrograde fractions through the shunt and neo-aorta arch), and systolic ejection times into the RVPAS and neo-aorta (which should reflect relative resistance to flow into the two vascular beds).4
Currently, there is no single measure that defines RV function by echocardiography. Two-dimensional assessment of RV volumes and ejection fraction is difficult due to the complex geometry of the chamber. The systolic to diastolic duration ratio, as calculated from the tricuspid regurgitation spectral Doppler signal, has been shown to be an indicator of global RV function in children with HLHS, with an increasing ratio correlated with poorer RV function.5 The calculation of the systolic to diastolic duration ratio is made by measuring the systolic duration (from onset to cessation of regurgitant flow from the tricuspid insufficiency jet) and diastolic duration (time when there is no tricuspid insufficiency flow signal). These intervals can also be calculated from spectral Doppler flow patterns in the RVPAS (measuring systolic antegrade and diastolic retrograde time intervals) of infants with HLHS who have undergone the Sano modification for stage I palliation. This new ratio is attractive because it would be available and easily obtained by echocardiography in every infant with an RVPAS; this is in contrast to calculation of the ratio from tricuspid regurgitation, where a measureable Doppler signal is available in only about 80% of infants with HLHS.5
The Pediatric Heart Network Single Ventricle Reconstruction (SVR) trial compared outcomes in 549 infants undergoing a Norwood procedure randomized to either MBTS or RVPAS at 15 North American centers.6 As part of the SVR trial, 2-dimensional and Doppler echocardiographic studies were evaluated by a core laboratory.3 Echocardiographic indices were measured with the purpose of assessing the effect of MBTS versus RVPAS at 4 stages during the trial. Correlation of neo-aortic and RVPAS flow patterns with clinical outcomes in the SVR cohort has not been previously examined. In the present study, we have analyzed Doppler indices of neo-aortic and RVPAS flow to more fully characterize the SVR cohort. We specifically wanted to compare these Doppler flow patterns with rates of transplant-free survival, length of hospital stay, indices of RV size and systolic/global function, and degree of tricuspid regurgitation prior to and following the staged surgical palliations performed during the first 14 months of life.
METHODS
Study Design
The SVR study design7, primary outcome6 (incidence of death or cardiac transplantation at 12 months after randomization) and secondary outcomes6 (morbidity during hospitalizations for the Norwood and stage II procedures, unintended cardiovascular interventions and rate of serious adverse events through 12 months, angiographically-derived pulmonary artery size prior to the stage II procedure), as well as risk factors for mortality and cardiac transplantantion8 have been previously published. Secondary echocardiographic markers of outcome, including indices of RV function, cardiac and vascular dimensions, valve annulus dimensions and function, and neo-aortic flow patterns in survivors of the Norwood procedure, have been previously summarized and were shown to be similar for subjects with MBTS and RVPAS by 14 months of age.3
Echocardiographic Analysis
An echocardiography core laboratory at the Medical College of Wisconsin reviewed 2D/Doppler echocardiograms performed at each clinical center to compare indices between shunt groups at 4 pre-designated time intervals during the study: 1) baseline (prior to the Norwood procedure) at a mean of 2.1 ± 3.4 days of age, 2) post-Norwood (either at time of discharge or at approximately 30 days of age if still hospitalized) at a mean of 22.1 ± 12.6 days of age, 3) pre-stage II (during the pre-operative evaluation for the stage II procedure) at a mean of 4.7 ± 1.5 months of age, and 4) 14 months of age (end of study visit) at a mean of 14.3 ± 1.2 months. Core lab procedures for image analysis and data management have been previously described.3
Five Doppler indices were the focus of this study:
Neo-aortic cardiac index, calculated as stroke volume across the neo-aortic valve: [(neo-aortic velocity time integral) x (neo-aortic valve area)] x heart rate in beats per min indexed to body surface area (BSA) in m2.
Descending aortic retrograde fraction, calculated as percentage of retrograde diastolic flow in the descending aorta, using pulsed wave Doppler from the suprasternal notch window to measure velocity time integrals of antegrade systolic and retrograde diastolic flow in the descending aorta: (diastolic velocity time integral /systolic velocity time integral) × 100.
RVPAS retrograde fraction, calculated as percentage of retrograde diastolic flow in the RVPAS, using pulsed wave Doppler to measure velocity time integrals of antegrade systolic and retrograde diastolic flow within the mid-portion of the shunt: (diastolic velocity time integral /systolic velocity time integral) × 100.
Ratio of RVPAS to neo-aortic ejection times, calculated as the ratio of the durations of systolic flow from the Doppler jets at the mid-portion of the shunt and at the neo-aortic annulus: RVPAS systolic ejection time/neo-aortic systolic ejection time.
Systolic to diastolic duration ratio in subjects with an RVPAS, calculated as the ratio of the systolic duration (from onset to cessation of antegrade flow within the shunt) to diastolic duration (from cessation to onset of antegrade flow within the shunt) obtained from pulsed Doppler tracings in the mid-portion of the RVPAS (Figure 1): systolic duration/diastolic duration.
Figure 1.
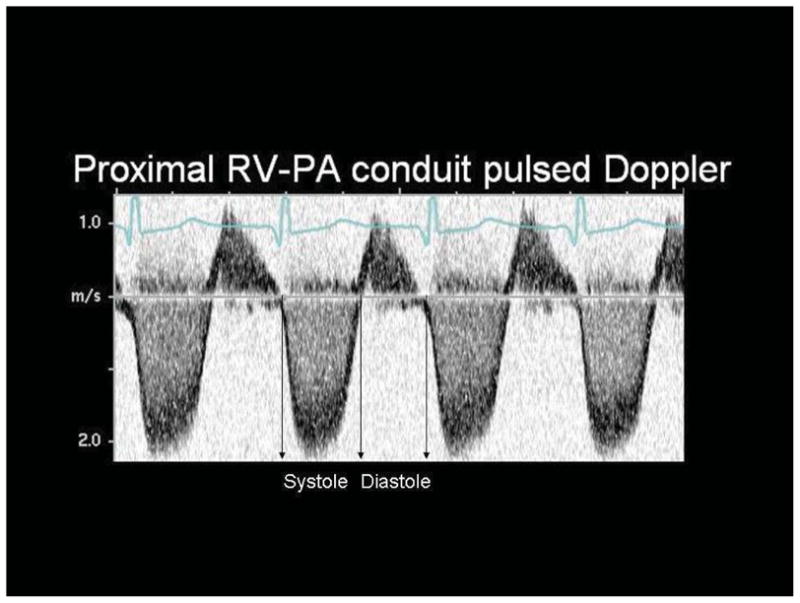
Systolic and diastolic time intervals obtained from flow signals in the mid RV-to-pulmonary artery shunt (RVPAS) with pulsed wave Doppler echocardiography in an infant with hypoplastic left heart syndrome after the Norwood procedure. The systolic duration is defined as the time from onset to cessation of antegrade flow (the flow signal below the baseline) and the diastolic duration as the time from cessation to onset of antegrade flow within the shunt (see arrows).
Outcomes to compare to these Doppler measures include:
-
Transplant-free survival for the following intervals:
Interstage (Norwood discharge to stage II admission).
Baseline and post-Norwood echo studies to stage II admission.
Pre-stage II echo study to stage II discharge.
Stage II discharge to latest follow-up.
Long-term (from the time of each echo study to latest follow-up).
Length of intensive care unit stay after Norwood (for the baseline and post-Norwood echo studies); length of intensive care unit stay after stage II (for the pre-stage II echo).
Length of hospital stay after Norwood (for the baseline and post-Norwood echo studies); length of hospital stay after stage II (for the pre-stage II echo).
Indexed RV end-diastolic and end-systolic volumes, RV ejection fraction and RV fractional area change (calculated as previously described3) from each echo study for the RVPAS and MBTS sub-groups.
Severity of tricuspid regurgitation, graded by color Doppler jet width, creating two groups defined as those with <moderate regurgitation (jet width <2.5 mm) and those with ≥moderate regurgitation (jet width ≥2.5 mm) from each echo study for RVPAS and MBTS sub-groups.
Myocardial performance index (MPI), calculated two ways; from blood flow Doppler and annular Doppler tissue imaging (DTI) measures as previously described,3 from each echo study for RVPAS and MBTS sub-groups.
Statistical Analysis
Summary descriptive statistics of echocardiographic indices are presented (by shunt type in place at the end of the Norwood operation) at each echo interval. Hazard ratios and p-values were calculated from a Cox proportional hazards regression predicting time to death or transplant for each time interval as a function of each Doppler variable. For the six continuous echocardiographic outcomes (right ventricular end diastolic volume indexed to BSA, right ventricular end systolic volume indexed to BSA, right ventricular ejection fraction, right ventricular area change expressed as a percentage, MPI using both the pulsed Doppler and DTI calculations), separate univariate regressions were performed for each combination of the Doppler measure as a predictor of the echocardiographic outcome. For the one dichotomous outcome (at least moderate tricuspid valve regurgitation), separate logistic regressions were performed predicting at least moderate tricuspid valve regurgitation as a function of each of the Doppler measures. Correlations and regression results with a p-value <0.05 were considered significant. All analyses were conducted using SAS version 9.2 (SAS Institute, Inc., Cary, NC).
RESULTS
There were 549 SVR trial subjects; as previously described,6 five additional subjects who did not undergo the Norwood procedure and one subject who withdrew from the trial in week 1 were excluded. A total of 268 infants undergoing a Norwood procedure received the MBTS and 281 infants received the RVPAS; all were included in this study cohort except for 17 subjects who had both shunts in place after the initial Norwood procedure or who crossed over to a different shunt after the initial Norwood procedure. In addition, 3 subjects underwent biventricular repair after the initial Norwood procedure and are also excluded from these analyses.
All available data from the remaining 529 SVR subjects’ baseline, post-Norwood, pre-Stage II and 14 month echocardiograms are included in the study cohort. Fifteen subjects underwent heart transplantation prior to their 14 month visit, and so only echocardiographic data collected prior to the date of heart transplantation are included in these analyses. Mean follow-up for the entire cohort from the time of initial pre-Norwood echocardiogram was 3.0 ± 2.1 years (median 3.9 years). Table 1 summarizes the number of protocol echocardiograms obtained at each stage throughout the trial (the primary reasons for failure to obtain an echocardiogram being death or transplant) and the number of echocardiograms which had at least 1 Doppler measure available for analysis. Table 2 summarizes the mean values for each Doppler measure at each time interval for both shunt groups, and Tables 3 and 4 summarize the correlation between the Doppler measures and transplant-free survival during the different intervals for each shunt group. A brief summary of the findings for each Doppler measure as it relates to RV function and clinical outcomes is described below.
Table 1.
Number of subjects with at least one Doppler measurement available at each echo study interval
| Visit | Echocardiogram | Doppler Assessment |
|---|---|---|
| Pre-Norwood | 529 | 315 |
| Post-Norwood | 468 | 433 |
| Pre-Stage II | 379 | 356 |
Table 2.
Mean values±SD for all Doppler measures at each study interval by shunt type
| MBTS | RVPAS | P-value | |
|---|---|---|---|
|
|
|||
| Pre-Norwood | |||
| Neoaortic cardiac index, L/min/m2 | 9.37 ± 3.69 (n = 166) | 8.67 ± 3.25 (n = 148) | 0.07 |
| Post-Norwood | |||
| Neoaortic cardiac index, L/min/m2 | 8.10 ± 2.67 (n = 198) | 4.43 ± 2.04 (n = 190) | <.001 |
| Descending aortic retrograde fraction | 0.45 ± 0.16 (n = 195) | 0.04 ± 0.12 (n = 187) | <.001 |
| RVPAS retrograde fraction | N/A | 0.23 ± 0.08 (n = 161) | - |
| Ratio of RVPAS to neoaortic ejection times | N/A | 1.49 ± 0.20 (n = 155) | - |
| Systolic/diastolic duration ratio | N/A | 1.41 ± 0.34 (n = 170) | - |
| Pre-Stage 2 | |||
| Neoaortic cardiac index, L/min/m2 | 9.42 ± 3.10 (n = 145) | 6.15 ± 2.37 (n = 171) | <.001 |
| Descending aortic retrograde fraction | 0.41 ± 0.19 (n = 129) | 0.06 ± 0.14 (n = 158) | <.001 |
| RVPAS retrograde fraction | N/A | 0.20 ± 0.07 (n = 126) | - |
| Ratio of RVPAS to neoaortic ejection times | N/A | 1.43 ± 0.17 (n = 126) | - |
| Systolic/diastolic duration ratio | N/A | 1.64 ± 0.55 (n = 134) | - |
Table 3.
Correlation of Doppler measures (mean±SD) at each study interval with interstage survival, survival to stage II discharge, and long-term survival for the MBTS cohort
| Interstage death/heart transplant | Death/heart transplant from date of echo to stage II surgery | Long-term death/heart transplant from date of echo | ||||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | |
| Pre-Norwood Predictors: | ||||||
| Neoaortic cardiac index, L/min/m2 | 9.17±4.32 (n=22) | 9.54±3.78 (n=112) | 8.93±3.69 (n=47) | 9.55±3.69 (n=119) | 9.05±3.49 (n=58) | 9.55±3.80 (n=108) |
| HR=0.97 P=0.58 | HR=0.96 P=0.28 | HR=0.97 P=0.37 | ||||
| Post-Norwood Predictors: | ||||||
| Neoaortic cardiac index, L/min/m2 | 7.55±2.02 (n=39) | 8.24±2.73 (n=136) | 7.71±2.40 (n=53) | 8.24±2.76 (n=145) | 7.91±2.76 (n=68) | 8.20±2.63 (n=130) |
| HR=0.91 P=0.15 | HR=0.93 P=0.22 | HR=0.96 P=0.36 | ||||
| Descend aortic retrograde fraction | 0.43±0.16 (n=38) | 0.45±0.14 (n=135) | 0.46±0.21 (n=50) | 0.45±0.15 (n=145) | 0.45±0.19 (n=66) | 0.45±0.15 (n=129) |
| HR=0.54 P=0.59 | HR=1.58 P=0.61 | HR=1.21 P=0.81 | ||||
| Death/heart transplant from date of echo to stage II discharge | Long-term death/heart transplant from date of stage II discharge | Long-term death/heart transplant from date of echo | ||||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | |
| Pre-Stage II Predictors: | ||||||
| Neoaortic cardiac index, L/min/m2 | 6.43±1.18 (n=5) | 9.53±3.10 (n=140) | 9.28±2.16 (n=9) | 9.54±3.16 (n=131) | 8.26±2.30 (n=14) | 9.54±3.16 (n=131) |
| HR=0.58 P=0.06 | HR=0.97 P=0.76 | HR=0.86 P=0.12 | ||||
| Descend aortic retrograde fraction | 0.49±0.09 (n=6) | 0.41±0.19 (n=123) | 0.31±0.14 (n=10) | 0.41±0.19 (n=113) | 0.38±0.15 (n=16) | 0.41±0.19 (n=113) |
| HR=13.02 P=0.21 | HR=0.02 P=0.05 | HR=0.32 P=0.43 | ||||
MBTS=modified Blalock Taussig shunt; HR=hazard ratio
Table 4.
Correlation of Doppler measures (mean±SD) at each study interval with interstage survival, survival to stage II discharge, and long-term survival for the RVPAS cohort
| Interstage death/heart transplant | Death/heart transplant from date of echo to stage II surgery | Long-term death/heart transplant from date of echo | ||||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | |
| Pre-Norwood Predictors: | ||||||
| Neoaortic cardiac index, L/min/m2 | 11.21±5.50 (n=4) | 8.79±3.21 (n=120) | 8.00±3.55 (n=25) | 8.81±3.18 (n=123) | 8.34±3.36 (n=44) | 8.81±3.20 (n=104) |
| HR=1.19 P=0.18 | HR=0.92 P=0.24 | HR=0.96 P=0.38 | ||||
| Post-Norwood Predictors: | ||||||
| Neoaortic cardiac index, L/min/m2 | 4.37±1.99 (n=12) | 5.58±2.08 (n=167) | 4.87±2.38 (n=15) | 4.41±2.02 (n=173) | 4.21±1.88 (n=49) | 4.51±2.09 (n=141) |
| HR=1.22 P=0.042 | HR=1.08 P=0.43 | HR=0.95 P=0.52 | ||||
| Descend aortic retrograde fraction | 0.00±0.00 (n=12) | 0.04±0.12 (n=162) | 0.00±0.00 (n=16) | 0.04±0.12 (n=169) | 0.04±0.11 (n=49) | 0.04±0.12 (n=138) |
| HR=0.00 P=0.99 | HR=0.00 P=0.99 | HR=1.29 P=0.83 | ||||
| RVPAS retrograde fraction | 0.22±0.09 (n=9) | 0.23±0.08 (n=140) | 0.25±0.09 (n=13) | 0.23±0.08 (n=146) | 0.24±0.07 (n=43) | 0.23±0.08 (n=118) |
| HR=2.38 P=0.83 | HR=37.22 P=0.25 | HR=5.24 P=0.36 | ||||
| Ratio of RVPAS to neoaortic ejection time | 1.56±0.12 (n=9) | 1.48±0.19 (n=135) | 1.58±0.18 (n=13) | 1.48±0.19 (n=140) | 1.50±0.18 (n=42) | 1.49±0.20 (n=113) |
| HR=4.68 P=0.36 | HR=4.63 P=0.25 | HR=1.27 P=0.75 | ||||
| Systolic/diastolic time ratio | 1.68±0.20 (n=9) | 1.40±0.33 (n=149) | 1.56±0.31 (n=13) | 1.40±0.34 (n=155) | 1.45±0.30 (n=44) | 1.40±0.35 (n=126) |
| HR=10.22 P=0.010 | HR=2.90 P=0.16 | HR=1.42 P=0.41 | ||||
| Death/heart transplant from date of echo to stage II discharge | Long-term death/heart transplant from date of stage II discharge | Long-term death/heart transplant from date of echo | ||||
|---|---|---|---|---|---|---|
| Yes | No | Yes | No | Yes | No | |
| Pre-Stage II Predictors: | ||||||
| Neoaortic cardiac index, L/min/m2 | 6.49±1.40 (n=6) | 6.13±2.40 (n=165) | 5.67±2.27 (n=28) | 6.23±2.43 (n=137) | 5.82±2.15 (n=34) | 6.23±2.43 (n=137) |
| HR=1.01 P=0.97 | HR=0.91 P=0.32 | HR=0.94 P=0.44 | ||||
| Descend aortic retrograde fraction | 0.00±0.00 (n=6) | 0.06±0.14 (n=152) | 0.08±0.14 (n=23) | 0.06±0.14 (n=129) | 0.06±0.13 (n=29) | 0.06±0.14 (n=129) |
| HR=0.00 P > .99 | HR=2.40 P=0.48 | HR=1.30 P=0.83 | ||||
| RVPAS retrograde fraction | 0.22±0.06 (n=5) | 0.20±0.07 (n=121) | 0.20±0.07 (n=16) | 0.20±0.08 (n=105) | 0.20±0.06 (n=21) | 0.20±0.08 (n=105) |
| HR=76.37 P=0.49 | HR=1.09 P=0.98 | HR=2.87 P=0.72 | ||||
| Ratio of RVPAS to neoaortic ejection time | 1.53±0.05 (n=4) | 1.43±0.17 (n=122) | 1.44±0.14 (n=18) | 1.42±0.18 (n=104) | 1.46±0.13 (n=22) | 1.42±0.18 (n=104) |
| HR=37.33 P=0.19 | HR=1.66 P=0.69 | HR=2.53 P=0.40 | ||||
| Systolic/diastolic time ratio | 1.58±0.30 (n=5) | 1.64±0.56 (n=129) | 1.62±0.37 (n=19) | 1.64±0.58 (n=110) | 1.61±0.35 (n=24) | 1.64±0.58 (n=110) |
| HR=0.93 P=0.94 | HR=0.97 P=0.95 | HR=0.94 P=0.87 | ||||
Neo-aortic cardiac output
Indexed neo-aortic cardiac output was significantly higher in the MBTS compared to the RVPAS cohort at both interstage intervals (Table 2). In the RVPAS cohort, the 12 subjects who died or required heart transplant before stage II surgery had a lower post-Norwood neo-aortic cardiac index (5.6 vs. 4.4 L/min/m2; hazard ratio 1.22; 95% confidence interval 1.01 – 1.48; p=0.04) than the interstage transplant-free survivors. In addition, a higher pre-stage II neo-aortic cardiac index correlated with increased RV end-diastolic (R=0.25; p=0.007) and end-systolic volume (R=0.25l; p=0.008) at 14 months in the RVPAS cohort. In the MBTS cohort, the 5 patients who died or required heart transplant before discharge following stage II surgery had a trend towards a lower pre-stage II neo-aortic cardiac index (6.4 compared to 9.5 L/min/m2 in the survivors), but this did not reach significance as a predictor of death or heart transplant during this period (hazard ratio 0.58; 95% confidence interval 0.33 – 1.02; p=0.06). No other differences in survival, length of hospital stay, or standard echo indices (RV size, ejection fraction, fractional area change, MPI, or degree of tricuspid regurgitation) correlated with neo-aortic cardiac output for either the RVPAS or MBTS cohort at any interval.
Descending aortic retrograde fraction
The descending aortic retrograde fraction was significantly higher in subjects with a MBTS (45% post-Norwood and 41% pre-stage II) compared to those with a RVPAS (4% post-Norwood and 6% pre-stage II). The descending aortic retrograde fraction was not associated with transplant-free survival or length of stay for subjects with either shunt type at any interval; in fact, the retrograde fraction at the post-Norwood echocardiogram was similar in the MBTS group for survivors and those who died or required transplant during the interstage period (45 vs. 43%), during the interval to stage II surgery (45 vs. 46%), and at latest long term follow-up (45 vs. 45%). For the RVPAS cohort, higher post-Norwood descending aortic retrograde fraction correlated with higher RV end-diastolic volume (R=0.19; p=0.035) at pre-stage II echo studies, and higher pre-stage II descending aortic retrograde fraction correlated with higher RV end-diastolic (R=0.36; p<0.001) and end-systolic volume (R=0.25; p=0.011) at 14 months. The number of RVPAS subjects with a measureable retrograde fraction, however, was small. RV volumes did not correlate with descending aortic retrograde fraction for the MBTS cohort, but a higher pre-stage II retrograde fraction did correlate with lower RV ejection fraction (R=−0.24; p=0.032) at 14 months for that group. No other differences in RV size or functional indices (ejection fraction, fractional area change, MPI, or degree of tricuspid regurgitation) correlated with descending aortic retrograde fraction for either group at any interval.
RVPAS retrograde fraction
The RVPAS retrograde fraction was 23±8% at the post-Norwood study and 20±7% at the pre-stage II study. The RVPAS retrograde fraction did not correlate with transplant-free survival or length of hospital stay for the RVPAS subjects at any interval. Similar to the descending aortic retrograde fraction for the MBTS, the RVPAS retrograde fraction percentage estimated at the post-Norwood echocardiogram was similar for survivors and non-survivors for the intervals from the post-Norwood study to stage II surgery (23 vs. 25%), during the interstage period (23 vs. 22%), and at long term follow-up (23 vs. 24%). RV size and functional indices also did not correlate with RVPAS retrograde fraction except at the post-Norwood study, where a higher fraction correlated with higher MPI as calculated by blood flow Doppler (R=0.22; p=0.015) and lower MPI as calculated by annular DTI (R=−0.3; p=0.027) at the pre-stage II echocardiogram.
RVPAS to neo-aortic ejection time ratio
The RVPAS ejection time was prolonged relative to the neo-aortic ejection time at both the interstage intervals for the RVPAS cohort, with a ratio of 1.49±0.20 at the pre-Norwood study and 1.43±0.17 at the pre-stage II study. The RVPAS to neo-aortic ejection time ratio did not correlate with transplant-free survival, length of hospital stay, or RV size/functional indices for the RVPAS subjects at any interval. Although the ratio tended to be lower in subjects who survived the interstage period to stage II surgery (1.48 vs. 1.58; p=0.25), it was almost identical when compared between the 113 long-term survivors who had this measure and the 42 who died or were transplanted (1.50 vs.1.49; p=0.78).
Systolic to diastolic duration ratio
Systolic duration was prolonged relative to diastolic duration at both the post-Norwood (1.41±0.34) and pre-stage II (1.64±0.55) studies for the RVPAS cohort. A lower post-Norwood systolic to diastolic duration ratio correlated with better interstage transplant-free survival (hazard ratio 10.22; 95% confidence interval 1.76 – 59.27; p=0.01 with a mean value of 1.40 for the survivors compared to 1.68 for those who died or required transplantation during that interval). In addition, a ratio >1.6 at the post-Norwood echocardiogram was associated with significantly increased risk of death or transplantation during the interstage period (hazard ratio 5.42; 95% confidence interval 1.35 – 21.73; p=0.017). This ratio was not a significant predictor of long-term death or need for transplant (1.40 vs. 1.45; hazard ratio 1.42; 95% confidence interval 0.62 – 3.26; p=0.41). No other differences in transplant-free survival, length of hospital stay, or RV size/functional indices correlated with the systolic to diastolic duration ratio for the RVPAS subjects at the post-Norwood or pre-stage II intervals.
DISCUSSION
This is the first study to characterize neo-aortic and RVPAS Doppler flow patterns and to assess their impact on clinical outcomes and RV function in a large cohort of infants with single RV anomalies after Norwood palliation during the first year of life. The physiology that results after the Norwood procedure presents unique hemodynamic burdens to the single RV that vary by the type of shunt used to supply pulmonary blood flow. These burdens have the potential to impact indices of RV function, as well as patient recovery and survival after surgery. A previous SVR publication3 reported that echocardiographic indices of cardiac size and function after the Norwood procedure were similar between groups for the MBTS and RVPAS survivors by 14 months of age. The few interstage differences when the shunt was in place (smaller neo-aortic annulus dimensions, shorter neo-aortic systolic ejection times, lower neo-aortic cardiac index, decreased peak aortic arch velocities and increased Doppler-calculated MPI in the RVPAS compared to the MBTS) resolved by 14 months with removal of the shunt after stage II palliation. The present analysis specifically focused on Doppler-derived quantitative measures of neo-aortic cardiac output, volume of retrograde diastolic flow in the descending aorta, volume of retrograde flow in the RVPAS, and ratios of duration of flow into the RVPAS and neo-aorta, as well as RV systolic to diastolic duration. We will discuss findings for each of the Doppler measures in more detail below.
Neo-aortic cardiac output
Flow across the neo-aortic valve should reflect systemic output in patients after the Norwood procedure with an RVPAS and total RV output after the Norwood procedure with a MBTS, thus it is not surprising that we found significantly lower indexed neo-aortic output in the RVPAS compared to the MBTS cohort at both interstage intervals. This difference has been previously described for the SVR cohort.3 We also identified better interstage transplant-free survival for the RVPAS subjects with higher post-Norwood output, and better stage II surgical survival to discharge for the MBTS subjects with higher pre-stage II output, although the numbers of non-survivors was small. In addition, significantly increased RV volumes at 14 months in those RVPAS subjects was observed in association with increased neo-aortic cardiac output at the pre-stage II echocardiogram, but that correlation is difficult to explain from better systemic output alone. It seems more likely that this dilatation is secondary to additional chronic volume-loading abnormalities that require higher RV output, such as development of aortopulmonary collaterals (which were not quantified in this study).
For most of the variables, echocardiographic estimates of neo-aortic cardiac output appear to have limited correlation with clinical outcomes (length of hospital stay or transplant-free survival) or echocardiographic indices of RV size/function and tricuspid valve function at any stage or with either shunt type. The lack of correlation between echo-estimated neo-aortic cardiac output and clinical outcomes/RV functional status emphasizes the influence of other factors8 (gestational age, presence of obstructed pulmonary venous return, presence of genetic syndromes, HLHS anatomic subtype, shunt patency) that impact outcome. Importantly, estimates of cardiac output by echocardiography can be unreliable because of errors in technique (requiring accurate estimates of neo-aortic valve cross-sectional area and laminar flow measured at the valve annulus). These errors likely led to the high-volume neo-aortic flow found in this study, which exceed the expected neo-aortic cardiac output for this patient population. This error may be exaggerated when assessing flow through the pulmonary (neo-aortic) valve because of dynamically changing annular size during systole compared to assessment through the native aortic valve.9
Descending aortic retrograde fraction
The MBTS results in continuous runoff from the aorta into the pulmonary arteries after the Norwood procedure,10 and this physiology was reflected in the significant descending aortic retrograde diastolic flow seen in the MBTS cohort in this study. Although increased retrograde flow could result in more RV volume overload and potentially lower systemic (and coronary) diastolic pressure, echocardiographic estimates of retrograde descending aortic flow had limited correlation with clinical outcomes or echocardiographic indices of RV size and function in this group of patients. This is surprising, as the MBTS cohort would seem to be at highest risk for adverse events related to the diastolic steal. Altered coronary perfusion secondary to diastolic steal may help explain the decreased RV ejection fraction found at 14 months in those MBTS subjects with higher descending aortic retrograde flow. The overall lack of correlation between descending aortic retrograde flow and RV functional status/clinical outcomes again emphasizes the multiple factors that contribute to the RV hemodynamic burden after Norwood with a MBTS. As expected, significant retrograde flow was unusual in the RVPAS cohort.
RV-PA conduit retrograde fraction
The diastolic retrograde flow through the RVPAS places an additional volume load on the single RV in infants after the Norwood procedure; an increased retrograde fraction could potentially impact RV size and function as well as clinical outcome. In the SVR cohort, we found no correlation between the Doppler-derived RVPAS retrograde fraction and any index of RV size or function; in addition, adverse clinical outcomes did not correlate with the degree of retrograde flow estimated by Doppler. The predicted retrograde fraction for the group generally ranged from 20–25%, suggesting a mild degree of conduit regurgitation despite the absence of a valved conduit. This may help explain our previously published finding from the SVR cohort that identified smaller RV size in the RVPAS cohort compared to the MBTS cohort,3 as the impact of the RVPAS retrograde flow appears to be minimal when the shunt is in place. Better early survival, both interstage11 and at 14 months,6 for the RVPAS group would also suggest that conduit retrograde flow has little negative impact on outcome during the first year of life.
RVPAS to neo-aortic ejection time ratio
Doppler patterns that compare the duration of flow into the systemic and pulmonary beds from a single ventricle should be influenced by the relative resistance to flow, with expected longer duration of systolic flow into the lower resistance pulmonary outflow (RVPAS) compared to the systemic outflow (neo-aorta). The lower resistance allows earlier initiation of flow during the “pre-ejection” isovolumic contraction phase and prolonged duration of flow into the RVPAS. This physiology of prolonged flow into the RVPAS compared to the neo-aorta was confirmed in this study, with mean RVPAS to neo-aortic ejection time ratios at both the post-Norwood and pre-stage II echocardiograms of 1.49 and 1.43, respectively.
Abnormal systolic time intervals (characterized by prolonged pre-ejection and shortened outflow ejection times) have been associated with pulmonary vascular disease (when assessing pulmonary systolic flow12) and poorer outcomes (when assessing aortic systolic flow13,14) in adults with acquired left ventricular dysfunction. Similarly, infants with elevated systemic vascular resistance and/or decreased RV systolic function after Norwood would also be expected to have abnormal systolic intervals with shorter neo-aortic systolic flow duration relative to pulmonary flow duration. Since this physiology suggests altered RV contractility and/or an unbalanced resistance to flow, it would be expected that a higher RVPAS to neo-aortic ejection time ratio would predict worse outcomes and poorer indices of RV function in our cohort. The fact that we did not find these correlations suggests that this ratio does not directly reflect the relative flow distribution or RV contractility, and that variations in ejection times are likely dynamic and can be influenced by other factors (like elevated pulmonary resistance and ventricular activation pattern) as well as conditions imposed by the echo study itself (such as level of sedation).
Systolic to diastolic duration ratios
The RV systolic to diastolic duration ratio has been found to correlate with indices of ventricular function and differentiate normal children from those with restrictive cardiomyopathy,15 dilated cardiomyopathy,16,17 and pulmonary hypertension.18 In addition, this ratio was predictive of outcome in children with pulmonary hypertension.18 In children with HLHS during staged palliations, the systolic to diastolic duration ratio was significantly increased compared to normal children (1.65 vs. 0.85)5, and was significantly higher in those patients with qualitatively decreased RV function compared to those with normal estimated function. Our study assessed the systolic to diastolic duration ratio using spectral Doppler interrogation of the RVPAS flow. This technique is attractive because the flow pattern in the RVPAS is easily and consistently obtained by echocardiography in all patients. Using this technique, we found that the systolic to diastolic ratio was significantly elevated at the post-Norwood (mean 1.41) and pre-stage II (mean 1.64) echocardiograms compared to published values for normal children (0.95).19 In addition, this ratio was prolonged in those subjects who did not survive the interstage period, with a ratio >1.6 at the post-Norwood echocardiogram predicting a significantly increased risk of death or transplantation during the interstage period. While long-term survival, length of hospital stay, and indices of RV function did not correlate with this index, the systolic to diastolic duration ratio may help the clinician identify high-risk infants during the interstage period.
LIMITATIONS
Careful training in protocol image acquisition was provided and reinforced at all clinical sites in this multi-institutional trial to optimize appropriate image capture. However, the more innovative measurements needed to assess the echo-Doppler patterns reviewed in the present study were not possible in all the subjects at each echocardiographic study because of incomplete or inadequate image acquisition. The impact of this limitation in the characterization of neo-aortic and RVPAS flow patterns for the SVR cohort is unclear but emphasizes the challenge of multi-institutional echocardiographic trials that require extensive data acquisition, particularly when obtaining those data from infants with complex heart disease.
Doppler-derived estimates of RVPAS flow, although obtained by pulsed Doppler in the mid-conduit (so that the cross-sectional area where the flow is sampled could be assumed to be stable), are likely affected by the varying degrees of turbulence during systole and diastole within the restrictive shunt. Finally, multiple comparisons between variables, as performed in this analysis, increase the likelihood that differences will be found between groups by chance, and so statistical significance at a p value of 0.05 may overestimate relevant correlations.
CONCLUSIONS
This is the first study to characterize neo-aortic and RVPAS flow patterns in a large group of infants with single right ventricle anomalies after initial staged palliations and correlate those patterns with clinical outcome and echocardiographic indices of RV functional status. After the Norwood procedure, infants appear to tolerate significant descending aortic retrograde flow (in those with a MBTS) and conduit retrograde flow (in those with an RVPAS) while the shunt is in place with little correlation between the Doppler-estimated retrograde fraction and transplant-free survival up to 3 years of age, duration of hospital stay, or RV/tricuspid valve function during the first year of life. Doppler estimates of neo-aortic cardiac index and the ratio of RVPAS to neo-aortic ejection times also had limited clinical correlation with outcome or RV functional indices. However, a lower neo-aortic cardiac output and higher systolic to diastolic duration ratio at the post-Norwood echocardiogram correlated with poorer interstage transplant-free survival in those with an RVPAS and may provide clinicians with additional non-invasive tools to help identify patients at risk.
Acknowledgments
Funding Sources
Supported by U01 grants from the National Heart, Lung, and Blood Institute (HL068269, HL068270, HL068279, HL068281, HL068285, HL068292, HL068290, HL068288, HL085057, HL109781, HL109737). The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute
Abbreviations
- HLHS
Hypoplastic left heart syndrome
- RV
right ventricle
- MBTS
modified Blalock-Taussig shunt
- RVPAS
right ventricle-to-pulmonary artery shunt
- SVR
Single Ventricle Reconstruction
- DTI
Doppler tissue imaging
- MPI
myocardial performance index
Footnotes
Clinical Trial Registration #: NCT00115934
Disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Peter C Frommelt, Medical College of Wisconsin, Milwaukee, WI.
Eric Gerstenberger, New England Research Institute, Watertown, MA.
Jeanne Baffa, Alfred I. DuPont Hospital for Children, Wilmington, DE.
William L Border, Emory University School of Medicine, Atlanta, GA.
Tim J Bradley, Hospital for Sick Children, Toronto, ON.
Steven Colan, Children’s Hospital of Boston, Boston, MA.
Jessica Gorentz, Medical College of Wisconsin, Milwaukee, WI.
Haleh Heydarian, Cincinnati Children’s Hospital, Cincinnati, OH.
J Blaine John, Congenital Heart Institute of Florida/Pediatrix, Tampa, FL.
Wyman W Lai, Columbia University Medical Center, New York, NY.
Jami Levine, Boston Children’s Hospital, Boston, MA.
Jimmy C Lu, University of Michigan, Ann Arbor, MI.
Rachel T. McCandless, University of Utah.
Stephen Miller, Duke University, Durham, NC.
Arni Nutting, Medical University of South Carolina, Charleston, SC.
Richard G Ohye, University of Michigan, Ann Arbor, MI.
Gail D. Pearson, National Heart, Lung, and Blood Institute/NIH, Bethesda, MD.
Pierre C Wong, Children’s Hospital of Los Angeles, CA.
Meryl S Cohen, The Children’s Hospital of Philadelphia, Philadelphia, PA.
References
- 1.Norwood WI, Lang P, Hansen DD. Physiologic repair of aortic atresia-hypoplastic left heart syndrome. N Engl J Med. 1983;308:23–6. doi: 10.1056/NEJM198301063080106. [DOI] [PubMed] [Google Scholar]
- 2.Sano S, Ishino K, Kawada M, Arai S, Kasahara S, Asai T, et al. Right ventricle-pulmonary artery shunt in first-stage palliation of hypoplastic left heart syndrome. J Thorac Cardiovasc Surg. 2003;126:504–10. doi: 10.1016/s0022-5223(02)73575-7. [DOI] [PubMed] [Google Scholar]
- 3.Frommelt PC, Guey LL, Minich LL, Bhat M, Bradley TJ, Colan SD, et al. Does Initial Shunt Type for the Norwood Procedure Impact Echocardiographic Measures of Cardiac Size and Function during Infancy? The Single Ventricle Reconstruction Trial. Circulation. 2012;125:2630–2638. doi: 10.1161/CIRCULATIONAHA.111.072694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Frommelt PC, Sheridan DC, Mussatto KA, Hoffman GM, Ghanayem NS, Frommelt MA, Tweddell JS. Effect of shunt type on echo indices after initial palliations for hypoplastic left heart syndrome: BT shunt vs. RV-PA conduit. J Am Soc Echocardiogr. 2007;20:1364–73. doi: 10.1016/j.echo.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 5.Friedberg M, Silverman N. The systolic to diastolic duration ratio in children with Hypoplastic left heart syndrome: a novel Doppler index of right ventricular function. J Am Soc Echocardiogr. 2007;20:749–755. doi: 10.1016/j.echo.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 6.Ohye RG, Sleeper LA, Mahony L, Newburger JW, Pearson GD, Lu M, et al. Comparison of shunt types in the Norwood procedure for single-ventricle lesions. N Engl J Med. 2010;362(21):1980–92. doi: 10.1056/NEJMoa0912461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mahony L, LA, Sleeper LA, Anderson PA, Gersony WM, McCrindle BW, Minich LL, et al. The Pediatric Heart Network: a primer for the conduct of multicenter studies in children with congenital and acquired heart disease. Pediatr Cardiol. 2006;27:191–198. doi: 10.1007/s00246-005-1151-9. [DOI] [PubMed] [Google Scholar]
- 8.Tweddell JS, Sleeper LA, Ohye RG, Williams IA, Mahony L, Pizarro C, et al. Intermediate-term mortality and cardiac transplantation in infants with single-ventricle lesions: Risk factors and their interaction with shunt type. J Thorac Cardiovasc Surg. 2012;144:152–159. doi: 10.1016/j.jtcvs.2012.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stewart WJ, Jiang L, Mich R, Pandian N, Guerrero JL, Weyman AE. Variable effects of changes in flow rate through the aortic, pulmonary and mitral valves on valve area and flow velocity: impact on quantitative Doppler flow calculations. J Am Coll Cardiol. 1985;6(3):653–62. doi: 10.1016/s0735-1097(85)80127-3. [DOI] [PubMed] [Google Scholar]
- 10.Rychik J, Bush DM, Spray TL, Gaynor JW, Wernovsky G. Assessment of pulmonary/systemic blood flow ratio after first-stage palliation for hypoplastic left heart syndrome: development of a new index with the use of Doppler echocardiography. J Thorac Cardiovasc Surg. 2000;120:81–87. doi: 10.1067/mtc.2000.106840. [DOI] [PubMed] [Google Scholar]
- 11.Ghanayem NS, Allen KR, Tabbutt S, Atz AM, Clabby ML, Cooper DS, et al. Interstage mortality after the Norwood procedure: Results of the multicenter Single Ventricle Reconstruction trial. J Thorac Cardiovasc Surg. 2012;144(4):896–906. doi: 10.1016/j.jtcvs.2012.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scapellato F, Temporelli PL, Eleuteri E, Corra U, Imparato A, Giannuzzi P. Accurate noninvasive estimation of pulmonary vascular resistance by Doppler echocardiography in patients with chronic failure heart failure. J Am Coll Cardiol. 2001;37(7):1813–9. doi: 10.1016/s0735-1097(01)01271-2. [DOI] [PubMed] [Google Scholar]
- 13.Garrard CL, Jr, Weissler AM, Dodge HT. The relationship of alterations in systolic time intervals to ejection fraction in patients with cardiac disease. Circulation. 1970;42:455–462. doi: 10.1161/01.cir.42.3.455. [DOI] [PubMed] [Google Scholar]
- 14.Chao TF, Sung SH, Cheng HM, Yu WC, Wang KL, Huang CM, Chen CH. Electromechanical activation time in the prediction of discharge outcomes in patients hospitalized with acute heart failure syndrome. Internal Medicine. 2010;49(19):2031–7. doi: 10.2169/internalmedicine.49.3944. [DOI] [PubMed] [Google Scholar]
- 15.Friedberg MK, Silverman NH. The systolic to diastolic duration ratio in children with heart failure secondary to restrictive cardiomyopathy. J Am Soc Echocardiogr. 2006;19:1326–1331. doi: 10.1016/j.echo.2006.05.024. [DOI] [PubMed] [Google Scholar]
- 16.Friedberg MK, Silverman NH. Cardiac ventricular diastolic and systolic duration in children with heart failure secondary to idiopathic dilated cardiomyopathy. Am J Cardiol. 2006;97:101–105. doi: 10.1016/j.amjcard.2005.07.127. [DOI] [PubMed] [Google Scholar]
- 17.Patel DR, Cui W, Gambetta K, Roberson DA. A comparison of Tei index versus systolic to diastolic ratio to detect left ventricular dysfunction in pediatric patients. J Am Soc Echocardiogr. 2009;2:152–158. doi: 10.1016/j.echo.2008.11.021. [DOI] [PubMed] [Google Scholar]
- 18.Alkon J, Humpl T, Manlhiot C, McCrindle BW, Reyes JT, Friedberg MK. Usefulness of the right ventricular systolic to diastolic duration ratio to predict functional capacity and survival in children with pulmonary arterial hypertension. Am J Cardiol. 2010;106(3):430–6. doi: 10.1016/j.amjcard.2010.03.048. [DOI] [PubMed] [Google Scholar]
- 19.Sarnari R, Kamal RY, Friedberg MK, Silverman NH. Doppler assessment of the ratio of the systolic to diastolic duration in normal children: relation to heart rate, age and body surface area. J Am Soc Echocardiogr. 2009;22:928–932. doi: 10.1016/j.echo.2009.05.004. [DOI] [PubMed] [Google Scholar]


