Abstract
Purpose
The aim of this study was to evaluate the efficacy and safety of photodynamic therapy (PDT) combined with intravitreal vascular endothelial growth factor (VEGF) inhibitors compared to those of PDT alone in the treatment of polypoidal choroidal vasculopathy (PCV).
Methods
A systematic search of Pubmed, Embase, and the Cochrane Library was performed to identify all comparative studies that compared the outcomes of the two approaches. Outcomes of interest included visual outcomes, anatomic variables, and adverse events.
Results
Two randomised controlled trials and nine retrospective studies including a total of 543 cases were identified. At three and six months post-injection, no significant difference in visual acuity was found in the combined therapy group compared with the PDT monotherapy group, with pooled weighted mean differences (WMDs) of 0.074 (−0.021, 0.17) at three months and 0.082 (−0.013, 0.18) at six months. However, the mean changes in visual acuity at month 12 in the combined therapy group were significantly better than those in the PDT monotherapy group, with pooled WMDs of 0.11 (0.012, 0.21). Similar efficacy was found at 24 months (WMD: 0.21; 95%CI: 0.054, 0.36; P = 0.008). Patients in the combined therapy group also might benefit from reduced retinal haemorrhage (OR: 0.32; 95% CI: 0.14, 0.74; P = 0.008). Polyp regression, recurrence of PCV, central retinal thickness reduction, and pigment epithelial detachment resolution did not differ significantly between the two treatments.
Conclusions
Combined treatment appeared to result in better visual acuity and lower retinal haemorrhage. However, combined treatment did not affect the resolution and recurrence of lesions. Given the inherent limitations of the included studies, future well-designed RCTs are awaited to confirm and update the findings of this analysis.
Introduction
Polypoidal choroidal vasculopathy (PCV) is a sight-threatening disease, which is relatively prevalent in Asian populations [1]. About half of the eyes that did not undergo treatment had persistent leakage or repeated bleeding with vision loss. Pathogenesis of PCV is not fully understood, but vascular endothelial growth factor (VEGF) may have a role in pathogenesis. VEGF concentrations in the aqueous were found to be markedly increased in PCV eyes compared to controls. Treatment strategies for PCV include thermal laser photocoagulation, verteporfin photodynamic therapy (PDT), anti- VEGF therapies, and the combination therapy of PDT with anti-VEGF. However, there is still no consensus regarding the most effective treatment for PCV [2], [3]. Currently, PDT is widely used in the treatment of PCV, as various studies have demonstrated that PDT can result in visual improvement [4]–[7]. However, haemorrhagic complications after PDT have been reported in up to 30% of eyes, and repeated PDT results in significant choroidal hypoperfusion [5], [7]–[9]. With the introduction of anti-VEGF drugs in ophthalmology community, intravitreal anti-VEGF agents were widely used for neovascular disease such as wet age related macular degeneration and PCV. Unlike for age related macular degeneration, anti-VEGF compounds by themselves do not work well in PCV. Thus, combination therapy comprising PDT and anti-VEGF drugs, such as bevacizumab and ranibizumab, become another treatment choice for PCV. Because increased expression of VEGF has been found in PCV patients following PDT, the combined therapy has been thought to result in additional or complementary effects [2].
To date, several studies comparing PDT combined with anti-VEGF drugs and PDT monotherapy have been conducted [8], [10]–[19]. However, most are small series with conflicting results, and no definitive conclusions regarding objective differences in outcomes have been reached. For example, Gomi [19] and colleagues reported significantly better results with PDT plus anti-VEGF therapy compared with PDT monotherapy one year after treatment. However, according to the study of Rouvas [8] and colleagues, PDT resulted in a significantly better outcome than PDT with ranibizumab after one year of followup. Therefore, we performed a systematic review and meta-analysis of the available published literature to compare the outcomes of the two approaches.
Methods
This study was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Checklist S1) [20]. All stages of study selection, data extraction, and quality assessment were performed independently by two reviewers (W.W. and M.H). Any disagreement was resolved via discussion and consensus.
1. Literature search
A systematic search of Pubmed, Embase, and the Cochrane Library was performed to identify relevant studies up to September 2013. No time or language restrictions were applied. The following terms, adapted for each database, were used for the searches: (“polypoidal choroidal vasculopathy” OR PCV) AND (“angiogenesis inhibitors” OR “endothelial growth factors” OR VEGF OR lucentis OR ranibizumab OR bevacizumab OR avastin) AND (“photodynamic therapy” OR PDT).The Related Articles function was also used to broaden the search, and the computer search was supplemented with manual searches of the reference lists of all retrieved studies, review articles, and conference abstracts.
2. Inclusion and exclusion criteria
All available randomised controlled trials (RCTs) and non-randomized comparative studies (cohort or case–control studies) that compared combined PDT with anti-VEGF therapy with PDT monotherapy in all age groups, and that had at least one of the quantitative outcomes mentioned in the next section of this paper, were included. Editorials, letters to the editor, review articles, case reports, meeting abstracts, and animal experimental studies were excluded. The studies on PDT combined with anti-inflammatory compounds and triple therapy were also excluded.
3. Data extraction
The data were extracted independently by two reviewers (W.W. and M.H.). Disagreements were resolved through discussion. The following information was extracted from each study: first author; year of publication; study design; inclusion and exclusion criteria; number of patients in each group; characteristics of the study population; and outcomes of interest. The numbers of withdrawals and patients reporting adverse events were also recorded. For publications reporting on the same study population, the article reporting the results of the last end point was included, and data that could not be obtained from this publication were obtained from the others.
4. Outcome measures
The following outcomes were used to compare combined therapy and PDT alone. (1) Visual outcomes: mean visual acuity (VA) change at months 3, 6, 12, and 24; and proportion of eyes with improved, stable, and deteriorated vision after each treatment at months 12 and 24. After assessing VA at each follow-up visit, the patients were categorised into three groups based on their VA change from baseline: improved, stable, and deteriorated VA. (2) Anatomical outcomes: mean change in central retinal thickness (CRT) at months 6; regression rates of polyps at months 3 and 12; resolution of pigment epithelial detachment (PED) at 12-month followup; and recurrence rate of PCV. (3) Adverse events: incidence of retinal haemorrhage.
5. Quality assessment
The methodological quality of RCTs was assessed using the Cochrane Risk of Bias Tool [21]. The methodological quality of observational studies was assessed using the modified Newcastle–Ottawa scale [22], which consists of three factors: patient selection, comparability of the study groups, and assessment of outcome. A score of 0–9 (allocated as stars) was allocated to each study except RCTs. RCTs and observational studies achieving seven or more stars were considered to be of high quality.
6. Statistical analysis
Weighted mean differences (WMDs) and odds ratios (ORs) were used to compare continuous and dichotomous variables, respectively. All outcomes were reported with 95% confidence intervals (CIs). Considering the different clinical characteristics among study groups and the variation in sample sizes, we assumed that heterogeneity was present even when no statistical significance was identified; therefore, we decided to combine data by using a random effects model to achieve more conservative estimates. The change in VA of each eligible arm of the individual studies was calculated as the difference between the value at baseline and those at the different follow-up times, and the variance was computed as the weighted mean of their variances. The WMD was then computed as the between-treatment difference in the visual changes from baseline.
Statistical heterogeneity among studies was assessed using the chi-squared test with significance set at P<0.10. The percentage of heterogeneity was evaluated using the I2 statistic, which ranges from 0% to 100%, with 0% representing no heterogeneity and larger values representing greater heterogeneity (I2 = 0–25% indicates no or mild heterogeneity; I2 = 25–50% indicates moderate heterogeneity; I2 = 50–75% indicates large heterogeneity; and I2 = 75–100% indicates extreme heterogeneity) [23].
Subgroup analysis was performed according to type of anti-VEGF agents, frequency of anti-VEGF agent, patient history of previous treatment, and intevals between PDT and anti-VEGF therapy. Sensitivity analysis was performed by excluding each study, one by one, and recalculating the combined estimates based on the remaining studies. Only outcomes of interest that were reported in more than three studies were included in the sensitivity analysis. Potential publication bias was evaluated by Begg's and Egger's tests. All analyses were performed with Stata Version 12.0 (StataCorp, College Station, TX). A p value<0.05 was considered significant, except where otherwise specified.
Results
1. Characteristics of eligible studies
Eleven studies [8], [10]–[19] including 543 cases (253 cases of combined PDT with anti-VEGF therapy and 290 cases of PDT monotherapy) fulfilled the predefined inclusion criteria and were included in the final analysis (Figure 1). The characteristics of the included studies are shown in Tables 1 and 2. Among the included studies, there were two small-sample RCTs and nine retrospective comparative studies.PCV was confirmed by ICGA in all studies. The inclusion criteria of the patients in these studies were summarized in table S1. The ICGA and OCT were used in the same way in all subcomponents of these studies. All of the retrospective comparative studies scored seven stars or higher and were considered to be of high quality.
Figure 1. Flow diagram of included studies for this meta-analysis.
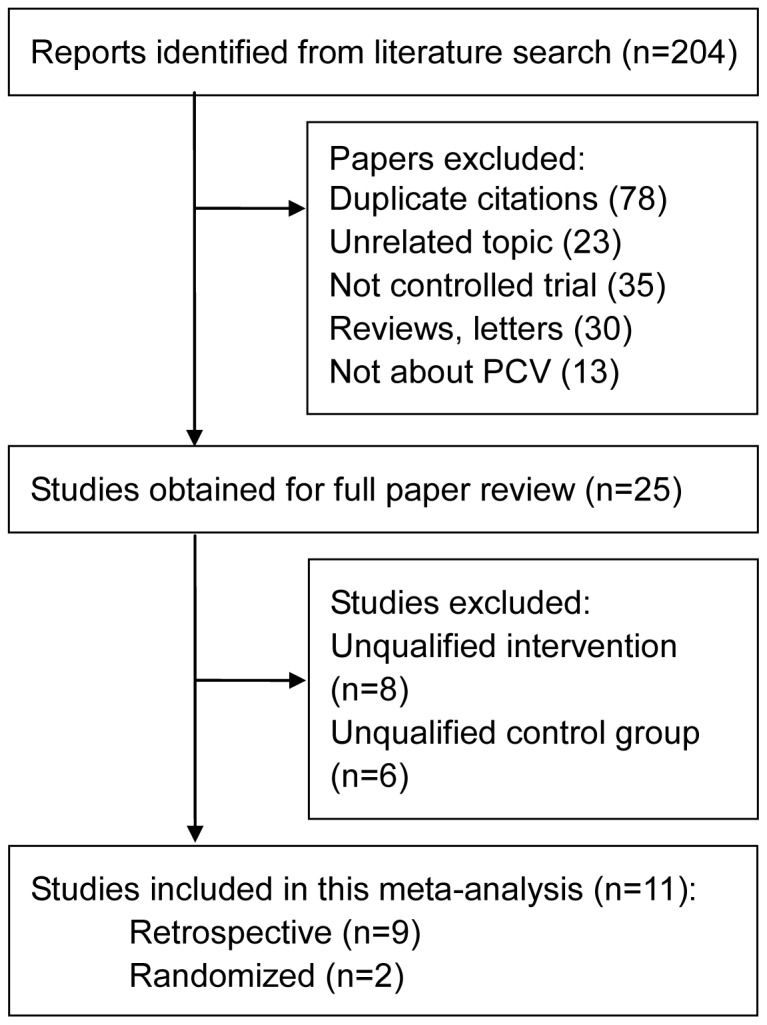
Table 1. Characteristics of studies included in the meta-analysis.
| Trial | Design | Centre | Location | Follow-up | No. of eyes | Mean Age(year) | Sex (M/F) | |||
| Combine | PDT | Combine | PDT | Combine | PDT | |||||
| Lee (2013) | RCT | 1 | Korea | 3 m | 12 | 8 | 63.68±8.78 | 66.33±7.85 | 11/1 | 5/3 |
| Saito (2013) | Retro | 1 | Japan | ≥24 m | 25 | 32 | 74.0±8.6 | 75.0±6.5 | 17/8 | 27/5 |
| Lee (2012) | Retro | 1 | Taiwan | ≥24 m | 36 | 33 | 67.8±9.8 | 65.6±9.5 | 21/15 | 18/15 |
| Koh (2012) | RCT | 7 | Hong Kong, Singapore, South Korea, Taiwan, Thailand | 6 m | 19 | 21 | 63.8±8.30 | 62.2±9.77 | 11/8 | 15/16 |
| Kim (2011) | Retro | 1 | Korea | >12 m | 20 | 19 | 64.8±8.3 | 65.9±8.4 | 13/6 | 15/5 |
| Maruko (2011) | Retro | 1 | Japan | 6 m | 11 | 16 | 71 | 71.8 | 6/5 | 12/4 |
| Rouvas (2011) | Retro | 2 | Greece | 12 m | 9 | 11 | 64.67 | 62.9 | 4/5 | 5/6 |
| Gomi (2010) | Retro | 1 | Japan | >12 m | 61 | 85 | 70.9±7.1 | 70.9±6.8 | 45/16 | 68/17 |
| Lai (2011) | Retro | 1 | Hong Kong | ≥12 m | 16 | 12 | 71.3±9.8 | 65.6±11.0 | 8/8 | 10/2 |
| Sakurada (2013) | Retro | 1 | Japan | ≥24 m | 24 | 34 | 73.2±7.4 | 70.1±7.1 | 16/8 | 25/9 |
| Kang (2013) | Retro | 1 | Korea | ≥24 m | 20 | 19 | 70.00±7.75 | 66.21±9.00 | NA | NA |
RCT = randomised controlled trials; Retro = retrospective comparative studies; Combine = PDT plus intravitreal anti-VEGF inhibitors; PDT = photodynamic alone; M/F = male/female; NA = not available.
Table 2. Characteristics of lesions and treatment exposures included in the meta-analysis.
| Study | Group | Lesion GLD (µm) | Interventions | Number of treatments (mean±SD) (range) |
| Lee (2013) | Combine | NA | PDT+IVR 0.5 mg (<1 hour after PDT) | 1PDT,1IVR |
| PDT | NA | PDT | 1PDT | |
| Saito (2013) | Combine | 4074±1459 | IVR 0.5 mg+PDT (1 or 2 days after IVR) | 1.4PDT, 4.5IVR |
| PDT | 4867±1855 | PDT (6 mg/m2) | 2.6PDT | |
| Lee (2012) | Combine | 2619±843 | IVB 1.25 mg+ PDT (1 week after IVB) | 2.25PDT, 2.42IVB |
| PDT | 2842±1092 | PDT (6 mg/m2) | 2.55PDT | |
| Koh (2012) | Combine | <5400 | PDT+IVR 0.5 mg (<24 hours after PDT) | 1.4(1-4)PDT, 3.9(3-6)IVR |
| PDT | <5400 | PDT (6 mg/m2) + sham | 1.7(1-4)PDT | |
| Kim (2011) | Combine | 3287.5 ±1335.9 | PDT +IVB 1.25 mg (on the same day) | 1.30±0.47 (1-2)PDT, 2.90±1.41(1-5)IVB |
| PDT | 3626.3±1334.6 | PDT (6 mg/m2) | 1.89±0.94 (1-4)PDT | |
| Maruko (2011) | Combine | 2905±1122 | IVR 0.5 mg + PDT (1-2 day after IVR) | 1PDT, 3IVR |
| PDT | 3013±1059 | PDT (6 mg/m2) | 1PDT | |
| Rouvas (2011) | Combine | NA | IVR 0.5 mg+PDT (7±2 days after IVR) | 1.67(1-2)PDT, 5(3-6)IVR |
| PDT | NA | PDT (6 mg/m2) | 1.82(1-3)PDT | |
| Gomi (2010) | Combine | 2626±1138 | IVB 1.25 mg +PDT (1 day after IVB) | 1.43PDT, 1.92IVB |
| PDT | 2521±996 | PDT (6 mg/m2) | 1.66PDT | |
| Lai (2011) | Combine | 3490±1170 | PDT+ IVR 0.5 mg (30 min after IVR) | 1.2(1-2)PDT, 3.4(3-6)IVR |
| PDT | 2580±707 | PDT (6 mg/m2) | 1.7(1-4)PDT | |
| Sakurada (2013) | Combine | 2039±847 | IVR 0.5 mg+PDT (1 week after IVR) | 1.54(1-3)PDT, 1.71(1-4)IVR |
| PDT | 2364±716 | PDT (6 mg/m2) | 1.42(1-3)PDT | |
| Kang (2013) | Combine | 2815±910.12 | PDT +IVB 0.5 mg (on the same day) | 1.67±0.65PDT,11.00±2.61IVB |
| PDT | 2810.87±974.10 | PDT (6 mg/m2) | 2.56±0.38 PDT |
Combine = PDT plus anti-VEGF inhibitors; PDT = photodynamic alone; NA = not available; GLD = greatest linear dimension; SD = standard deviation; IVR = intravitreal ranibizumab; IVB = intravitreal bevacizumab.
2. Visual outcomes
VA was the most important criterion for evaluating efficacy. In the included studies, VA was measured using the logMAR scale and reported as the mean change in logMAR units from baseline for each group. In the combined PDT with anti-VEGF therapy group, the mean VA improved continuously during the two years of treatment when compared with baseline VA. The pooled WMDs at 3, 6, 12, and 24 months were 0.14 (0.07, 0.21), 0.17 (0.11, 0.24), 0.19 (0.12, 0.26), and 0.21 (0.11, 0.30), respectively. In the PDT monotherapy group, the mean VA improved at three and six months after initial treatment. However, it deteriorated after six months, and at 12 and 24 months, it was not significantly different from baseline VA.
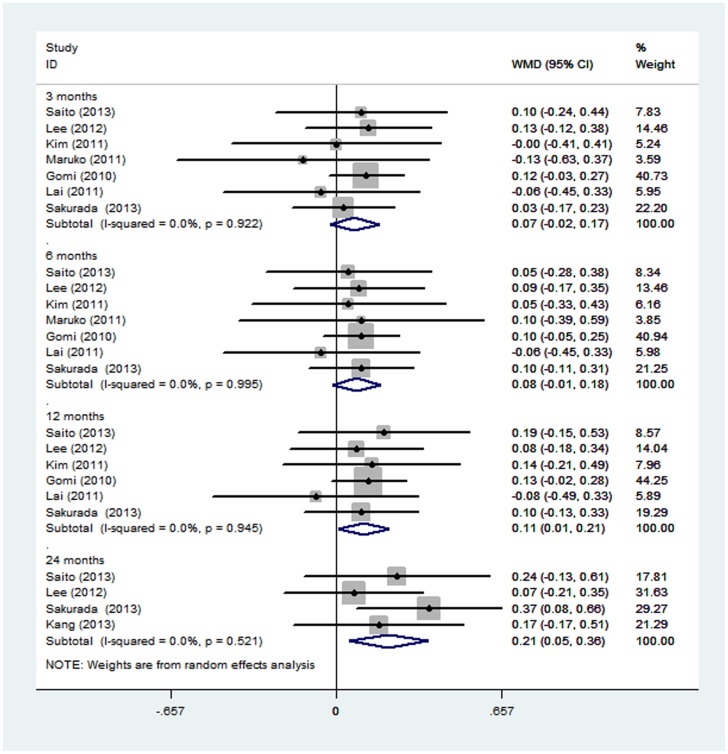
LogMAR VA improvements in the combined PDT with anti-VEGF therapy group versus those in the PDT monotherapy group are shown in Figure 2 and Table 3. At three and six months post-injection, no significant difference in VA was found in the combined therapy group compared with the PDT monotherapy group, with pooled WMDs of 0.074 (−0.021, 0.17) at three months and 0.082 (−0.013, 0.18) at six months. However, the mean changes in BCVA at month 12 in the combined PDT with anti-VEGF therapy group were significantly better than those of the PDT monotherapy group, with pooled WMDs of 0.11 (0.012, 0.21). Similar efficacy was found at 24 months (WMD: 0.21; 95%CI: 0.054, 0.36; p = 0.008). There was no evidence of heterogeneity across trials.
Figure 2. Forest plot displaying the pooled summary estimates of visual acuity in the combined PDT and anti-VEGF therapy group versus the PDT monotherapy group.
VA = visual acuity.
Table 3. Results of meta-analysis comparison of combined therapy and PDT monotherapy.
| Outcome of interest | Studies (n) | WMD/OR (95% CI) | P | Study heterogeneity | ||
| ?2 | P | I2 | ||||
| Mean logMAR change in combined PDT and anti-VEGF therapy group (followup vs baseline) | ||||||
| 3 months | 7 | 0.14 (0.07, 0.21) | <0.001 | 4.84 | 0.565 | 0.00% |
| 6 months | 7 | 0.17 (0.11, 0.24) | <0.001 | 2.38 | 0.881 | 0.00% |
| 12 months | 6 | 0.19 (0.12, 0.26) | <0.001 | 4.92 | 0.426 | 0.00% |
| 24 months | 4 | 0.21 (0.11, 0.30) | <0.001 | 1.23 | 0.746 | 0.00% |
| Mean logMAR change in PDT therapy group (followup vs baseline) | ||||||
| 3 months | 7 | 0.06 (−0.01, 0.12) | 0.075 | 4.76 | 0.575 | 0.00% |
| 6 months | 7 | 0.09 (0.02, 0.15) | 0.010 | 4.07 | 0.667 | 0.00% |
| 12 months | 6 | 0.06 (−0.01, 0.13) | 0.075 | 4.93 | 0.424 | 0.00% |
| 24 months | 4 | 0.00 (−0.12, 0.12) | 0.972 | 1.9 | 0.592 | 0.00% |
| LogMAR improvements in both groups (combined PDT and anti-VEGF group vs PDT monotherapy group) | ||||||
| 3 months | 7 | 0.074 (−0.021, 0.17) | 0.127 | 1.98 | 0.922 | 0.00% |
| 6 months | 7 | 0.082 (−0.013, 0.18) | 0.092 | 0.67 | 0.995 | 0.00% |
| 12 months | 6 | 0.11 (0.012, 0.21) | 0.028 | 1.2 | 0.945 | 0.00% |
| 24 months | 4 | 0.21 (0.054, 0.36) | 0.008 | 2.26 | 0.521 | 0.00% |
| LogMAR change as categorical variable | ||||||
| Proportion of eyes with improved vision | ||||||
| final visit | 6 | 1.91 (1.14, 3.18) | 0.013 | 2.8 | 0.73 | 0.00% |
| 12 months | 3 | 2.33 (1.07, 5.07) | 0.033 | 1.67 | 0.434 | 0.00% |
| 24 months | 3 | 1.64 (0.83, 3.22) | 0.154 | 0.68 | 0.71 | 0.00% |
| Proportion of eyes with deteriorated vision | ||||||
| final visit | 8 | 0.77 (0.41, 1.46) | 0.423 | 5.42 | 0.609 | 0.00% |
| 12 months | 5 | 0.87 (0.38, 1.98) | 0.741 | 1.35 | 0.854 | 0.00% |
| 24 months | 3 | 0.42 (0.08, 2.29) | 0.317 | 3.86 | 0.145 | 48.10% |
| Proportion of eyes with stable vision | ||||||
| final visit | 4 | 0.98 (0.66, 1.46) | 0.926 | 1.41 | 0.702 | 0.00% |
| 12 months | 9 | 0.83 (0.47, 1.46) | 0.512 | 5.52 | 0.700 | 0.00% |
| 24 months | 5 | 1.15 (0.67, 1.99) | 0.617 | 3.44 | 0.487 | 0.00% |
| Anatomical outcomes | ||||||
| CRT reduction at 6 months | 4 | 26.19 (−15.38, 67.76) | 0.217 | 1.81 | 0.614 | 0.00% |
| Resolution of PED at 12 months | 4 | 2.18 (0.48, 9.89) | 0.311 | 7.31 | 0.063 | 59.00% |
| Regression of polyps at 3 months | 9 | 1.43(0.9, 2.27) | 0.130 | 8.19 | 0.415 | 2.30% |
| Regression of polyps at 6 months | 4 | 1.80 (0.66, 4.87) | 0.248 | 2.07 | 0.557 | 0.00% |
| Recurrence rate of PCV | 6 | 0.95 (0.59, 1.53) | 0.840 | 1.87 | 0.867 | 0.00% |
| Adverse events | ||||||
| Incidence of retinal haemorrhage | 5 | 0.32 (0.14, 0.74) | 0.008 | 4.22 | 0.378 | 5.10% |
Combine = PDT plus intravitreal anti-VEGF inhibitors; PDT = photodynamic alone; NA = not available; logMAR = logarithm of the minimal angle of resolution; CRT = central retinal thickness; PED = pigment epithelial detachment; PCV = polypoidal choroidal vasculopathy; WMD = weighted mean difference; OR = odds ratio; CI = confidence interval; χ2 = chi-square statistic; P = P-value; I2 = I-square heterogeneity statistic.
When VA change was treated as a categorical variable, the percentages of improved, stable, and deteriorated VA were compared at 12 months, 24 months, and final visits. The rates of improved VA were significantly higher in the combined PDT with anti-VEGF therapy group than in the PDT monotherapy group at 12 months and final visits. However, the rates did not differ significantly at 24 months. Although the pooled results favoured the combined PDT with anti-VEGF therapy group in terms of stable and deteriorated VA, the differences were not statistically significant at any of the time points (Table 2).
3. Anatomical outcomes
CRT was defined as the distance between the internal limiting membrane and the inner surface of the retinal pigment epithelium, and measured at the fovea. CRT changes from baseline to month six were reported in four studies including 145 patients; the change in CRT was higher in the combined PDT with anti-VEGF therapy group than the PDT monotherapy group, but this difference was not statistically significant (WMD: 26.19; 95% CI: −15.38, 67.76; p = 0.217).
Four studies reported data for the frequency of PED resolved at 12 months. Analysis of these data showed no significant difference between the combined PDT with anti-VEGF and PDT monotherapy groups (OR: 2.18; 95% CI: 0.48, 9.89; p = 0.311).
Regression rates of polyps at three and six months were available for nine and four studies, respectively; there were no significant differences between the two groups (OR: 1.43; 95% CI: 0.90, 2.27; p = 0.130; and OR: 1.80; 95% CI: 0.66, 4.87; p = 0.248, respectively).
Recurrences of PCV during the follow-up periods were noted in six studies. Although the PDT monotherapy group showed more recurrences, there was no significant difference in recurrence rate between the groups (OR: 0.95; 95%CI: 0.59, 1.53; p = 0.840).
4. Adverse events
Retinal haemorrhage was the most common complication. Five studies including 340 patients reported frequency of retinal haemorrhage, and the pooled data showed a significant difference favouring the combined PDT with anti-VEGF therapy group (OR: 0.32; 95% CI: 0.14, 0.74; p = 0.008) (Table 2).
5. Subgroup analysis, sensitivity analysis and publication bias
There was no statistically significant difference in the mean changes of BCVA at 3 months and 6 months between combined PDT and anti-VEGF therapy group and PDT monotherapy group at all subgroups (Table 4). Visual outcome results were not significantly influenced by type of anti-VEGF agents, frequency of intravitreal anti-VEGF therapy, inteval between PDT and anti-VEGF therapy, and previous intevention history. Because of unadequate number of studies, subgroup analysis for other outcomes were not available. To evaluate the robustness of the results, each study in the meta-analysis was excluded in turn to reflect the influence of individual studies on the pooled estimates. The pattern of differences was similar to that of the original analysis, suggesting high stability of the meta-analysis results (data not shown). Begg's tests (all p>0.05) and Egger's tests (all p>0.05) showing no evidence of publication bias (data not shown).
Table 4. Subgroup analysis comparing combined PDT with anti-VEGF therapy group with PDT monotherapy group for change in LogMAR from baseline.
| Subgroup | Studies(n) | WMD(95%CI) | P | Test for Heterogeneity | ||
| ?2 | P | I2 | ||||
| LogMAR improvements at 3 months | ||||||
| All | 7 | 0.07 (−0.02, 0.17) | 0.127 | 1.98 | 0.922 | 0.00% |
| Ranibizumab used | 4 | 0.02 (−0.14, 0.17) | 0.838 | 0.73 | 0.867 | 0.00% |
| Bevacizumab used | 4 | 0.11 (−0.01, 0.23) | 0.072 | 0.31 | 0.856 | 0.00% |
| Protocol of anti-VEGF was 3+PRN | 4 | 0.06 (−0.11, 0.23) | 0.501 | 1.27 | 0.736 | 0.00% |
| Protocol of anti-VEGF was 1+PRN | 3 | 0.08 (−0.03, 0.2) | 0.165 | 0.66 | 0.720 | 0.00% |
| All patients were treatment-naïve | 5 | 0.10 (−0.01, 0.21) | 0.085 | 1.16 | 0.885 | 0.00% |
| Some patients receive intervention previously | 2 | 0.01 (−0.17, 0.19) | 0.904 | 0.16 | 0.687 | 0.00% |
| PDT 1-7 days after intravitreal anti-VEGF therapy | 5 | 0.09 (−0.01, 0.19) | 0.090 | 1.34 | 0.855 | 0.00% |
| PDT and anti-VEGF therapy on the same day | 2 | −0.03 (−0.32, 0.25) | 0.826 | 0.04 | 0.836 | 0.00% |
| LogMAR improvements at 6 months | ||||||
| All | 7 | 0.08 (−0.01, 0.18) | 0.092 | 0.67 | 0.995 | 0.00% |
| Ranibizumab used | 4 | 0.07 (−0.09, 0.22) | 0.400 | 0.53 | 0.911 | 0.00% |
| Bevacizumab used | 3 | 0.09 (−0.03, 0.22) | 0.138 | 0.06 | 0.972 | 0.00% |
| Protocol of anti-VEGF was 3+PRN | 4 | 0.05 (−0.12, 0.22) | 0.545 | 0.44 | 0.932 | 0.00% |
| Protocol of anti-VEGF was 1+PRN | 3 | 0.10 (−0.02, 0.21) | 0.104 | 0.06 | 0.971 | 0.00% |
| All patients were treatment-naïve | 5 | 0.09 (−0.02, 0.2) | 0.122 | 0.12 | 0.998 | 0.00% |
| Some patients receive intervention previously | 2 | 0.07 (−0.12, 0.25) | 0.486 | 0.51 | 0.477 | 0.00% |
| PDT 1-7 days after intravitreal anti-VEGF therapy | 5 | 0.09 (−0.01, 0.2) | 0.071 | 0.08 | 0.999 | 0.00% |
| PDT and anti-VEGF therapy on the same day | 2 | 0 (−0.28, 0.27) | 0.976 | 0.16 | 0.693 | 0.00% |
PDT = photodynamic therapy; logMAR = logarithm of the minimal angle of resolution; 3+PRN = 3 initial monthly + as needed injection; 1+PRN = 1 initial monthly + as needed injection.
Discussion
Among the currently available treatment modalities for PCV, PDT alone or PDT combined with VEGF inhibitors therapy seem the most promising [24], [25]. This meta-analysis of two RCTs and nine retrospective studies including 543 patients, comparing the efficacy of combined PDT with anti-VEGF therapy and PDT monotherapy, showed that combined PDT with anti-VEGF therapy was superior to PDT monotherapy in terms of visual outcome and complication. We found no significant differences in CRT reduction, polyp regression, PCV recurrence, or PED resolution.
The results showed that PDT alone will not be the best option to treat PCV, mainly until 6 months. PDT monotherapy causes regression of polyps and reduces fluid leakage, but polypoidal lesions have high recurrence rates over long periods. Furthermore, PDT is associated with the risk of submacular hemorrhages in PCV, which is confirmed in this study. The papers used in this meta-analysis did not explore the parameters of PDT and outcomes of treatment, which prevent our intution for disscussion. Anti-VEGF drugs reduced exudative fluid and suppressed upregulation of VEGF, but the vascular lesions did not regress. Thus, the combination of PDT and an anti-VEGF agent seems to be a rational approach.
In our study, the mean VA changes in the combined PDT with anti-VEGF therapy group were superior to those of the PDT monotherapy group at the 12-month and 24-month follow-up time points. Furthermore, the improvement in mean VA seemed to decrease with time in the PDT monotherapy group. In patients treated with PDT monotherapy, the improvement of mean VA did not reach statistical significance after six months. Theoretically, several additional PDT sessions would be necessary to treat PCV recurrences during longer follow-up periods [26]. It means an increase in the risks induced by PDT, such as subretinal haemorrhage and ischemic damage of normal choroidal tissue. PDT combined with an anti-VEGF drug can reduce those side effects of PDT [27]. Therefore, eyes in the combined therapy group had more potential to maintain better VA over the long term.
A similar rate of resolution of the original PCV vasculature was observed in the two treatments; the recurrence rates were also similar. This seemed reasonable, because various trials have shown that anti-VEGF agents are effective in reducing leakage, resolving fluids, and improving VA, but ineffective for polyp regression [7]–[9], [15], [28], [29]. Thus, the resolution of polypoidal lesions may be attributed mainly to PDT alone.
Retinal haemorrhage is one of the major problems associated with PCV treatment [8], [10]–[19]. In this study, we found a significantly lower rate of retinal haemorrhage in the combined PDT with anti-VEGF treatment group. It has been reported that PDT induces ischemia in the choroid, and that inflammatory responses around the RPE result in enhanced expression of VEGF [30]. Intravitreal injection of an anti-VEGF agent could therefore block the adverse effects induced by the increased VEGF expression, which might account for the lower retinal haemorrhage rate observed in the combined PDT group [31]. The reduced risk of haemorrhage induced by anti-VEGF inhibitors should encourage ophthalmologists to choose the combined therapy.
Although combined therapy showed better results than mono therapy, combined therapy was not sufficient to improve the results obtained mainly after 12 and 24 months of evaluation. Several studies have reported that the efficacy of PDT and PDT combined with intravitreal anti-VEGF agents decreased with time [11], [14], [24], [32]. Resolution of the original PCV vasculature was observed at a similar rate between the treatments, and the recurrence rates also were similar.Therefore, decrease in benefit at 12 months seems to be because of recurrences of exudative changes or atrophic tissue changes related to persistent lesions. In addition, the possible influence of cumulative harmful effect of PDT cannot be excluded. To maintain the beneficial effects of combination therapy with long-term follow-up, further investigation will be needed.
To our knowledge, this is the first meta-analysis comparing combined therapy and PDT monotherapy in the treatment of PCV. While heterogeneity is often a concern in a meta-analysis, little evidence of heterogeneity was observed throughout our study. This finding can be partially explained by the following facts: all of the studies used common indications and measurements of outcomes; all of the studies, except one [8], were conducted in Asian countries, where populations share much in terms of genetic background, lifestyle, and dietary patterns; and the baseline characteristics of most of the studies were comparable. To assess any impact of a single study on the effect estimates, we performed a sensitivity analysis by iteratively removing one study at a time to assess the stability of the meta-analysis results; the results were similar to those of the initial analysis. Although a meta-analysis of RCTs only would be ideal, the limited number of RCTs prevented us from reaching any definitive conclusions based on those studies alone.
The present meta-analysis has some limitations that must be taken into account. First, all the included studies were retrospective, except for two RCTs with small sample sizes. Inadequate random sequence generation and blinding could result in selection bias, as patients with worse visual prognoses might be offered the combination treatment. Nonetheless, the major characteristics of the eyes in the two groups were comparable at baseline, and therefore, selection bias was less likely to occur. Second, the combination group used either bevacizumab or ranibizumab as an anti-VEGF agent, and there might be a difference between the two agents when treating PCV. However, the results of the subgroup analysis showed that the effect of different anti-VEGF agents led to similar VA changes. Recent studies also have demonstrated that these two agents have similar efficacy in treating both age-related macular degeneration and PCV [33]–[36]. Third, because the studies included in the analysis were mostly conducted at major institutions, the patients evaluated might not reflect actual patient populations in the community. Fourth, while computer-based literature searching is essential, it is possible that not all of the relevant studies were identified, because ‘grey literature’ was not included in this study. Fifth, PDT combined with anti-inflammatory compounds and the combination therapy involving PDT, anti-VEGF and an anti-inflammatory agent did not evaluated. Further studies are necessary to evaluate these protocols for treating PCV. Finally, given that the treatment of PCV is not limited to two years, more data are needed from studies of longer duration in order to determine the efficacy and safety of combination therapy over the long term.
In conclusion, the present meta-analysis suggests that combination of PDT and anti-VEGF therapy results in better long-term visual outcomes and lower incidence rates of retinal haemorrhage than PDT monotherapy. The two treatments appear to be equivalent in terms of polyp regression and recurrence, CRT reduction, and PED resolution. Nevertheless, despite our rigorous methodology, the inherent limitations of the included studies should be considered, and conclusions drawn from our pooled results should be interpreted with caution. Future large-volume, well-designed RCTs with extensive follow-up are awaited to confirm and update the findings of this analysis.
Supporting Information
The inclusion criteria of the patients in the studies included in the meta-analysis.
(DOCX)
PRISMA checklist.
(DOC)
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by the National Natural Science Foundation of China (81170849, 81371008). No additional external funding was received. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sakurada Y, Yoneyama S, Imasawa M, Iijima H (2013) Systemic risk factors associated with polypoidal choroidal vasculopathy and neovascular age-related macular degeneration. Retina 33: 841–845. [DOI] [PubMed] [Google Scholar]
- 2. Imamura Y, Engelbert M, Iida T, Freund KB, Yannuzzi LA (2010) Polypoidal choroidal vasculopathy: a review. Surv Ophthalmol 55: 501–515. [DOI] [PubMed] [Google Scholar]
- 3. Gomi F, Tano Y (2008) Polypoidal choroidal vasculopathy and treatments. Curr Opin Ophthalmol 19: 208–212. [DOI] [PubMed] [Google Scholar]
- 4.Nowak-Sliwinska P, van den Bergh H, Sickenberg M, Koh AH (2013) Photodynamic Therapy for Polypoidal Choroidal Vasculopathy. Prog Retin Eye Res. [DOI] [PubMed]
- 5. Spaide RF, Donsoff I, Lam DL, Yannuzzi LA, Jampol LM, et al. (2012) Treatment of polypoidal choroidal vasculopathy with photodynamic therapy. 2002. Retina 32 Suppl 1: 529–535. [DOI] [PubMed] [Google Scholar]
- 6. Tomita K, Tsujikawa A, Yamashiro K, Ooto S, Tamura H, et al. (2012) Treatment of polypoidal choroidal vasculopathy with photodynamic therapy combined with intravitreal injections of ranibizumab. Am J Ophthalmol 153: 68–80. [DOI] [PubMed] [Google Scholar]
- 7. Oishi A, Kojima H, Mandai M, Honda S, Matsuoka T, et al. (2013) Comparison of the Effect of Ranibizumab and Verteporfin for Polypoidal Choroidal Vasculopathy: 12-Month LAPTOP Study Results. Am J Ophthalmol 156: 644–651. [DOI] [PubMed] [Google Scholar]
- 8. Rouvas AA, Papakostas TD, Ntouraki A, Douvali M, Vergados I, et al. (2011) Photodynamic therapy, ranibizumab, and ranibizumab with photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Retina 31: 464–474. [DOI] [PubMed] [Google Scholar]
- 9. Inoue M, Arakawa A, Yamane S, Kadonosono K (2013) Long-term outcome of intravitreal ranibizumab treatment, compared with photodynamic therapy, in patients with polypoidal choroidal vasculopathy. Eye (Lond) 27: 1013–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee MY, Lee WK, Baek J, Kwon OW, Lee JH (2013) Photodynamic Therapy Versus Combination Therapy in Polypoidal Choroidal Vasculopathy: Changes of Aqueous Vascular Endothelial Growth Factor. Am J Ophthalmol. 156: 343–8. [DOI] [PubMed] [Google Scholar]
- 11. Saito M, Iida T, Kano M, Itagaki K (2013) Two-year results of combined intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy. Graefes Arch Clin Exp Ophthalmol. 251: 2099–110. [DOI] [PubMed] [Google Scholar]
- 12. Sakurada Y, Iijima H (2013) Two-Year Results of Photodynamic Therapy With or Without Intravitreal Ranibizumab for Polypoidal Choroidal Vasculopathy. J Ocul Pharmacol Ther. 29: 832–6. [DOI] [PubMed] [Google Scholar]
- 13. Kang HM, Koh HJ (2014) Two-Year Outcome after Combination Therapy for Polypoidal Choroidal Vasculopathy: Comparison with Photodynamic Monotherapy and Anti-Vascular Endothelial Growth Factor Monotherapy. Ophthalmologica. 231: 86–93. [DOI] [PubMed] [Google Scholar]
- 14. Lee YA, Yang CH, Yang CM, Ho TC, Lin CP, et al. (2012) Photodynamic therapy with or without intravitreal bevacizumab for polypoidal choroidal vasculopathy: two years of follow-up. Am J Ophthalmol 154: 872–880. [DOI] [PubMed] [Google Scholar]
- 15. Koh A, Lee WK, Chen LJ, Chen SJ, Hashad Y, et al. (2012) EVEREST study: efficacy and safety of verteporfin photodynamic therapy in combination with ranibizumab or alone versus ranibizumab monotherapy in patients with symptomatic macular polypoidal choroidal vasculopathy. Retina 32: 1453–1464. [DOI] [PubMed] [Google Scholar]
- 16. Kim SJ, Yu HG (2011) Efficacy of combined photodynamic therapy and intravitreal bevacizumab injection versus photodynamic therapy alone in polypoidal choroidal vasculopathy. Retina 31: 1827–1834. [DOI] [PubMed] [Google Scholar]
- 17. Lai TY, Lee GK, Luk FO, Lam DS (2011) Intravitreal ranibizumab with or without photodynamic therapy for the treatment of symptomatic polypoidal choroidal vasculopathy. Retina 31: 1581–1588. [DOI] [PubMed] [Google Scholar]
- 18. Maruko I, Iida T, Sugano Y, Saito M, Sekiryu T (2011) Subfoveal retinal and choroidal thickness after verteporfin photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 151: 594–603. [DOI] [PubMed] [Google Scholar]
- 19. Gomi F, Sawa M, Wakabayashi T, Sasamoto Y, Suzuki M, et al. (2010) Efficacy of intravitreal bevacizumab combined with photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 150: 48–54. [DOI] [PubMed] [Google Scholar]
- 20. Moher D, Liberati A, Tetzlaff J, Altman DG (2009) Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. J Clin Epidemiol 62: 1006–1012. [DOI] [PubMed] [Google Scholar]
- 21.Higgins JP, Green S (2011) Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 [updated March, 2011]: The Cochrane Collaboration. http://handbook.cochrane.org/
- 22.Wells G, Shea B, O'Connell D, Peterson J, Welch V, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp
- 23. Higgins JP, Thompson SG, Deeks JJ, Altman DG (2003) Measuring inconsistency in meta-analyses. BMJ 327: 557–560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Koh AH, Chen LJ, Chen SJ, Chen Y, Giridhar A, et al. (2013) Polypoidal choroidal vasculopathy: evidence-based guidelines for clinical diagnosis and treatment. Retina 33: 686–716. [DOI] [PubMed] [Google Scholar]
- 25. Englander M, Kaiser PK (2013) Combination therapy for the treatment of neovascular age-related macular degeneration. Curr Opin Ophthalmol 24: 233–238. [DOI] [PubMed] [Google Scholar]
- 26. Sato T, Kishi S, Matsumoto H, Mukai R (2013) Comparisons of outcomes with different intervals between adjunctive ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy. Am J Ophthalmol 156: 95–105. [DOI] [PubMed] [Google Scholar]
- 27. Gemmy CC, Yeo I, Li X, Mathur R, Lee SY, et al. (2013) Argon laser with and without anti-vascular endothelial growth factor therapy for extrafoveal polypoidal choroidal vasculopathy. Am J Ophthalmol 155: 295–304. [DOI] [PubMed] [Google Scholar]
- 28. Song MH, Ryu HW, Roh YJ (2011) One-year results of intravitreal ranibizumab with or without photodynamic therapy for polypoidal choroidal vasculopathy. Ophthalmologica 226: 119–126. [DOI] [PubMed] [Google Scholar]
- 29. Lai TY, Chan WM, Liu DT, Luk FO, Lam DS (2008) Intravitreal bevacizumab (Avastin) with or without photodynamic therapy for the treatment of polypoidal choroidal vasculopathy. Br J Ophthalmol 92: 661–666. [DOI] [PubMed] [Google Scholar]
- 30. Ruamviboonsuk P, Tadarati M, Vanichvaranont S, Hanutsaha P, Pokawattana N (2010) Photodynamic therapy combined with ranibizumab for polypoidal choroidal vasculopathy: results of a 1-year preliminary study. Br J Ophthalmol 94: 1045–1051. [DOI] [PubMed] [Google Scholar]
- 31. Saito M, Iida T, Kano M (2012) Combined intravitreal ranibizumab and photodynamic therapy for polypoidal choroidal vasculopathy. Retina 32: 1272–1279. [DOI] [PubMed] [Google Scholar]
- 32. de Crecchio G, Chan RV, Manzi G, Romano MR (2011) Polypoidal choroidal vasculopathy: recent advances in therapy. Curr Drug Targets 12: 206–211. [DOI] [PubMed] [Google Scholar]
- 33. Krebs I, Schmetterer L, Boltz A, Told R, Vecsei-Marlovits V, et al. (2013) A randomised double-masked trial comparing the visual outcome after treatment with ranibizumab or bevacizumab in patients with neovascular age-related macular degeneration. Br J Ophthalmol 97: 266–271. [DOI] [PubMed] [Google Scholar]
- 34. Kodjikian L, Souied EH, Mimoun G, Mauget-Faysse M, Behar-Cohen F, et al. (2013) Ranibizumab versus Bevacizumab for Neovascular Age-related Macular Degeneration: Results from the GEFAL Noninferiority Randomized Trial. Ophthalmology. 120: 2300–9. [DOI] [PubMed] [Google Scholar]
- 35. Chakravarthy U, Harding SP, Rogers CA, Downes SM, Lotery AJ, et al. (2013) Alternative treatments to inhibit VEGF in age-related choroidal neovascularisation: 2-year findings of the IVAN randomised controlled trial. Lancet 382: 1258–1267. [DOI] [PubMed] [Google Scholar]
- 36. Martin DF, Maguire MG, Fine SL, Ying GS, Jaffe GJ, et al. (2012) Ranibizumab and bevacizumab for treatment of neovascular age-related macular degeneration: two-year results. Ophthalmology 119: 1388–1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The inclusion criteria of the patients in the studies included in the meta-analysis.
(DOCX)
PRISMA checklist.
(DOC)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.