Abstract
This study evaluated bacterial etiology and antibiotic susceptibility in patients diagnosed with community-acquired perforated appendicitis over a 12-year-period. We retrospectively reviewed records of adult patients diagnosed with perforated appendicitis at an 800-bed teaching hospital between January 2000 and December 2011. In total, 415 culture-positive perforated appendicitis cases were analyzed. Escherichia coli was the most common pathogen (277/415, 66.7%), followed by Streptococcus species (61/415, 14.7%). The susceptibility of E. coli to ampicillin, piperacillin/tazobactam, ceftriaxone, cefepime, amikacin, gentamicin, and imipenem was 35.1%, 97.1%, 97.0%, 98.2%, 98.9%, 81.8%, and 100%, respectively. The overall susceptibility of E. coli to quinolones (ciprofloxacin or levofloxacin) was 78.7%. During the study period, univariate logistic regression analysis showed a significant decrease in E. coli susceptibility to quinolones (OR = 0.91, 95% CI 0.84–0.99, P = 0.040). We therefore do not recommend quinolones as empirical therapy for community-acquired perforated appendicitis.
Introduction
Acute appendicitis is one of the most common abdominal surgical emergencies; it is also typically a community-acquired infection. Despite the generally favorable outcome, complicated appendicitis, such as perforated appendicitis, is associated with increased morbidity compared with simple acute appendicitis [1], [2]. Because Escherichia coli and Bacteroides fragilis are most commonly associated with appendicitis, antibiotic therapies are generally selected to target these bacteria [3], [4].
For adult patients with community-acquired complicated intra-abdominal infections of mild-to moderate severity, the use of ticarcillin-clavulanate, cefoxitin, ertapenem, moxifloxacin, or tigecycline as single-agent therapy or combinations of metronidazole with cefazolin, cefuroxime, ceftriaxone, cefotaxime, levofloxacin, or ciprofloxacin are recommended by Infectious Diseases Society of America (IDSA) guidelines [5]. However, with increased E. coli resistance to quinolones, investigation of local microbiologic findings had been proposed when selecting empirical therapies [5].
Because previous literature has reported a proportionally greater ratio of extended-spectrum β-lactamase (ESBL) and quinolone-resistant E. coli among bacteria responsible for community-acquired abdominal infections in Asia compared to other regions, careful selection of empirical antibiotics is particularly important in Asia [6]–[8]. We therefore conducted a study of the local microbiological profile and changes in antibiotic resistance in community-acquired perforated appendicitis over the past 12 years. These results may help us to inform selection of empirical antibiotic treatments for community-acquired complicated appendicitis.
Materials and Methods
Patient selection and data collection
We retrospectively reviewed the records of adult patients (age ≥18 years) who were diagnosed to have perforated appendicitis at Ulsan University Hospital, an 800-bed teaching hospital, between January 2000 to December 2011. Hospital charts and follow-up records were reviewed.
Definitions
Perforated appendicitis was defined as either gross or microscopic evidence of appendiceal perforation. The appendix was not considered to be ruptured by the mere presence of suppurative peritoneal fluid or gangrenous appendicitis without microscopic evidence of perforation.
Community-acquired appendicitis was defined as appendicitis that occurred within 48 hours of hospital admission. Patients were excluded from the study if they had at least 1 of the following health care risk factors: 1) presence of an invasive device at time of admission, 2) history of MRSA infection or colonization, 3) history of surgery, hospitalization, dialysis, or residence in a long-term care facility in the 12 months preceding the culture date [5].
Systemic inflammatory response syndrome (SIRS), sepsis, severe sepsis, and septic shock were defined as described elsewhere [9].
Specimen culture, species identification, and susceptibility testing
Specimens were obtained by swabbing the suppurative peritoneal fluid or periappendiceal abscess. In some cases, specimens were obtained by swabbing the lumen of appendix or by retrieving the suppurative peritoneal fluid via syringe aspiration. The swab specimens were transported to the laboratory in a transport medium (Amies transport medium without Charcoal; Asan Pharmaceuticals Co., Ltd., Hwasung, Korea). The specimens were either dispatched to the microbiology laboratory directly or stored in the operating room until the next day if collected after the working hours. The specimens were inoculated on blood agar, chocolate agar, and MacConkey agar plates. Samples were not inoculated into anaerobic culture. An automated VITEK 2 system (bioMerieux, Inc. Durham, NC, USA) was used to identify pathogens and perform ESBL susceptibility testing. The Vitek 2 ESBL test has 6 wells containing cefepime at 1 µg/mL, cefotaxime at 0.5 µg/mL, and ceftazidime at 0.5 µg/mL alone and in combination with clavulanic acid (10 µg/mL, 4 µg/mL, and 4 µg/mL, respectively); growth rate in each well is quantitatively assessed with an optical scanner. The proportional growth reduction (over 50%) in wells containing cephalosporin plus clavulanic acid compared with those containing cephalosporin alone was considered evidence of ESBL production. Susceptibility testing results were interpreted according to the National Committee for Clinical Laboratory Standards (CLSI) guidelines published in 2009 [10]. However, cephalosporin susceptibility results of ESBL-positive strains were interpreted on the basis of the strains’ respective minimal inhibitory concentration (MIC) breakpoints.
Ethics statement
This retrospective study was approved by the Institutional Review Board (IRB) committee of Ulsan University Hospital. Written consent given by the patients was waived by the approving IRB.
Statistical analysis
Statistical analyses were performed by using IBM SPSS Statistics for Windows, version 21 (IBM Corp., Armonk, NY, USA). The Chi-squared test was used to compare frequencies. A univariate logistic regression model was used to calculate odds ratios (OR), 95% confidence intervals, and p-values. The significance level was set at 0.05.
Results
Study population and clinical characteristics
Of 3,379 patients, 567 (16.7%) who received appendectomies during the study period were diagnosed with perforated appendicitis. Of these, we discarded 4 cases of health care-associated infection, 6 without confirmatory cultures, and 142 culture-negative cases; in total, we analyzed 415 culture-positive perforated appendicitis cases. The average length of hospitalization was 9.1±5.1 days. Patient ages ranged between 20 years and 94 years (mean 48.6±17.0 years), with 51.1% (212/415) men (Table 1). A majority of patients (404, 97.3%) underwent open appendectomy via a McBurney incision; laparotomy with a low midline incision was performed in 9 patients (2.2%) and laparoscopic appendectomy was performed in 2 patients (0.5%). The most common underlying disease was hypertension, reported in 56 patients (13.5%). Severe sepsis or septic shock was observed in 70 patients (16.8%), while 1 patient (0.2%) died of sepsis after mechanical ileus. Post-operative complications included wound infection in 18 patients (4.3%), abdominal abscesses or peritonitis in 7 patients (1.6%), and mechanical ileus in 6 patients (1.4%). A combination therapy comprising cephalosporin and metronidazole was the most frequent empirical antibiotic treatment.
Table 1. Baseline characteristics of patients with perforated appendicitis.
| Characteristics | Number (%) |
| Number of culture positive patients | 415 |
| Hospital day (Mean ± SD) | 9.1±5.1 |
| Age (Mean ± SD) | 48.6±17.0 |
| Sex | |
| male | 212 (51.1) |
| female | 203 (48.9) |
| Operation method | |
| laparoscopic | 2 (0.5) |
| McBurney | 404 (97.3) |
| laparotomy | 9 (2.2) |
| Underlying disease | |
| hypertension | 56 (13.5) |
| diabetes mellitus | 26 (6.3) |
| hepatitis B virus | 16 (3.9) |
| solid cancer | 12 (2.9) |
| Initial manifestation | |
| infection without SIRS | 73 (17.6) |
| sepsis | 272 (65.7) |
| severe sepsis | 67 (16.1) |
| septic shock | 3 (0.7) |
| In-hospital mortality | |
| alive | 414 (99.8) |
| death | 1 (0.2) |
| Infectious complication | |
| wound infection | 18 (4.3) |
| intra-abdominal abscess or peritonitis | 7 (1.6) |
| mechanical ileus | 6 (1.4) |
| Antibiotics | |
| 1st (or 2nd) generation cephalosporin + metronidazole | 215 (51.8) |
| 3rd generationcephalosporin + metronidazole | 193 (46.5) |
| ciprofloxacin + metronidazole | 4 (1.0) |
| piperacillin/tazobactam | 3 (0.7) |
SIRS = systemic inflammatory response syndrome.
Microbiological features
The most commonly isolated bacteria was E. coli (277 isolates, 66.7%), followed by Streptococcus spp. (61, 14.7%), Enterococcus spp. (32, 7.7%), Klebsiella spp. (25, 6.0%), and Pseudomonas aeruginosa (24, 5.8%) (Table 2). More than 2 organisms were isolated in 75 cases (18.0%).
Table 2. Distribution of bacterial species.
| Species | Number (%)f | |
| Gram negative organism | Escherichia coli | 277 (66.7) |
| Klebsiella speciesa | 25 (6.0) | |
| Pseudomonas aeruginosa | 24 (5.8) | |
| Other gramnegative organismb | 45 (10.8) | |
| Gram positive organism | Streptococcus speciesc | 61 (14.7) |
| Enterococcus speciesd | 32 (7.7) | |
| Staphylococcus aureus | 6 (1.4) | |
| Other gram positive organisme | 23 (5.5) |
Includes: K. pneumoniae, K. oxytoca.
Includes: Achromobacter xylosoxidans, Acinetobacter lwoffii, Aeromonas hydrophila, Comamonas testosteroni, Hafnia alvei, Proteus mirabilis, Raoultella planticola, Serratia species, Enterobacter cloacae.
Includes: S. alactolyticus, S. anginosus, S. cristatus, S. constellatus, S. gordonii, S. intermedius, S. mitis, S. salivarius, S. sanguinis, Viridans Streptococci.
Includes: E. avium, E. faecalis, E. faecium, E. gallinarum, E. hirae, E. raffinosus.
Includes: Gemella morbillorum, Lactococcus garvieae, Leuconostoc mesenteroides, Pediococcus pentosaceus.
Polymicrobial infection: 75 cases (18.0%).
Antibiotic susceptibilities of isolated organisms
Data on antibiotic susceptibilities of isolated organisms showed that E. coli had 78.7% susceptibility to quinolones (ciprofloxacin or levofloxacin). Susceptibilities to ampicillin, aztreonam, ampicillin/sulbactam, amoxicillin/clavulanic acid, piperacillin/tazobactam, cefazolin, cefoxitin, ceftriaxone, cefepime, trimethoprim/sulfamethoxazole, amikacin, gentamicin, tobramycin, and imipenem were 35.1%, 95.2%, 41.4%, 83.5%, 97.1%, 89.8%, 97.7%, 97.0%, 98.2%, 65.6%, 98.9%, 81.8%, 83.4%, and 100%, respectively (Table 3). ESBL-producing strains accounted for 3.9% of E. coli species. Streptococcus species showed 68.9% susceptibility to penicillin, and 100% susceptibility to ceftriaxone. Enterococcus species were 71.9% susceptible to penicillin. The susceptibilities of P. aeruginosa to piperacillin/tazobactam, cefepime, quinolones, amikacin, and imipenem were 95.2%, 100%, 87.5%, 100%, and 95.8%, respectively.
Table 3. Antibiotic susceptibilities of isolated organisms that caused perforated appendicitis.
| E. coli (total) | E. coli (non-ESBL) | E. coli (ESBL) | Streptococcusspecies | Enterococcus species | P. aeruginosa | |
| Antibiotic | (n = 277) | (n = 266) | (n = 11) | (n = 61) | (n = 32) | (n = 24) |
| Penicillin | 42/61(68.9) | 23/32(71.9) | ||||
| Ampicillin | 97/276 (35.1) | 97/265 (36.6) | 0/11(0) | 24/27(88.8) | ||
| Aztreonam | 220/231 (95.2) | 217/220 (98.6) | 3/11(27.2) | |||
| Ampicillin/sulbactam | 84/203 (41.4) | 84/200 (42.0) | 0/3 (0) | |||
| Amoxicillin/clavulanic acid | 61/73 (83.5) | 55/65 (84.6) | 6/8(75.0) | |||
| Piperacillin/tazobactam | 240/247 (97.1) | 229/236 (97.0) | 11/11 (100) | 20/21 (95.2) | ||
| Cefazolin | 248/276 (89.8) | 248/265 (93.5) | 0/11(0) | |||
| Cefoxitin | 264/270 (97.7) | 254/259 (98.0) | 10/11 (90.9) | |||
| Ceftriaxone | 267/275 (97.0) | 262/264 (99.2) | 5/11(45.4) | 39/39(100) | 2/24 (8.3) | |
| Cefepime | 227/231 (98.2) | 220/220 (100) | 7/11(63.6) | 22/22 (100) | ||
| Quinolone | 218/277 (78.7) | 215/266 (80.8) | 3/11(27.2) | 25/28(89.2) | 21/24 (87.5) | |
| Trimethoprim/sulfamethoxazole | 181/276 (65.6) | 177/265 (66.7) | 4/11(36.3) | 34/47(72.3) | 11/17(64.7) | 1/24 (4.1) |
| Amikacin | 274/277 (98.9) | 264/266 (99.2) | 10/11 (90.9) | 24/24 (100) | ||
| Gentamicin | 226/276 (81.8) | 221/265 (83.4) | 5/11(45.4) | 24/24 (100) | ||
| Tobramycin | 231/277 (83.4) | 227/266 (85.3) | 4/11(36.3) | 24/24 (100) | ||
| Vancomycin | 60/61(98.3) | 30/32(93.7) | ||||
| Imipenem | 276/276 (100) | 265/265 (100) | 11/11 (100) | 11/11(100) | 23/26(88.5) | 23/24 (95.8) |
Comparisons of bacterial species and E. coli isolate antibiotic susceptibilities by clinical severity
We compared the bacterial species and antibiotic susceptibilities of E. coli isolates according to the clinically indicated severity (Table 4). The cases were redistributed into two major groups: “sepsis” and “severe sepsis.” Infected patients without SIRS and the patients with sepsis were grouped together in the “sepsis” group, whereas the patients with severe sepsis and septic shock were grouped together in the “severe sepsis” group. A total of 345 patients (83.1%) were included in the sepsis group and 70 (16.9%) were included in the severe sepsis group. E. coli isolates were found more frequently in the severe sepsis group (74.3%) than in the sepsis group (65.2%), but the difference was not statistically significant. The isolation rates of the other species were also not significantly different between groups. There were no statistically significant differences in E. coli susceptibility to all antibiotics between groups.
Table 4. Comparisons of bacterial species and antibiotic susceptibilities of E. coli between the sepsis group and the severe sepsis group.
| Sepsisa (%) | Severe sepsisb (%) | P-value | |
| Species | |||
| E. coli | 225/345 (65.2) | 52/70 (74.3) | 0.142 |
| P. aeruginosa | 20/345 (5.8) | 4/70 (5.7) | 0.978 |
| Streptococcus species | 52/345 (15.1) | 9/70 (12.9) | 0.633 |
| Enterococcus species | 25/345 (7.3) | 7/70 (10.0) | 0.431 |
| Antibiotics susceptibilities of E. coli | |||
| Piperacillin/tazobactam | 197/202 (97.5) | 43/45 (95.6) | 0.472 |
| Cefoxitin | 214/220 (97.3) | 50/50 (100) | 0.238 |
| Ceftriaxone | 215/223 (96.4) | 52/52 (100) | 0.166 |
| Cefepime | 184/187 (98.4) | 43/44 (97.7) | 0.760 |
| Ciprofloxacin or levofloxacin | 176/225 (78.2) | 42/52 (80.8) | 0.686 |
Infection without SIRS (systemic inflammatory response syndrome) & sepsis.
Severe sepsis & septic shock.
Changes in E. coli antimicrobial susceptibility according to the year
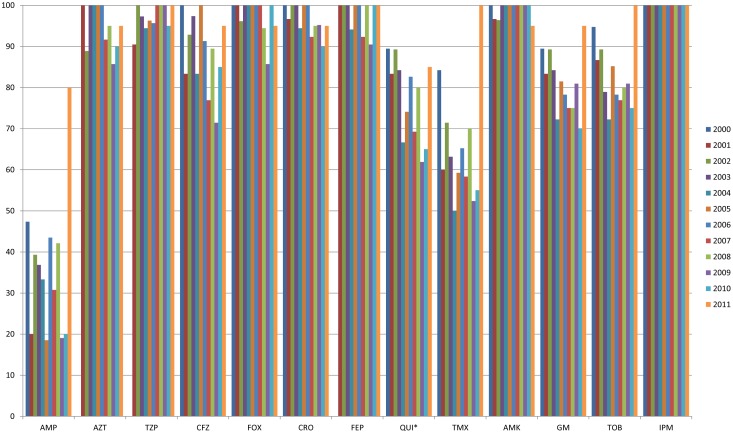
Yearly changes in E. coli antimicrobial susceptibility during the study period were examined (Fig. 1). Univariate logistic regression analysis showed that E. coli susceptibility to quinolones significantly decreased, with annual susceptibility rates of 89.4%, 83.3%, 89.2%, 84.2%, 66.6%, 74.0%, 82.6%, 69.2%, 80.0%, 61.9%, 65.0%, and 85.0%, during the period of 2000 to 2011 (OR = 0.91, 95% CI 0.84–0.99, P = 0.040). In particular, E. coli susceptibility to cefoxitin (P = 0.052) and ceftriaxone (P = 0.054) decreased during the study period, but the change was not statistically significant. Nor were any statistically significant changes observed in E. coli susceptibility to other antibiotics such as ampicillin (P = 0.235), aztreonam (P = 0.168), piperacillin/tazobactam (P = 0.645), cefazolin (P = 0.126), cefepime (P = 0.393), trimethoprim/sulfamethoxazole (P = 0.732), amikacin (P = 0.835), gentamicin (P = 0.389), and tobramycin (P = 0.645).
Figure 1. Change of antimicrobial susceptibility among E. coli during the 12-year-period.
AMP, ampicillin; AZT, aztreonam; TZP, piperacillin/tazobactam; CFZ, cefazolin; FOX, cefoxitin; CRO, ceftriaxone; FEP, cefepime; QUI, quinolone; TMX, trimethoprim/sulfamethoxazole; AMK, amikacin; GM, gentamicin; TOB, tobramycin; IPM, imipenem. * During the study period, there was a significant decrease in antimicrobial susceptibility on univariate logistic regression analysis (P = 0.040).
Discussion
This study evaluated microbiological profiles and antibiotic susceptibilities of pathogens isolated from cases of perforated appendicitis. The flora detected in complicated intra-abdominal infection differs between community-acquired and nosocomial infections. We considered appendicitis suitable for studying community-acquired bacterial infections since this illness is largely community-acquired. In fact, only 4 patients discarded from analysis owing to health care-associated infections. P. aeruginosa isolates in this study showed overall high levels of antibiotic susceptibility with no multidrug-resistant strains, supporting the idea that appendicitis is more commonly a community-acquired rather than nosocomial infection [11].
E. coli was the most common pathogen identified in this study (66.7% of all isolates), similar to findings in previous appendicitis literature [3], [12]. Similarly, Streptococcus and Enterococcus species were the most frequently isolated gram-positive organisms [12], [13]. The ratio of ESBL-producing E. coli was 3.9%, within previously reported ranges of 3.5–15.4% [8], [14]. The isolation rate of E. coli was greater in the severe sepsis group, although this difference was not statistically significant. Some studies have reported that P. aeruginosa is a commonly isolated strain in appendicitis, with an isolation rate of 19–32%; however, this was not the case in the current study [12], [15].
Although E. coli showed a high susceptibility rate of 97% to second- and third-generation cephalosporins that are most commonly used for empirical antibiotic treatment, the susceptibility decreased during the study period, albeit without statistical significance (P = 0.052 and P = 0.054, respectively). The susceptibility to quinolones was 78.7%, with a statistically significant (P = 0.040) decrease during the study period. Previous studies by Bochicchio et al (2006) and Rob et al (2013) reported that the susceptibility rate of E. coli, isolated from appendicitis samples, to quinolones was 71.4–85.6% [6], [8]. The E. coli susceptibility to quinolones and cephalosporins reported by Rob et al (2013) was lower than that reported by Bochicchio et al (2006). This may be attributable to E. coli’s increased resistance to the antibiotics or the lowered MIC breakpoint for cephalosporins set by the CLSI guidelines. For most antibiotics, E. coli susceptibility rates observed in this study were similar to those reported by Bochicchio et al (2006), with the susceptibility rate to quinolones being slightly lower. Both previous studies found high susceptibilities to carbapenem, amikacin, and piperacillin/tazobactam; in this study, ESBL-producing organisms were particularly sensitive to piperacillin/tazobactam (12/12, 100%). The susceptibility of Streptococcus species to penicillin was 68.9%, and all strains were susceptible to ceftriaxone. P. aeruginosa isolated in this study was highly susceptible to amikacin, cefepime, piperacillin/tazobactam, and carbapenem, but was slightly less susceptible to quinolones (87.5%).
All patients undergoing operation for appendicitis should receive antimicrobial therapy [16]. Appropriate antimicrobial therapy includes agents effective against facultative and aerobic gram-negative organisms and anaerobic organisms. There are data that inadequate empiric antibiotic therapy results in increased morbidity or treatment failure in complicated appendicitis [17], [18]. If resistance to a given antibiotic is present in 10%–20% or more of isolates of a common intra-abdominal pathogen in the community, use of that agent should be avoided [5]. A report in Taiwan proposed that a quinolone be used to treat community-acquired complicated intra-abdominal infections, as E. coli was found to be 82–85% susceptible to ciprofloxacin and levofloxacin [19]. In this study, however, the resistance rate of E. coli to quinolones is >20%; therefore, its use as an empirical antibiotic is not advisable in Korea. Second- and third-generation cephalosporins appeared to be an appropriate treatment for this application according to our results. Although third-generation cephalosporins might be a better treatment choice because that Streptococcus species showed 100% susceptibility to ceftriaxone, further studies are needed to thoroughly trace variations in susceptibility, given that the decrease in E. coli susceptibility, observed during the study period, was not statistically significant. Piperacillin/tazobactam and carbapenem might be considered to treat P. aeruginosa or ESBL-producing organisms in patients with signs of severe sepsis such as organ dysfunction. However, these species were not frequently isolated in all patient groups including the severe sepsis group of the current study, and spectrum of these antibiotics may be too broad. E. coli also showed high susceptibility to amikacin, but concerns remain regarding use of aminoglycoside antibiotics owing to their nephrotoxicity and ototoxicity. Considering the high resistance of E. coli to ampicillin and ampicillin/sulbactam–and the questionable significance of enterococci as pathogens in complicated intra-abdominal infections–these antibiotics are not recommended for treating perforated appendicitis.
On the basis of evidence that culture testing of intraoperative specimens does not affect the prognosis of patients with perforated appendicitis, many institutions may not perform routine culture testing [20], [21]. However, considering the current reality of increasing antibiotic resistance, routine culture testing might be useful to identify changes in susceptibility and to select appropriate antibiotics [5]. Anaerobic cultures are not necessary for patients with community-acquired intra-abdominal infection if empiric antimicrobial therapy active against common anaerobic pathogens is provide [5]. Although anaerobic bacteria culturing was not performed in this study, previous reports on anaerobic culture showed that Bacteroides fragilis, along with E. coli, was the most commonly isolated pathogen in appendicitis [14], [20]. In past studies of appendicitis that conducted anaerobic susceptibility testing, B. fragilis was found to be more than 95% susceptible to metronidazole [4], [14], [22]. Anaerobic bacteria culturing could be considered for future studies if an increase in anaerobic bacterial resistance to metronidazole is observed.
The retrospective nature of the present study might have resulted in intrinsic bias and the data may not represent the entire population because data was collected from a single institution. However, considering that the quinolone resistance rate we observed was similar to that reported in previous studies conducted in Korea [23], [24]–which involved community-acquired E. coli bacteremia originated from various infections including intra-abdominal infection–we speculated that quinolone resistance rate among E. coli causing intra-abdominal infection in Korea should be similar to the one determined in this study.
In conclusion, E. coli was the most commonly identified pathogen in patients with perforated appendicitis. The quinolone resistance rate was >20% in E. coli isolated from community-acquired perforated appendicitis. The isolates were decreasingly susceptible to quinolones during the study period. We advise against the use of quinolones as a first line antibiotic therapy in community-acquired perforated appendicitis in Korea.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Lau WY, Wong SH (1981) Randomized, prospective trial of topical hydrogen peroxide in appendectomy wound infection. High risk factors. Am J Surg 142: 393–397. [DOI] [PubMed] [Google Scholar]
- 2. Schmit PJ, Hiyama DT, Swisher SG, Bennion RS, Thompson JE Jr (1994) Analysis of risk factors of postappendectomy intra-abdominal abscess. J Am Coll Surg 179: 721–726. [PubMed] [Google Scholar]
- 3. Bennion RS, Baron EJ, Thompson JE Jr, Downes J, Summanen P, et al. (1990) The bacteriology of gangrenous and perforated appendicitis–revisited. Ann Surg 211: 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Lau WY, Teoh-Chan CH, Fan ST, Yam WC, Lau KF, et al. (1984) The bacteriology and septic complication of patients with appendicitis. Ann Surg 200: 576–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Solomkin JS, Mazuski JE, Bradley JS, Rodvold KA, Goldstein EJ, et al. (2010) Diagnosis and management of complicated intra-abdominal infection in adults and children: guidelines by the Surgical Infection Society and the Infectious Diseases Society of America. Clin Infect Dis 50: 133–164. [DOI] [PubMed] [Google Scholar]
- 6. Bochicchio GV, Baquero F, Hsueh PR, Paterson DL, Rossi F, et al. (2006) In vitro susceptibilities of Escherichia coli isolated from patients with intra-abdominal infections worldwide in 2002–2004: results from SMART (Study for Monitoring Antimicrobial Resistance Trends). Surg Infect (Larchmt) 7: 537–545. [DOI] [PubMed] [Google Scholar]
- 7. Paterson DL, Rossi F, Baquero F, Hsueh PR, Woods GL, et al. (2005) In vitro susceptibilities of aerobic and facultative Gram-negative bacilli isolated from patients with intra-abdominal infections worldwide: the 2003 Study for Monitoring Antimicrobial Resistance Trends (SMART). J Antimicrob Chemother 55: 965–973. [DOI] [PubMed] [Google Scholar]
- 8. Lob SH, Badal RE, Bouchillon SK, Hawser SP, Hackel MA, et al. (2013) Epidemiology and susceptibility of Gram-negative appendicitis pathogens: SMART 2008–2010. Surg Infect (Larchmt) 14: 203–208. [DOI] [PubMed] [Google Scholar]
- 9. Bone RC, Sibbald WJ, Sprung CL (1992) The ACCP-SCCM consensus conference on sepsis and organ failure. Chest 101: 1481–1483. [DOI] [PubMed] [Google Scholar]
- 10.Clinical and Laboratory Standards Institute (2009) Performance standards for antimicrobial susceptibility testing. Nineteenth informational supplement. Document M100–S19. Wayne, PA: CLSI.
- 11. Montravers P, Lepape A, Dubreuil L, Gauzit R, Pean Y, et al. (2009) Clinical and microbiological profiles of community-acquired and nosocomial intra-abdominal infections: results of the French prospective, observational EBIIA study. J Antimicrob Chemother 63: 785–794. [DOI] [PubMed] [Google Scholar]
- 12. Guillet-Caruba C, Cheikhelard A, Guillet M, Bille E, Descamps P, et al. (2011) Bacteriologic epidemiology and empirical treatment of pediatric complicated appendicitis. Diagn Microbiol Infect Dis 69: 376–381. [DOI] [PubMed] [Google Scholar]
- 13. Chen CY, Chen YC, Pu HN, Tsai CH, Chen WT, et al. (2012) Bacteriology of acute appendicitis and its implication for the use of prophylactic antibiotics. Surg Infect (Larchmt) 13: 383–390. [DOI] [PubMed] [Google Scholar]
- 14. Chan KW, Lee KH, Mou JW, Cheung ST, Sihoe JD, et al. (2010) Evidence-based adjustment of antibiotic in pediatric complicated appendicitis in the era of antibiotic resistance. Pediatr Surg Int 26: 157–160. [DOI] [PubMed] [Google Scholar]
- 15. Fallon SC, Hassan SF, Larimer EL, Rodriguez JR, Brandt ML, et al. (2013) Modification of an evidence-based protocol for advanced appendicitis in children. J Surg Res 185: 273–277. [DOI] [PubMed] [Google Scholar]
- 16.Andersen BR, Kallehave FL, Andersen HK (2001) Antibiotics versus placebo for prevention of postoperative infection after appendectomy. Cochrane Database Syst Rev: CD001439. [DOI] [PubMed]
- 17. Yellin AE, Heseltine PN, Berne TV, Appleman MD, Gill MA, et al. (1985) The role of Pseudomonas species in patients treated with ampicillin and Sulbactam for gangrenous and perforated appendicitis. Surg Gynecol Obstet 161: 303–307. [PubMed] [Google Scholar]
- 18. Berne TV, Yellin AW, Appleman MD, Heseltine PN (1982) Antibiotic management of surgically treated gangrenous or perforated appendicitis. Comparison of gentamicin and clindamycin versus cefamandole versus cefoperazone. Am J Surg 144: 8–13. [DOI] [PubMed] [Google Scholar]
- 19. Lau YJ, Chen YH, Huang CT, Lee WS, Liu CY, et al. (2012) Role of moxifloxacin for the treatment of community-acquired [corrected] complicated intra-abdominal infections in Taiwan. J Microbiol Immunol Infect 45: 1–6. [DOI] [PubMed] [Google Scholar]
- 20. Kokoska ER, Silen ML, Tracy TF Jr, Dillon PA, Kennedy DJ, et al. (1999) The impact of intraoperative culture on treatment and outcome in children with perforated appendicitis. J Pediatr Surg 34: 749–753. [DOI] [PubMed] [Google Scholar]
- 21. Foo FJ, Beckingham IJ, Ahmed I (2008) Intra-operative culture swabs in acute appendicitis: a waste of resources. Surgeon 6: 278–281. [DOI] [PubMed] [Google Scholar]
- 22. Wojcik-Stojek B, Bulanda M, Martirosian G, Heczko P, Meisel-Mikolajczyk F (2000) In vitro antibiotic susceptibility of Bacteroides fragilis strains isolated from excised appendix of patients with phlegmonous or gangrenous appendicitis. Acta Microbiol Pol 49: 171–175. [PubMed] [Google Scholar]
- 23. Lee S, Han SW, Kim KW, Song do Y, Kwon KT (2014) Third-generation cephalosporin resistance of community-onset Escherichia coli and Klebsiella pneumoniae bacteremia in a secondary hospital. Korean J Intern Med 29: 49–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Park S, Park J, Lee S (2005) Analysis on the etiology and prognostic factors of community-acquired bacteremia in a community-based tertiary hospital. Infect Chemother 37: 255–264. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.