Abstract
Background
Fasting during the month of Ramadan is a religious obligation for Muslims who represent 20% of the world population. This study explores the safety of fasting for a whole month among patients with chronic kidney disease (CKD) with the possible risk of dehydration and hyperviscosity leading to deterioration of kidney functions and vascular thrombosis.
Methods
We followed CKD patients with stable kidney function who chose to fast during the month of Ramadan. A group of nonfasting CKD patients served as controls. Serum creatinine was recorded at the beginning of the month, after 1 week of fasting, at the end of the month and 3 months later. Patients were followed for major adverse cardiovascular events (MACE).
Results
A total of 131 CKD patients were recruited and included in two groups: fasting and nonfasting (mean baseline estimated glomerular filtration rate 27.7, SD 13 and 21.5, SD 11.8 mL/min/1.73 m2, respectively). A rise of serum creatinine was noted during fasting in 60.4% of patients by Day 7 and was associated with intake of renin angiotensin aldosterone system antagonists [relative risk (RR) 2, P = 0.002]. Adverse cardiovascular events were observed in six patients in the fasting cohort and were associated with a rise of serum creatinine after 1 week of fasting (P = 0.009) and the presence of pre-existing cardiovascular disease (RR 15, P = 0.001); the latter association was confirmed by logistic regression analysis. Only one event was recorded in the nonfasting group, P = 0.036.
Conclusions
MACE occurred more frequently among fasting CKD patients with pre-existing cardiovascular disease and were predicted by an early rise of serum creatinine.
Keywords: acute kidney injury, chronic kidney disease, fasting, myocardial infarction
Introduction
Every year, nephrologists around the world have to face their socio-medical duties towards their Muslim chronic kidney disease (CKD) patients who wish to fulfill their religious obligation of fasting during the month of Ramadan. Muslims form almost 20% of the world population and inhabit all corners of the globe, thus clinicians need to be familiar with this spiritual obligation as well as its impact on health [1, 2]. During their fast, Muslims are expected to avoid drinking and eating from dawn till sunset every day for 1 lunar month. According to Islamic religion, sick people are frankly exempted from fasting if a ‘well-informed’ physician decides that fasting may adversely affect their health. This bases the patient's decision to fast or not to fast on the physician's recommendation rather than on a religious scholar's advice [2, 3]. However, as suggested by epidemiological studies on diabetics, most patients insist on fasting against medical advice [4].
Ramadan is the ninth month of the lunar year (Hijri) year which is 11 days shorter than the solar year causing the month to rotate around all four seasons once every 33 years. This has resulted in Ramadan crawling toward the hot weather of early autumn and summer over the past few years. In 2012, Ramadan coincided with July; in Cairo, this meant that daily fasting continued for 16 h at an average daytime temperature of 35°C, sometimes reaching 43°C [5–9]. This exposes patients to the risks of dehydration and hyperviscosity predisposing to further kidney injury and thrombosis of their calcified blood vessels [2, 10].
Very little is known about the safety of exposing CKD patients to fasting under such climatic conditions. Exceptionally few studies have reported on this, and those that have, included very small numbers of patients, selected exceptionally fit patients with minimal comorbidities, did not include a matched nonfasting control group and most of all were performed in the early 2000s when Ramadan occurred in late autumn and winter (shorter duration of fasting and lower temperatures). These studies suggested that changes in kidney function during fasting were insignificant or even improved by the end of the month. Moreover, previous studies did not report on medium or long-term changes in kidney function following fasting for the month of Ramadan, the factors associated with changes in kidney function or the cardiovascular outcomes of fasting among these patients with high cardiovascular risk [5, 11].
In this study, we report on a cohort of ‘real-life’ CKD patients who chose to fast during the month of Ramadan in summer of the past 3 years (without excluding patients with comorbidities if they insisted to fast after explaining the possible hazards). We monitored their kidney functions and cardiovascular outcomes and compared them with a control group of nonfasting CKD patients.
Materials and methods
All patients with CKD who presented to a nephrology clinic in a tertiary referral center during the month preceding the month of Ramadan of Hijri years 1530–2 (2009–11) were asked to participate in the study. Patients were only included if they agreed to be enrolled and had records of stable serum creatinine (defined as fluctuations of <10% in at least three readings during the 3 months prior to the month of Ramadan). Patients with evidence of acute cardiovascular disease or active infection as well as patients on dialysis and kidney transplant recipients were excluded.
Patients who chose to fast were clearly warned about theoretical possibilities of deterioration of kidney functions and advised to avoid excessive exposure to heat or overexertion in order to avoid dehydration. Patients were urged to monitor their serum creatinine regularly and report the results to us; they were strictly advised to stop fasting if the serum creatinine rose by >10% during follow-up. Patients who chose not to fast were included as a control group. Local research ethics committee approval was obtained.
Clinical and demographic data were collected. Changes in kidney function during and after the month of Ramadan were monitored: patients were instructed to attend the laboratory on four appointments set 2–4 h after breakfast to measure serum creatinine (i.e. after sunset for samples drawn during fasting in Ramadan): (i) Day 0 = within the 5 days preceding the month of Ramadan, (ii) Day 7 = after 1 week of fasting, (iii) Day 30 = within 5 days after the end of the month, (iv) late = 3 months after the end of Ramadan. Glomerular filtration rate was estimated by the abbreviated modification of diet in renal disease equation [12]. As for controls, serum creatinine was monitored on only three occasions (Days 0, 30 and after 3 months). All participants were followed for the occurrence of cardiovascular events viz. stroke, acute peripheral vascular disease (PVD) or acute coronary syndrome. These events were reported only if they were clinically overt and symptomatic then subsequently confirmed by objective investigations: brain imaging for stroke, arterial duplex for PVD, acute coronary syndrome was confirmed by electrocardiogram, cardiac troponin and/or typical chest pain dictating admission to an intensive care unit. Pre-existing cardiovascular disease was defined as stable heart failure, coronary heart disease, stroke or PVD documented at least 3 months before onset of the study. The occurrence of an acute cardiovascular event during the 3 months prior to recruitment was an exclusion criterion from the study. Serum creatinine was measured by a kinetic Jaffe reaction with a coefficient of variation of 4.45% at a mean of 1.6 mg/dL and 2.2% at 4.6 mg/dL (406.6 μmol/L) within run.
Statistical analysis
Statistical analysis was performed for the two patient groups based on their initial classification (intention to treat). Statistical Package for Social Analysis (SPSS) version 7.5 was used for data analysis. Data were summarized as mean, standard deviation and median. Based on the sample distribution and the relationship between different samples, comparisons were performed using Mann–Whitney U-test. Paired t-test was used to compare levels of serum creatinine at different time points to baseline values. Chi-squared test was used for categorical data and Fisher's exact test for cells with less than six categories. Spearman's correlation was used for bivariate analysis of correlation. Positive predictive value (PPV) and relative risk (RR) were calculated and reported when relevant. A P value of ≤0.05 was considered significant.
Results
Initially, we recruited 131 patients who consented to join the study and attended the initial evaluation. Twenty-five (11 from the fasting group and 14 from the nonfasting group) failed to show up on their follow-up dates and were excluded. The final cohort included only those participants who completed their scheduled follow-up appointments; these were 106 patients, 52 in the fasting group and 54 in the control group. The characteristics of both groups are shown in Table 1. Mean baseline estimated glomerular filtration rate (eGFR) in the fasting group was higher than in the nonfasting controls, 27.7 mL/min/1.73 m2 (SD 13) versus 21.5 mL/min/m2 mg% (SD 11.8), respectively, P = 0.008. Patients in the fasting group completed fasting for the whole month in 45 of all 52 instances; 6 stopped fasting after 1 week because of a rise in serum creatinine of >10%, 2 of whom also experienced a cardiovascular event and a seventh patient decided at his own will not to continue fasting after 2 weeks. In other cases with significant renal and/or cardiovascular events, patients insisted to continue fasting for the rest of the month against medical advice. All patients who started in the fasting group, but did not complete fasting for the month for any reason, were included in the final analysis of the fasting group.
Table 1.
Baseline characteristics of study participants
| Fasting | Nonfasting | P-value | |
|---|---|---|---|
| Age; years | 58.4; 12.4 | 60.9; 15.2 | 0.2 |
| Gender: males; no. (%) | 32 (60.4) | 27 (50) | 0.27 |
| Systolic BP | 151; 19.8 | 147; 24.7 | 0.27 |
| Diastolic BP | 85.9; 12.4 | 84; 12.9 | 0.34 |
| Hypertension: no. (%) | 45 (85) | 44 (81.5) | 0.7 |
| Diabetes mellitus: no. (%) | 22 (42.3) | 16 (29.6) | 0.17 |
| Cause of CKD: no. (%) | |||
| Diabetic nephropathy | 21 (40.4) | 16 (29.6) | |
| Hypertensive nephrosclerosis | 7 (13.4) | 12 (22) | |
| Obstructive uropathy | 7 (13.4) | 5 (9.3) | |
| PCKD | 6 (11.5) | 6 (11) | |
| Chronic glomerulonephritis | 6 (11.5) | 5 (9.3) | |
| Unknown | 5 (9.6) | 10 (18.5) | |
| Antihypertensive medications: no. (%) | 45 | 44 | 0.7 |
| Calcium channel blockers | 31 (59.6) | 23 (42.5) | |
| Diuretics | 29 (54.7) | 7 (13.5) | |
| RAAS blockers | 26 (50) | 18 (33.3) | |
| β-Blockers | 22 (42.3) | 10 (19) | |
| α-Blockers | 4 (7.7) | 0 | |
| CVD: no. (%) | 13 (25) | 16 (29.6) | 0.6 |
| CKD stage at baseline: no. (%) | |||
| Stage 3A | 5 | 1 | |
| Stage 3B | 14 | 16 | |
| Stage 4 | 23 | 17 | |
| Stage 5 | 10 | 20 | |
| Baseline creatininea | 2.8; 1.4 (247.5; 123.8) | 3.6; 1.8 (318.2; 159) | 0.012 |
| Baseline eGFR (mL/min/1.73 m2) | 27.7; 13 | 21.5; 11.8 | 0.008 |
Unless otherwise stated, data are reported as: mean; standard deviation. CVD, pre-existing cardiovascular disease; PCKD, polycystic kidney disease.
aFigures are in mg/dL (umol/L in parentheses).
Cardiovascular events
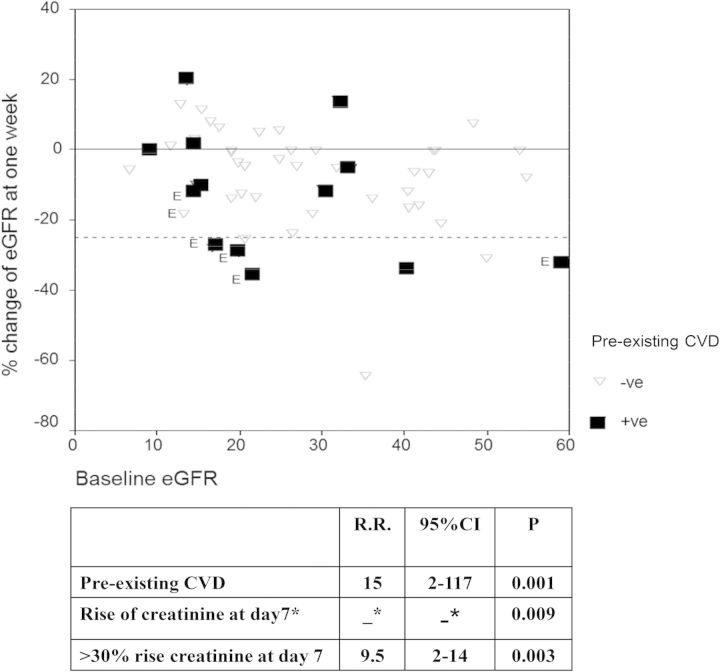
Six adverse cardiovascular events were observed in our fasting cohort during the month of Ramadan compared with only one patient in the control group, P = 0.036. The cardiovascular events in the fasting group included: two cases of acute coronary syndrome, two exacerbations of chronic peripheral vascular disease requiring surgical interference, one case of severe heart failure and one minor nonhemorrhagic stroke. All six events occurred in patients who experienced a rise in serum creatinine after the first week of fasting. In four cases, the rise in serum creatinine exceeded 30% compared with baseline values. Five of the six patients with events also had pre-existing cardiovascular disease (Figure 1).
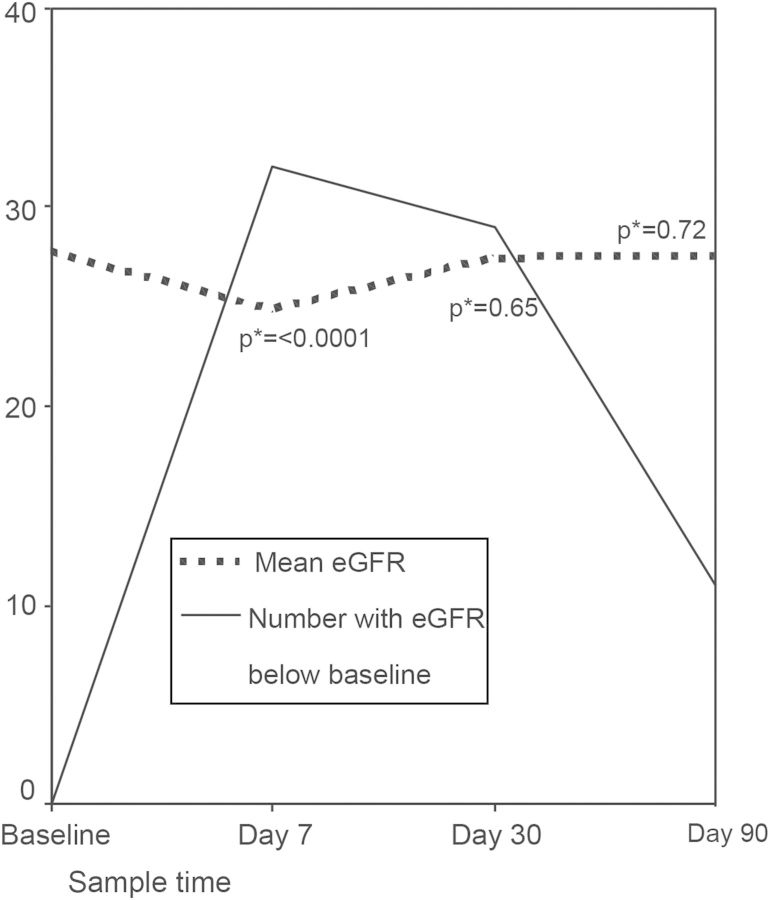
Fig. 1.
Covariates associated with cardiovascular events in the fasting group among patients with and without pre-existing CVD. CVD, cardiovascular disease; E, patients who suffered a cardiovascular event; RR, relative risk. *RR is mathematically ‘infinity’ because all of those suffering events experienced a rise in creatinine at Day 7.
Indeed, the only predictors of cardiovascular events were pre-existing cardiovascular disease and a rise of serum creatinine at Day 7. The sensitivity of any rise of serum creatinine to detect a cardiovascular event was 100% [95% confidence interval (CI) 51–100] with a specificity of 45% (95% CI 31–61) and PPV 19% (95% CI 8–38); P = 0.009. On the other hand, a rise in serum creatinine of ≥30% at Day 7 was more specific 89% (95% CI 75–95) and less sensitive 66.6% (95% CI 24–94) with a PPV of 44% (95% CI 15–77); P = 0.003. The predictive values of pre-existing cardiovascular disease to detect new events were: specificity 83% (95% CI 68–92; sensitivity 83% (95% CI 36–99) and PPV 38% (95% CI 18–64.5); P = 0.001. Age, baseline eGFR, blood pressure, CKD stage and type of antihypertensive medication were not related to the occurrence of these events. Logistic regression confirmed the association of the cardiovascular events with pre-existing cardiovascular disease (P = 0.01), the regression model included age, pre-existing cardiovascular disease, diabetes, initial eGFR and rise of serum creatinine after 1 week.
Serum creatinine remained higher than baseline at the end of the month in five of these six patients, P = 0.04. Serum creatinine did not reach baseline after 3 months of follow-up in three of these patients. One patient had to be put on temporary dialysis for 2 weeks. None of the patients died during the follow-up period.
Changes in serum creatinine
Among the fasting group, serum creatinine increased at Day 7 compared with baseline in 32 instances (60.4%), Figure 2. This corresponded to a mean change in eGFR of −8.5%, SD 15 (median −5.5%, ranging −64.2 to +20), P < 0.0001. The risk was higher in those on renin angiotensin aldosterone system (RAAS) antagonists and those on diuretics but lower in those on calcium channel blockers. The CKD class per se was not a risk factor for the occurrence of a drop in eGFR during fasting (Table 2, Figure 1). The magnitude of change in eGFR did not correlate with alterations in mean blood pressure during the follow-up period, R = 0.2, P = 0.4.
Fig. 2.
Changes of eGFR among fasting patients during the study and number of patients with reductions of eGFR below baseline. *P values refer to comparisons with baseline eGFR.
Table 2.
RR for rise in serum creatinine after the first week of fasting
| Any rise of serum creatinine |
Rise of serum creatinine >30% |
|||||
|---|---|---|---|---|---|---|
| RR | 95% CI | P-value | RR | 95% CI | P-value | |
| RAS blockers | 2 | 1.2–3.5 | 0.002 | 8.3 | 1.1–62 | 0.006 |
| CCB | 0.6 | 0.36–0.9 | 0.01 | 0.5 | 0.13–1.7 | 0.2 |
| Diuretics | 1.6 | 0.95–2.5 | 0.05 | 0.4 | 0.1–1.5 | 0.2 |
| Age >60 years | 1 | 0.6–1.5 | 0.9 | 0.3 | 0.07–1.4 | 0.09 |
| CKD Stage 4 or 5a | 0.7 | 0.2–2.3 | 0.6 | 1.4 | 0.3–6.5 | 0.7 |
| CVD | 1.1 | 0.7–1.8 | 0.6 | 1.8 | 0.5–5.8 | 0.4 |
| Diabetes | 1.2 | 0.7–1.8 | 0.4 | 1.9 | 0.6–6 | 0.3 |
| Gender, male | 1.3 | 0.7–2 | 0.3 | 1.3 | 0.4–4.6 | 0.7 |
| Hypertension | 1.7 | 0.4–6 | 0.3 | 0.6 | 0.2–2.5 | 0.5 |
RAS, renin angiotensin system; CCB, calcium channel blocker; CVD, pre-existing cardiovascular disease.
aCKD Stage 4 and 5 patients compared with Stage 3.
Marked changes in kidney functions in fasting patients, defined as ≥30% rise of serum creatinine, (which corresponded to ≥25% drop of eGFR) occurred in nine instances (17%) at Day 7. The risk was increased by RAAS antagonists but was not associated with other examined factors (Table 2 and Figure 1).
By the end of the month, serum creatinine in the fasting group was higher than baseline in 29 patients (54.7%), but the magnitude of deviation of eGFR from baseline was insignificant, P = 0.5. Marked elevations of serum creatinine, exceeding 30%, were noted at the end of the month in only seven instances (13.2%). Mean deviation from baseline eGFR was −3% (SD 17.8) among fasting compared with −1.3% (SD 24.5) in nonfasting patients, P = 0.9.
Three months after the end of Ramadan, serum creatinine remained elevated in 12 of 52 (23%) patients in the fasting group, not significantly different from controls 19/54 (35%), P = 0.17 (Figure 2). One patient in the fasting group had to be put on temporary dialysis and one patient in the control group was started on permanent dialysis during the period of follow-up.
Discussion
The risk of cardiovascular events is increased among CKD who fasted during the month of Ramadan. Pre-existing cardiovascular disease and early rise in serum creatinine were the main risk factors.
An outstanding point was the determination of most fasting patients to continue fasting against medical advice even defying their religious obligation to avoid fasting if deemed harmful. After 1 week of fasting, 21 of 52 patients suffered a 10% rise in serum creatinine above baseline but only 6 agreed to discontinue fasting; 4 patients continued fasting after experiencing a major cardiovascular event. This is reminiscent of the results of the EPIDIAR study which followed fasting diabetic patients and reported that 43% of Type 1 and 79% of Type 2 diabetics chose to fast during Ramadan despite a relatively high rate of complications [4].
The high rate of cardiovascular events that we found among fasting CKD patients was strongly associated with the presence of pre-existing cardiovascular disease as well as with a rise in serum creatinine after 1 week of fasting. Previous studies on fasting CKD patients excluded those with significant cardiovascular morbidities and did not report on this outcome [5, 11]. It is striking that more events were noted among the fasting patients despite having significantly better kidney functions than the nonfasting patients; this underscores the risk of fasting on cardiovascular events that may supersede the risk of lower kidney functions. Most studies on non-CKD patients reported no increase in cardiovascular events during Ramadan (including studies that investigated patients with pre-existing cardiovascular disease) [13–18], but obviously, CKD patients with cardiovascular disease seem to represent a different model of high-risk individuals. Fasting in hot humid conditions may lead to dehydration and hyperviscosity predisposing to thrombosis of the already diseased vessels of CKD patients which, compared with non-CKD patients, are frequently and markedly calcified [2, 10]. The association of cardiovascular events with deterioration of kidney function comes in agreement with the reported negative impact of acute kidney injury on cardiovascular outcomes in patients with pre-existing cardiac disease [19–21]. A possible argument against our interpretations is that fasting patients had numerically higher systolic blood pressure and more diabetics. This argument is refuted by the fact that these differences were of no statistical significance and, on the contrary, fasting patients (who suffered more cardiovascular events) were younger and had less baseline cardiovascular disease than the nonfasting controls (Table 1). It remains that the main significant difference was that the fasting patients had better kidney functions and despite this suffered worse cardiovascular outcomes.
Serum creatinine increased during the first week of fasting, and mostly returned to baseline by the end of the month; a trend that was not described in previous studies [5, 11]. Average maximum temperatures in Cairo during the months of August and September (during which the study was performed) were 35 and 32°C, respectively, sometimes exceeding 40°C [9]. The initial rise in serum creatinine may be attributed to dehydration and its anticipated effect on renal perfusion which was accentuated by RAAS inhibitors and diuretics. This effect was reversed by the end of the month, possibly by adaptations in renal hemodynamics, tubular function and homeostasis of fluid preservation [22, 23]. Data from this study are not sufficient to confirm the hypothesis that dehydration was the cause of the fluctuations of serum creatinine. Dehydration occurs during fasting at daytime and is reversed after breakfast at sunset. Theses cyclic daily alterations make it cumbersome to conveniently evaluate hydration status and its effects with sufficient confidence in a clinical cohort. However, we can confirm that the variations in kidney function were not accompanied by changes in blood pressure although, needless to say, this does not exclude the presence of dehydration.
This study is the first whistle blower drawing attention to the cardiovascular risk imposed on CKD patients by fasting during the month of Ramadan. The main strength of this study is that it is the largest controlled cohort addressing this demanding question on the safety of fasting for a whole month among CKD patients. It is also the only study reporting on the consequences of fasting during summer time and is the only one including ‘real-life’ CKD patients with frequent comorbidities rather than highly selected healthy patients. The relatively small number of the study is a weakness that needs to be overcome by larger cohort studies. Another point that needs to be taken care of in future studies is to objectively monitor hydration status and correlate it to the reported events e.g. by monitoring body weight changes at day and evening as well as changes in serum creatinine and hematocrit. Interventional studies could provide more definite answers, but it remains practically difficult to address this topic via interventional studies, and we have to rely on follow-up cohorts to provide the answers.
Conclusions
Fasting during the month of Ramadan is hazardous for CKD patients with pre-existing cardiovascular disease as it is associated with a high risk of acute cardiovascular events. Elevation of serum creatinine during the month of fasting is mostly transient, is accentuated by the intake of RAAS blockers and may pose further risk for the occurrence of cardiovascular events.
Conflict of interest statement
The authors have no conflicts of interests to claim regarding this work and have not received external funding to perform this study.
Acknowledgements
We thank Prof. Abdel Meguid El-Nahas, Sheffield Kidney Institute, for the time and effort that he offered to revise the manuscript and provide us with valuable feed-back.
References
- 1.2000. The Canadian Society of Muslims: Muslim population statistics [article online]. http://muslimcanada.org/muslimstats.html. (2012, date last accessed)
- 2.Al-Arouj M, Assaad-Khalil S, Buse J, et al. Recommendations for management of diabetes during Ramadan: update 2010. Diabetes Care. 2010;33:1895–1902. doi: 10.2337/dc10-0896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Surat Al-Bakkarah (Chapter 2), verses 183–5, The Holy Quran.
- 4.Salti I, Benard E, Detournay B, et al. (the EPIDIAR Study Group) A population-based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the Epidemiology of Diabetes and Ramadan 1422/2001 (EPIDIAR) study. Diabetes Care. 2004;27:2306–2311. doi: 10.2337/diacare.27.10.2306. [DOI] [PubMed] [Google Scholar]
- 5.Bernieh B, Al Hakim MR, Boobes Y, et al. Fasting Ramadan in chronic kidney disease patients: clinical and biochemical effects. Saudi J Kidney Dis Transpl. 2010;21:898–902. [PubMed] [Google Scholar]
- 6.Azizi F. Research in islamic fasting and health. Ann Saudi Med. 2002;22:186–191. doi: 10.5144/0256-4947.2002.186. [DOI] [PubMed] [Google Scholar]
- 7.Islamic calendar. Wikepedia. (online). http://en.wikipedia.org/wiki/Islamic_calendar. (2012, date last accessed)
- 8.Ramadan. Wikepedia (online). http://en.wikipedia.org/wiki/Ramadan. (2012, date last accessed)
- 9.BBC weather: Cairo. http://www.bbc.co.uk/weather/360630. (2012, date last accessed)
- 10.Nasrallah MM, El-Shehaby AR, Osman NA, et al. Endogenous soluble receptor of advanced glycation end-products (esRAGE) is negatively associated with vascular calcification in non-diabetic hemodialysis patients. Int Urol Nephrol. 2012;44:1193–1199. doi: 10.1007/s11255-011-0007-x. [DOI] [PubMed] [Google Scholar]
- 11.El-Wakil HS, Desoky I, Lotfy N, et al. Fasting the month of Ramadan by Muslims: could it be injurious to their kidneys? Saudi J Kidney Dis Transpl. 2007;18:349–354. [PubMed] [Google Scholar]
- 12.National Kidney Foundation: K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification and stratification. Am J Kidney Dis. 2002;39(Suppl 1):S1. [PubMed] [Google Scholar]
- 13.Bener A, Hamad A, Fares A, et al. Is there any effect of Ramadan fasting on stroke incidence? Singapore Med J. 2006;47:404–408. [PubMed] [Google Scholar]
- 14.Al Suwaidi J, Zubaid M, Al-Mahmeed WA, et al. Impact of fasting in Ramadan in patients with cardiac disease. Saudi Med J. 2005;26:1579–1583. [PubMed] [Google Scholar]
- 15.Al Suwaidi J, Bener A, Hajar HA, et al. Does hospitalization for congestive heart failure occur more frequently in Ramadan: a population-based study (1991–2001) Int J Cardiol. 2004;96:217–221. doi: 10.1016/j.ijcard.2003.06.018. [DOI] [PubMed] [Google Scholar]
- 16.Akhan G, Kutluhan S, Koyuncuoglu HR. Is there any change of stroke incidence during Ramadan? Acta Neurol Scand. 2000;101:259–261. doi: 10.1034/j.1600-0404.2000.101004259.x. [DOI] [PubMed] [Google Scholar]
- 17.Temizhan A, Dönderici O, Ouz D, et al. Is there any effect of Ramadan fasting on acute coronary heart disease events? Int J Cardiol. 1999;70:149–153. doi: 10.1016/s0167-5273(99)00082-0. [DOI] [PubMed] [Google Scholar]
- 18.Al Suwaidi J, Bener A, Suliman A, et al. A population based study of Ramadan fasting and acute coronary syndromes. Heart. 2004;90:695–696. doi: 10.1136/hrt.2003.012526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Coca SG, Yusuf B, Shlipak MG, et al. Long-term risk of mortality and other adverse outcomes after acute kidney injury: a systematic review and meta-analysis. Am J Kidney Dis. 2009;53:961–973. doi: 10.1053/j.ajkd.2008.11.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lindsay J, Apple S, Pinnow EE, et al. Percutaneous coronary intervention-associated nephropathy foreshadows increased risk of late adverse events in patients with normal baseline serum creatinine. Catheter Cardiovasc Interv. 2003;59:338–343. doi: 10.1002/ccd.10534. [DOI] [PubMed] [Google Scholar]
- 21.Rihal CS, Textor S, Grill DE, et al. Incidence and prognostic importance of acute renal failure after percutaneous coronary intervention. Circulation. 2002;105:2259–2264. doi: 10.1161/01.cir.0000016043.87291.33. [DOI] [PubMed] [Google Scholar]
- 22.Bakris GL, Weir MR. Angiotensinconverting enzyme inhibitor associated elevations in serum creatinine: is this a cause for concern? Arch Intern Med. 2000;160:685–693. doi: 10.1001/archinte.160.5.685. [DOI] [PubMed] [Google Scholar]
- 23.Salim S, Farooq N, Priyamvada S, et al. Influence of Ramadan-type fasting on carbohydrate metabolism, brush border membrane enzymes and phosphate transport in rat kidney used as a model. Br J Nutr. 2007;98:984–990. doi: 10.1017/S0007114507764759. [DOI] [PubMed] [Google Scholar]