Abstract
Objective
To address the need for nucleos(t)ide reverse transcriptase inhibitor (NRTI)-sparing regimens, we explored the virologic and pharmacokinetic characteristics of maraviroc plus ritonavir-boosted darunavir in a single-arm, open-label, 96-week study.
Methods
24 antiretroviral-naïve R5 HIV-1-infected participants received maraviroc 150 mg and DRV/r 800/100 mg (MVC/DRV/r) once-daily. The primary outcome was virologic failure (VF) = confirmed viral load (VL) >50 copies/mL at week 24 in the modified intent-to-treat population. To determine viral dynamics, participant-specific first- and second-phase empirical Bayes estimates were compared to decay rates from efavirenz plus lopinavir/ritonavir, lopinavir/ritonavir plus 2NRTIs and efavirenz plus 2NRTIs. Maraviroc plasma concentrations were determined at weeks 2, 4,12, 24 and 48.
Results
Baseline median (Q1, Q3) CD4 count and VL were 455 (299, 607) cells/mm3 and 4.62 (4.18, 4.80) log10 copies/mL, respectively. VF occurred in 3/24 participants (12.5 % [95% CI 2.7, 32.4]) at week 24. One of these resuppressed, yielding a week 48 VF rate of 2/24 (8.3 % [95% CI 1.0, 27.0]). The week 48 failures were 2 of the 4 (50%) participants with baseline VL >100,000 copies/mL. Week 96 VF rate was 2/20 (10 % [95% CI 1.2, 31.7]). Phase 1 decay was faster with MVC/DRV/r than reported for ritonavir-boosted lopinavir plus 2 NRTIs (p=0.0063) and similar to efavirenz-based regimens. Individual maraviroc trough concentrations collected between 20–28 hours post dose (n=59) was 13.7 to 130 ng/mL (Q1, 23.4 ng/mL; Q3, 46.5 ng/mL), and modeled steady-state concentration was 128 ng/mL.
Conclusion
MVC/DRV/r 150/800/100 mg once-daily has potential for treatment-naïve patients with R5 HIV-1.
Keywords: maraviroc, darunavir, nucleos(t)ide-sparing, pharmacokinetics, viral dynamics
INTRODUCTION
Although several nucleos(t)ide reverse transcriptase inhibitor (NRTI)-sparing regimens have been investigated for initial treatment of HIV [1–4], all recommended regimens worldwide include two NRTIs [5–9]. Effective NRTI-sparing regimens would provide options for individuals with transmitted NRTI resistance and renal impairment and avoid long-term NRTI toxicities [10]. Maraviroc (MVC) is a CCR5 receptor antagonist with activity against R5 HIV-1 [11], and possible though unproven immunomodulatory properties [12, 13]. Darunavir (DRV) is a protease inhibitor (PI) with a high barrier against resistance [14]. Both MVC and ritonavir-boosted DRV (DRV/r) have reliable cerebrospinal fluid penetration [15, 16], rare serious toxicities [15, 17] and are associated with robust CD4+ T (CD4) cell reconstitution [18, 19].
The recommended MVC dose when combined with NRTIs is 300 mg twice-daily [5]. Among treatment-naïve patients randomized to MVC 300 mg plus lamivudine/zidovudine twice-daily in the MERIT study, the probability of virologic success decreased when average MVC plasma concentration (Cavg) and trough concentrations (Ctrough) fell below 75 ng/mL and 25 ng/mL, respectively [20]. DRV/r inhibits cytochrome P450 (CYP) 3A4-mediated metabolism of MVC, resulting in a four-fold increase in MVC area under the plasma concentration-time curve (AUC) [11, 21]. The recommended MVC dose when co-administered with DRV/r is 150 mg twice-daily [5]. However, half of the recommended dose (150 mg once-daily) combined with DRV/r 800/100 mg daily produced median (IQR) MVC Ctrough of 43 (35–55) ng/mL in a clinical cohort [22]. In the MOTIVATE study, treatment-experienced patients were randomized to placebo, MVC 150 mg once-daily or MVC 150 mg twice-daily combined with an optimized background regimen that included several investigator chosen PIs but not darunavir [23]. At 48 weeks, plasma HIV-1 RNA concentration (viral load, VL) was < 50 copies/mL in 179/414 (43%) and 194/426 (46%) of participants on daily versus twice-daily MVC, respectively.
We conducted the single-arm MaravIroc plus Darunavir/ritonavir Study (MIDAS) [clinicalTrials.gov Identifier: NCT00993148] to explore whether once-daily MVC 150 mg plus DRV/r 800/100 mg (MVC/DRV/r) is an active NRTI-sparing regimen for initial treatment of R5 HIV-1. We also evaluated the early HIV-1 decay and MVC pharmacokinetics (PK) of this novel regimen.
METHODS
Study participants
Participants were treatment-naïve HIV-1-infected patients who were at least 18 years old with: i) VL of 5,000 to 500,000 copies/mL within 90 days prior to study entry; ii) R5 virus by the enhanced sensitivity Trofile assay (Trofile ES); and iii) CD4 count > 100 cells/mm3. We excluded patients with active hepatitis B, protocol-specified abnormal laboratory values, or any DRV resistance-associated mutation (V11I, V32I, L33F, I47V, I50V, I54L, I54M, T74P, L76V, I84V and L89V). Each participant was invited to participate in a viral dynamics substudy. Ethics review committees at each research site approved the study. Participants were provided a written informed consent. An independent Monitoring Committee reviewed the study after the first 15 patients reached week 12.
Study intervention
Each participant received open-label DRV 800 mg (two 400 mg tablets), ritonavir 100 mg (one capsule) and MVC 150 mg (one tablet) co-administered once-daily with food.
Procedures and assessments
At the first screening visit, a Trofile ES assay on plasma was performed (Monogram, Inc., San Francisco, California, USA). Participants with R5 virus only returned for the second screening visit where other eligibility criteria were assessed. Study entry (day 0) occurred within 90 days of the first screening evaluation. Subsequent evaluations occurred at weeks 1, 2, 4, 12, 24, 36 48, 60, 72, 84 and 96. VL was determined at entry and all subsequent evaluation time-points. Hematologic, liver function and blood chemistry tests were performed at entry and weeks 2, 4, 12, 24, 36, 48, 60, 72, 84 and 96. CD4 count was determined at entry and weeks 12, 24, 36, 48, 60, 72, 84 and 96. Fasting lipid levels were measured at entry and weeks 24, 48 and 96. Participants in the viral dynamics sub-study underwent additional VL determination on days 2, 4 and 10. Random samples for PK evaluation were collected and an adherence questionnaire was administered at weeks 2, 4,12, 24 and 48. Participants were classified as perfectly adherent if they reported taking study medications with food and had no missed doses in the preceding 4 days [24]. Participants with suspected virologic failure (VF) returned within 7–35 days for a failure confirmation visit where adherence was assessed and samples collected for VL, protease genotype, Trofile ES, MVC phenotypic assay, CD4 count, and PK evaluation.
VL was determined using the COBAS® AmpliPrep/COBAS® Taqman® HIV-1 assay (Roche). Resistance to PIs at the time of VF was assessed by genotyping the HIV-1 protease gene from plasma HIV-1 RNA. To isolate and sequence independent full-length env clones, viral RNA was extracted from patient plasma samples (QIAamp, Qiagen). Independent env gp160 amplicons were generated by nested PCR as previously described [25]. Tropism and MVC resistance testing were done at time of VF.
Maraviroc bioanalysis and pharmacokinetics
A validated protein precipitation method using acetonitrile (AcN) containing internal standard (MVC-d6) was employed to extract MVC from human plasma. An aliquot of the supernatant was further diluted with 0.5% trifluoroacetic acid to maintain signal intensity within the linear range of the instrument. Reversed phase chromatographic separation was performed on an XBridge™ C18 analytical column (2.1 × 50mm, 3.5mm) under isocratic conditions. A binary mobile phase consisting of 0.1% formic acid in water and 0.1% formic acid in acetonitrile (72:28) was used and provided adequate separation from other analytes in the assay. Detection and quantitation was achieved by multiple reaction monitoring (MRM), and MVC and internal standard (MVC-d6) were detected using the following transitions for protonated molecular products [M+H]+: m/z MVC 514.2 → 106.0; m/z MVC-d6 520.3 → 115.0. The dynamic range was 5 to 5,000 ng/mL using a 20 µL plasma sample. PK modeling was conducted using ADAPT 5 (Biomedical Simulations Resource, Los Angeles, CA). [26]. A two-compartment model was utilized and MVC absorption and clearance processes were assumed to be linear. Since few data points were available in the absorptive phase, the absorption rate constant (Ka) was fixed at 1.0 and no lag time was assessed. Covariates were not examined in this PK dataset.
Outcome measures
The primary outcome was VF (defined as confirmed plasma VL > 50 copies/mL) at week 24. Secondary outcome measures were VF at weeks 48 and 96, change in CD4 count, adherence to study treatment, MVC PK, early viral decay, incidence of grade ≥3 or any grade if it led to drug discontinuation, change in viral tropism or emergence of protease or MVC resistance.
Statistical methods
With a sample size of 25 participants, assuming a 10% participant loss by week 24, if the observed VF rate was between 15% and 25%, then the 95% confidence interval (CI) would have a width of ±15% to ±18%. The 95% CI width was calculated using large sample approximation assuming a binomial distribution. Efficacy analysis was based on a modified intent-to-treat (ITT) population, which included all participants who initiated MVC/DRV/r and censored participants at time of loss to follow-up or treatment modification if the last VL was < 50 copies/mL. VL < 50 copies/mL while on MVC/DRV/r was considered a success. In secondary analysis, participants lost to follow up or who had any treatment modification were considered failures.
Viral decay rates were estimated with a bi-exponential nonlinear mixed effects model using VL at days 0, 2, 4, 7, 10, 14 and 28 after initiating MVC/DRV/r. Models were fit to the data on a log10 scale to normalize the error distribution [27]. Participant-specific first- and second-phase empirical Bayes estimates were compared to decay rates from efavirenz (EFV) plus lopinavir/ritonavir (LPV/r), LPV/r plus 2NRTIs and EFV plus 2NRTIs arms of ACTG A5160s [28] and EFV plus 2NRTIs arm of ACTG A5166s [29] using the primary data. We used a 2-sided Wilcoxon rank sum test unadjusted for multiple comparisons (A5160s and A5166s decay curves were determined from data through week 8). Models were also fit through week 12 to investigate bias of decay estimates in comparison to A5160s and A5166s since week 8 VLs were not collected with MVC/DRV/r. Viral decay models through week 4 are reported to eliminate bias from censoring undetectable VL values (0% through week 4 vs. 27% through week 12).
RESULTS
Study Participants
A total of 46 antiretroviral naïve HIV-1-infected volunteers underwent screening at five U.S. research sites. Nine of these (20%) had non-R5 virus and 12 failed other eligibility criteria. Twenty-five participants with R5 HIV-1 enrolled in the study: median (Q1, Q3) age was 38 (31, 43) years, 88% were male, and 60% were White non-Hispanic. Baseline median CD4 count and VL were 455 (299, 607) cells/mm3 and 4.62 (4.18, 4.80) log10 copies/mL, respectively. VL was >100,000 copies/mL in 4 (16%) participants, 10,000–100,000 copies/mL in 16 (64%) participants, and <10,000 copies/mL in 5 (20%). Baseline resistance mutations were detected in 5 (20%) participants: 1 had PI (D30N) plus NRTI (L210W, M41L, T215C) mutations; 3 had NNRTI (K103N, Y181C) mutations only and 1 had NNRTI (Y181C) plus NRTI (M41L, T215D) mutations.
Virologic response
One participant did not initiate MVC/DRV/r and was not included in the analysis. Twenty four participants initiated MVC/DRV/r All the participants with confirmed VL > 50 copies/mL at or after week 24 are shown in Table 1. Participants A, B and D experienced VF at week 24; VF rate = 3/24 (12.5 % [95% CI 2.7, 32.4]). All these participants remained on MVC and one (Participant D) later resuppressed to VL <50 copies/mL. VF rate at week 48 was 2/24 (8.3 % [95% CI 1.0, 27.0]). The week 48 failures were 2 of the 4 participants (50%) with baseline VL >100,000 copies/mL. All the 20 participants with baseline VL <100,000 copies/mL had VL <50 copies/mL at week 48. In secondary analysis considering participants lost to follow up or who had any treatment modification as failures, VF rates at weeks 24 and 48 remained unchanged because none of the 24 participants who initiated MVC/DRV/r was lost to follow up or had treatment modification through week 48.
Table 1.
Participants with any viral load > 50 copies/mL at or after week 24 *
| HIV RNA copies/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baseline | Wk 2 |
Wk 4 |
Wk 12 |
Wk 24 |
Wk 36 |
Wk 48 |
Wk 60 |
Wk 72 |
Wk 84 |
Wk 96 |
Treatment modification** |
Tropism | Genotype | Maraviroc plasma Concentration (time post dose)*** |
|
| A | 277,373 | 3,775 | 1,951 | 219 | 82/67 | 119 | 78 | 249 | 89 | <50 | <50 | TDF/FTC, DRV/r (after the Wk 84 visit) | NR (Wk 4) | Failed to amplify (Wk 60) | 29.5 ng/mL (23 hours) at Wk 24 |
| B | 156,013 | 1,806 | 1,320 | 365 | 224/496 | 247 | 257 | 268 | 1505/3826 | <50 | <50 | EFV/TDF/FTC (at Wk 78) | R5 (Wk 4), NR(Wk 72) | Failed to amplify (Wk 72) | 52.1 ng/mL (22 hours) at Wk 24 |
| C | 166,567 | 1,791 | 338 | 51 | <50 | 94/98 | <50 | <50 | <50 | <50 | <50 | EFV/TDF/FTC (after the Wk 48 visit) | ND | ND | Sampling ND at Wk 36 |
| D | 7,903 | 438 | 204 | <50 | 989/114 | <50 | <50 | <50 | <50 | <50 | <50 | None | ND | ND | 109.0 ng/mL (10 hours) at Wk 24 |
| E | 15,150 | 439 | 300 | 58 | 50 | <50 | <50 | <50 | 139/<50 | <50 | 1815/165 | None | R5 (Wk 96) | Failed to amplify (Wk 96) | 167.5 ng/mL (9 hours) at Wk 96 |
Abbreviations: TDF/FTC, Truvada®; EFV/TDF/FTC, Atripla®; ND, not done; NR, not reportable; WK, week
All study participants remained on MVC/DRV/r through week 96 unless indicated in the treatment modification column. Some HIV RNA values are initial/confirmatory measurements at that timepoint
Participants A and C were switched before the VL results became available from weeks 84 and 48, respectively
Maraviroc trough concentration window is 20–28 hours post dose
To derive the week 96 VF rate, we censored 2 participants who were lost to follow up after week 48 (at week 72 and week 84, respectively) while their VLs were < 50 copies/mL on MVC/DRV/r (not shown in Table 1). In addition, we censored 2 other participants (A and C in Table 1) who switched from MVC/DRV/r while suppressed. Thus, the virologic failures at week 96 were Participants B and E in Table 1, yielding a VF rate of 2/20 (10 % [95% CI 1.2, 31.7]). In secondary analysis considering participants lost to follow up or who had any treatment modification as failures, VF at week 96 was 6/24 (25 % [95% CI 9.7, 46.7]). All the subjects with VF reported perfect adherence at that time point, except Patient E at week 96.
CD4 response and safety
Median (Q1, Q3) CD4 count change from baseline was +247 (119, 340) cells/mm3 and +216 (119, 346) cells/mm3 at weeks 48 and 96, respectively. The only grade 3 abnormality assessed as at least possibly related to the study regimen was LDL-cholesterol elevation in one participant. There were no grade 4 adverse events or study discontinuations due to adverse events.
Resistance
None of the participants with VF had any baseline resistance mutation. We limited Trofile ES, protease genotyping and phenotypic testing of MVC susceptibility to participants with VL > 200 copies/mL after week 48 (Patients A, B and E in Table 1). HIV-1 tropism remained R5 in the 2 samples that were successfully tested. Genotypic and phenotypic DRV and MVC resistance testing could not be performed because HIV-1 pol and envelop amplification failed with different amplification strategies in all tested samples most of which had too low plasma virus concentrations. To confirm RNA integrity, gag was successfully amplified from one of the two patients, suggesting that primer mismatch may also have played a role in our inability to amplify and sequence HIV-1 pol and envelope.
Viral dynamics
Fifteen participants enrolled in the viral dynamics sub-study with median (Q1,Q3) pretreatment VL of 4.6 (4.2,4.8) log10 copies/mL. As shown in Table 2, median phase 1 decay was faster with MVC/DRV/r than reported for LPV/r plus 2 NRTIs [28]. The faster decay corresponded to a shorter median half-life (1.0 day vs. 1.3 days, respectively). The median phase 1 decay rate in this study was not significantly different from the phase 1 decay rates reported for EFV plus LPV/r or EFV plus 2 NRTIs, respectively [28, 29]. Median phase 2 decay with MVC/DRV/r was slower than reported for EFV plus LPV/r, LPV/r plus 2NRTIs and EFV plus 2NRTIs, respectively in A5160s [28], and slower than the phase 2 decay rate reported for EFV plus 2 NRTIs in A5166s [29]. Population average (fixed effects) biexponential decay in VL is shown in Figure 1. Since VL was not collected at week 8 in the current study, a sensitivity analysis was conducted to determine if observed decay rates were affected by fitting a model to week 4. We found no significant differences between the first- and second-phase decay rates of models run through week 4 or through week 12 (p>0.7).
Table 2.
Phase 1 and phase 2 HIV-1 RNA decay parameters of MIDAS study (through week 4 or week 12) compared to historical controls receiving EFV plus LPV/r, LPV/r plus 2NRTIs and EFV plus 2NRTIs in ACTG A5160s [28] and EFV plus 2NRTIs in ACTG A5166s [29]
| Estimated decay parameter (per day) |
|||||
|---|---|---|---|---|---|
| Regimen | N | Median | (Q1, Q3) | Median half-life, days |
P-value* |
| Phase 1 | |||||
| MIDAS: MVC+DRV/r (weeks 0–4) | 15 | 0.69 | (0.58,0.76) | 1.00 | - |
| MIDAS: MVC+DRV/r (weeks 0–12) | 15 | 0.69 | (0.59,0.73) | 1.00 | 0.9349 |
| A5160s Arm A: EFV+LPV/r | 21 | 0.61 | (0.52, 0.68) | 1.14 | 0.2651 |
| A5160s Arm B: LPV/r+2NRTIs | 22 | 0.53 | (0.38, 0.66) | 1.31 | 0.0063 |
| A5160s Arm C: EFV+2NRTIs | 25 | 0.63 | (0.57, 0.70) | 1.09 | 0.3909 |
| A5166s Arm C: EFV+2NRTIs | 16 | 0.67 | (0.60, 0.73) | 1.03 | 0.5986 |
| Phase 2 | |||||
| MIDAS: MVC+DRV/r (weeks 0–4) | 15 | 0.021 | (0.009, 0.039) | 32.65 | - |
| MIDAS: MVC+DRV/r (weeks 0–12) | 15 | 0.025 | (0.020, 0.029) | 28.03 | 0.6529 |
| A5160s Arm A: EFV+LPV/r | 21 | 0.045 | (0.035, 0.048) | 15.32 | 0.0024 |
| A5160s Arm B: LPV/r+2NRTIs | 22 | 0.046 | (0.043, 0.049) | 15.02 | 0.0005 |
| A5160s Arm C: EFV+2NRTIs | 25 | 0.036 | (0.032, 0.042) | 19.49 | 0.0176 |
| A5166s Arm C: EFV+2NRTIs | 16 | 0.055 | (0.046, 0.070) | 12.60 | 0.0001 |
P-values come from a Wilcoxon rank sum test not adjusted for multiple comparisons of differences in decay estimates between the MIDAS study and arms from 2 comparison studies; decay estimates are reported from A5160s and A5166s from models fit through week 8; a model fit through week 12 of the MIDAS data is also shown as a comparison to the model run through week 4.
Figure 1.
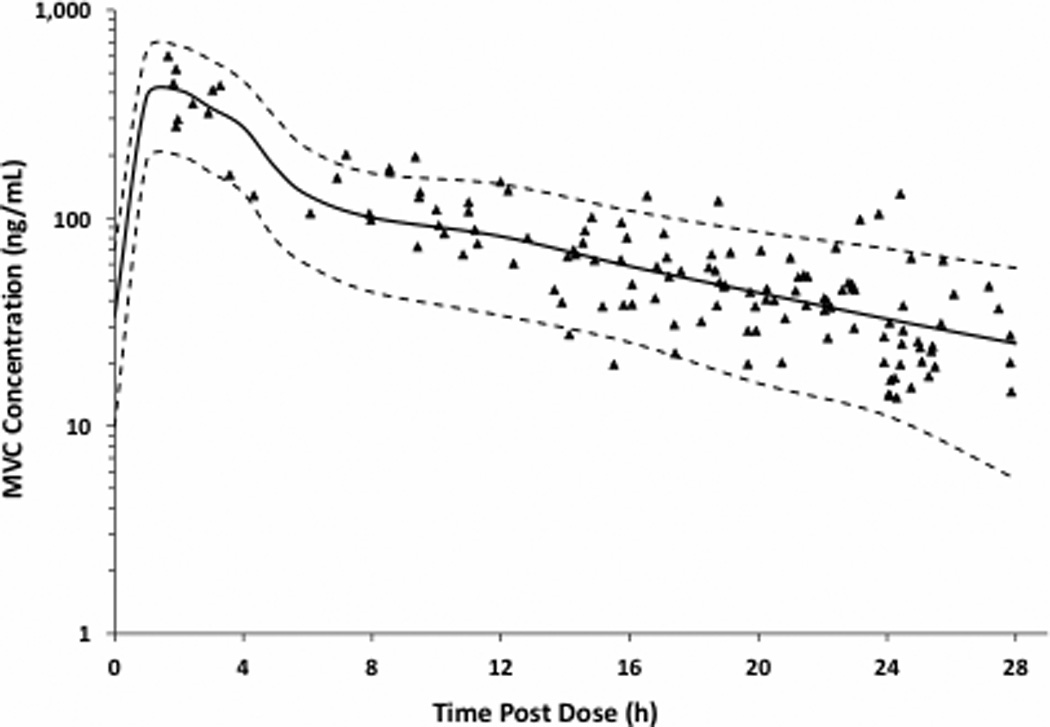
Bi-exponential VL decay curves in the MIDAS study and two different ACTG clinical trials with NRTI-sparing arms. Using primary data from two AIDS Clinical Trials Group studies: EFV plus LPV/r, LPV/r plus 2NRTIs and EFV plus 2NRTIs in A5160s [28] and EFV plus 2NRTIs in A5166s [29]
Median (Q1, Q3) transition time (the day and HIV-RNA level at which production of HIV-RNA decay from short- and longer-lived cells is equal) was longer with MVC/DRV/r (13 days (11,17)) compared to EFV plus LPV/r and EFV plus 2NRTIs in A5160s (12 days (11,13)) and EFV plus 2NRTIs in A5166s (11 days (10,13)). An earlier median transition time was observed when compared to the LPV/r plus 2NRTIs arm (14 days (12,19)). Median predicted VL at transition was higher for MVC/DRV/r compared to the two EFV plus 2NRTIs arms (2.79 log10 copies/mL vs. 2.65 and 2.78). Median predicted VL at transition was lower than EFV plus LPV/r and LPV/r plus 2NRTIs (2.93 and 2.95 log10 copies/mL, respectively).
Pharmacokinetics
A total of 145 MVC plasma concentration-time points were collected. Of these, 59 fell within the 20–28 hour Ctrough collection window and 133 were used for modeling. From the raw data, the average peak (between 1–4 hours post dose) was 363 ng/mL and the average (± standard deviation) Ctrough (between 20–28 hours post dose) was 39.3 ± 22.8 ng/mL. Overall, individual Ctrough values ranged from 13.7 to 130 ng/mL (Q1, 23.4 ng/mL; Q3, 46.5 ng/mL). A linear two-compartment model provided reasonable fits to the data (Figure 2). The modeled MVC clearance (CL/F) was 48 ± 8.4 L/h. Central distribution volume (Vc/F), intercompartmental clearance (CLd), and peripheral distribution volume (Vp/F) were 213±35 L, 42.5±21.6 L/h, and 278±167 L, respectively. The median modeled AUC24 was 3073 ng·h/mL and the steady-state concentration (Cavg) was 128 ng/mL. The population half-life (T1/2) was estimated to be 10.3±3.5 hours. The modeled MVC peak (Cmax at 2 hours post dose) and Ctrough (at 24 hours) concentrations were 415 and 36.1 ng/mL, respectively. VF was not explained by MVC plasma concentrations (Table 1).
Figure 2.
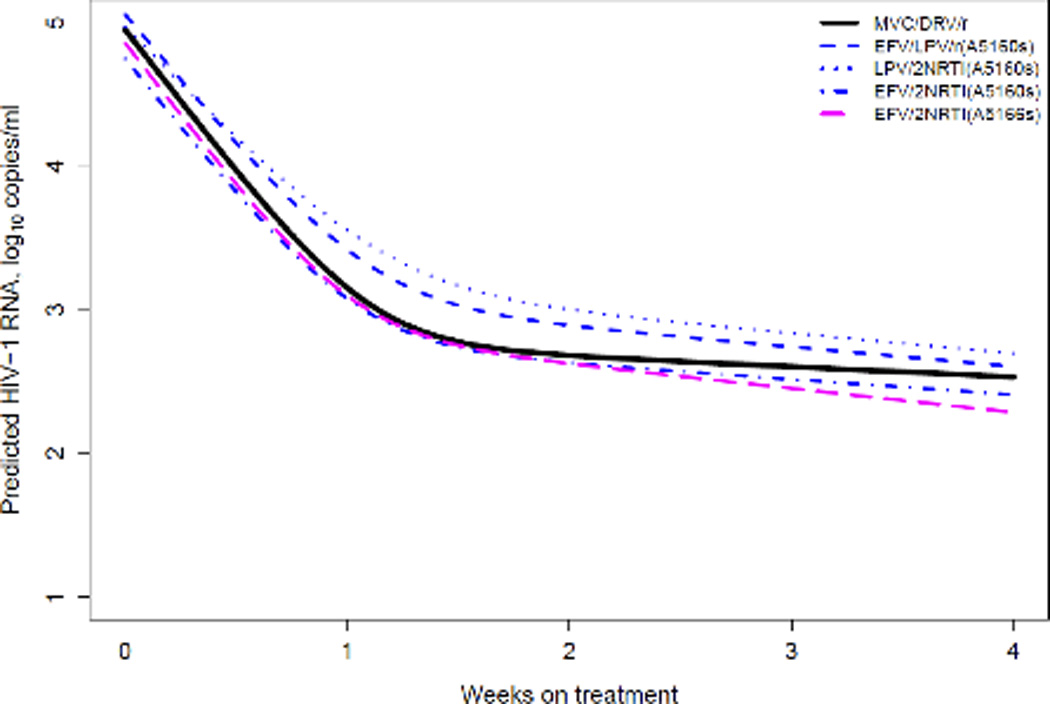
MVC concentration-time data in 24 subjects receiving 150 mg once-daily with DRV/r 800/100 mg once-daily. Solid line is the median simulated curve and dashed lines represent the 95th percentile confidence interval
DISCUSSION
The MIDAS study is the first to explore the virologic activity of the nucleos(t)ide-sparing regimen of MVC/DRV/r in treatment-naïve patients. Twenty-one of the twenty-four (87.5%) treatment-naïve participants treated with MVC/DRV/r 150/800/100 mg once-daily in this study had VL <50 copies/mL at week 24. At 48 weeks, VL was < 50 copies/mL in 22/24 participants (92%). Notably, both participants with VL > 50 copies/mL at week 48 had pretreatment VL >100,000 copies/mL but one of them (Patient A in Table 1) achieved VL < 50 copies/mL after almost two years on MVC/DRV/r. Virologic response to MVC/DRV/r was durable through week 96 with all but two participants (90%) maintaining viral suppression. CD4 counts increased by a median of 216 cells/mm3 from baseline to week 96. The regimen was well tolerated.
Of the NRTI-sparing regimens investigated in treatment-naïve patients to date, MVC 150 mg plus atazanavir/ritonavir 300/100 mg (MVC/ATV/r) has the closest antiretroviral drug composition to MVC/DRV/r. In Study A4001078, 44 of 59 (74.6%) patients treated with MVC/ATV/r had VL < 50 copies/mL at week 48 [30], dropping to (40/59) 67.8% at week 96 [30]. The corresponding suppression rates for atazanavir/ritonavir 300/100 mg plus fixed-dose tenofovir/emtricitabine were 83.6% and 82.0%, respectively. Hyperbilirubinemia was more common with MVC/ATV/r. These results coupled with the relatively limited CNS penetration of atazanavir [31] have reduced enthusiasm for MVC/ATV/r. In the SPARTAN study, atazanavir 300 mg twice daily plus raltegravir 400 mg twice-daily was associated with high rates of raltegravir resistance during VF and treatment-limiting hyperbilirubinemia [3]. Similarly, LPV/r 400/100 mg twice-daily plus EFV 600 mg daily was associated with high rates of NNRTI resistance during VF [1]. We recently reported that, among patients with pretreatment VL >100,000 copies/mL, DRV/r 800/100 mg once-daily plus raltegravir 400 mg twice-daily was associated with higher than expected rate of VF and a propensity for raltegravir resistance during VF [4]. LPV/r plus raltegravir was non-inferior to LPV/r plus tenofovir/emtricitabine at week 96 (66.3% versus 68.6%, respectively), but the mean baseline VL was relatively low (4.25 log10 copies/mL) in that study [2]. No two-drug NRTI-sparing regimen is currently recommended, although DRV/r plus raltegravir is being investigated further [ANRS 143; NCT01066962].
The average MVC Ctrough achieved with MVC/DRV/r 150/800/100 mg daily in MIDAS was 39.3 ng/mL. Although Ctrough >25 ng/mL was associated with a higher probability of virologic response in MERIT [20], it was not determinative of success in our study. The two participants (A and B) with Ctrough measurements at VF had levels >25 ng/mL. Also, none of three participants who had Ctrough < 25 ng/mL at 50–100% of assessed time-points experienced VF (data not shown). The modeled MVC Cavg of 128 ng/mL in the current study exceeds the Cavg (75 ng/mL) associated with virologic response in MERIT. MVC Cavg may have a better prognostic measure of virologic response than the Ctrough [20]. Overall, our pharmacokinetic results are consistent with other studies that investigated once-daily dosing of 150 mg MVC with DRV/r 800/100 mg [22, 32]. MVC plasma exposures with the 150 mg once-daily dose in MIDAS were also similar to levels achieved with the approved 300 mg twice-daily dose when administered in the absence of potent CYP3A4 inhibitors and/or inducers [33]. Given potential differences in virologic suppression provided by DRV/r versus lamivudine/zidovudine, it is possible that MVC Ctrough and Cavg that correlate with virologic success with MVC/DRV/r may differ from the levels identified in MERIT (MVC plus lamivudine/zidovudine), but our study was not designed to address this. All but two participants in our study had VL < 50 copies/mL at week 48. All MVC concentrations were quantifiable, indicating that all subjects were taking MVC at the time of plasma sampling. The DHHS suggests a minimum trough concentration of 50 ng/mL in treatment-experienced patients with VF [5].
By comparing virus decay during MVC/DRV/r treatment to previously reported decay rates with EFV- and LPV-containing regimens, we found that phase 1 decay (i.e., virus decay in the first 10 days of treatment) with MVC/DRV/r was faster than LPV plus two NRTIs and comparable to EFV plus two NRTIs [28, 29] and EFV plus LPV [28]. This is important because phase 1 virus decay rate, which reflects turnover of short-lived infected cells [34], correlates with subsequent virologic response [35], and can inform which experimental regimens merit further evaluation [28]. EFV-containing regimens have demonstrated faster phase 1 decay than LPV plus two NRTIs [28] and triple nucleoside ART [29]. In contrast to phase 1 decay, phase 2 decay rate reflects turnover of long-lived infected cells [34]. In our model, phase 2 decay was slower with MVC/DRV/r than EFV and LPV-containing regimens previously reported. One potential explanation for this is that ARV agents that act prior to viral integration such as EFV and MVC may increase the proportion of infected cells with longer half-life and thereby lower the apparent rate of phase 2 decay [28]. Indeed EFV has slower phase 2 decay than LPV, which acts after integration [28]. Overall, the virus decay pattern of MVC/DRV/r bears similarities with EFV-containing regimens, suggesting potent inhibition of infectious virion production.
Although the small number of participants and the single-arm design are limitations of our study, we have generated important virologic and PK data on MVC/DRV/r. Our ability to characterize emergent resistance during VF was limited by occurrence of very few virologic failures and the low level of viremia in most of those who did. Another limitation of our study is that few participants had advanced HIV infection (median CD4 count at entry was 455 cells/mm3, and those with CD4 <100 cells/mm3 were excluded). Therefore, our results may not apply to patients with very low CD4 counts. Finally, of the four participants with baseline VL >100,000 copies/mL in the study, two had VF at week 48 though one of them achieved viral suppression at week 84 on MVC/DRV/r. This is in contrast to a single VF among 20 subjects with VL < 100,000 copies/mL at baseline. While these are interesting observations, the small size of this study limits our ability to rigorously compare virologic responses in the different baseline VL strata. The small number of patients enrolled in this study and the variable blood sampling time limit the possibility to draw definitive conclusions on the potential association (or lack of association) between virologic outcome and MVC pharmacokinetics.
In conclusion, results of the MIDAS study support further evaluation of MVC/DRV/r 150/800/100 mg once-daily for initial treatment of R5 HIV-1. A large multicenter clinical trial (MODERN) is already underway [NCT01345630]. MODERN and other future studies should determine the virologic efficacy of MVC/DRV/r across baseline VL strata, and characterize the resistance consequences of VF in patients receiving MVC/DRV/r, the pharmacokinetic correlates of virologic success, and the impact of NRTI-sparing on metabolic complications associated with HIV and contemporary antiretroviral therapy.
ACKNOWLEDGMENT
Funding
This work was supported by an Investigator Initiated Study Grant to Northwestern University from Pfizer, which also provided maraviroc and ritonavir. Janssen provided darunavir
This study was conceived and developed by B.T, P.R, D.K, J.E and S.S. Lead investigators at the research sites were B.T (Northwestern University), S.S (University of Nebraska), O.A (Cook County Hospital, Chicago), J.L (Quest Research) and J.C (University of Miami). A.T was the study virologist, B.B. the study manager, and D.L was the statistician. All authors contributed to preparation of the manuscript and approved the final version.
MIDAS research staff: Northwestern University (Meredith Rathert, Byron Yip, Nina Lambert), University of Nebraska (Frances Van Meter), Quest Clinical Research (Devin Walsh), University of Miami (Tom Tanner), CORECenter (Julia Lee).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Meeting Presentations: XIX International AIDS Conference, July 2012, Washington DC; and the Conference of Retroviruses and Opportunistic Infections, March 2013, Atlanta, Georgia
REFERENCES
- 1.Riddler SA, Haubrich R, DiRienzo AG, Peeples L, Powderly WG, Klingman KL, et al. Class-sparing regimens for initial treatment of HIV-1 infection. N Engl J Med. 2008;358:2095–2106. doi: 10.1056/NEJMoa074609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Reynes J, Trinh R, Pulido F, et al. Lopinavir/ritonavir (LPV/r) combined with raltegravir (RAL) or tenofovir/emtricitabine (TDF/FTC) in antiretroviral (ARV)-naïve subjects: 96 week results of the PROGRESS Study. AIDS Res Hum Retroviruses. 2012;29:256–265. doi: 10.1089/aid.2011.0275. [DOI] [PubMed] [Google Scholar]
- 3.Kozal MJ, Lupo S, De Jesus E, et al. A nucleoside- and ritonavir-sparing regimen containing atazanavir plus raltegravir in treatment-naïve HIV-infected patients: SPARTAN study results. HIV Clin Trials. 2012;13:119–130. doi: 10.1310/hct1303-119. [DOI] [PubMed] [Google Scholar]
- 4.Taiwo B, Zheng L, Gallien S, et al. Efficacy of a nucleoside sparing regimen of darunavir/ritonavir plus raltegravir in treatment-naïve HIV-1-infected patients (ACTG A5262) AIDS. 2011;25:2113–2122. doi: 10.1097/QAD.0b013e32834bbaa9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panel on Antiretroviral Guidelines for Adults and Adolescents. Guidelines for the use of antiretroviral agents in HIV-1-infected adults and adolescents. Department of Health and Human Services; [Accessed 5 June, 2013]. (Last Updated February, 2013). Available at http://www.aidsinfo.nih.gov/contentfiles/lvguidelines/adultandadolescentgl.pdf. [Google Scholar]
- 6.Thompson MA, Aberg JA, Hoy F, et al. Antiretroviral treatment of adult HIV infection: 2012 recommendations of the International AIDS Society-USA panel. JAMA. 2012;308:387–402. doi: 10.1001/jama.2012.7961. [DOI] [PubMed] [Google Scholar]
- 7.WHO. [Accessed on January 30, 2013];Rapid Advice: antiretroviral therapy for HI infection in adults and adolescents. 2009 Available at http://www.who.int/hiv/pub/arv/rapid_advice_art.pdf.
- 8. [Accessed on January 30, 2013];European AIDS Clinical Society Guildelines. Version 6.1. 2012 Nov; Available at http://www.europeanaidsclinicalsociety.org/images/stories/EACS-Pdf/EacsGuidelines-v6.1-2edition.pdf.
- 9. [Accessed on January 30, 2013];British HIV Association Guidelines for the Treatment of HIV-1 Positive Adults with Antiretroviral Therapy. Available at http://www.bhiva.org/documents/Guidelines/Treatment/2012/120430TreatmentGuidelines.pdf.
- 10.Taiwo B, Murphy RL, Katlama C. Novel antiretroviral combinations in treatment-experienced patients with HIV infection: rationale and results. Drugs. 2010;70:1629–1642. doi: 10.2165/11538020-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 11.Abel S, Back DJ, Vourvahis M. Maraviroc: pharmacokinetics and drug interactions. Antivir Ther. 2009;14:607–618. [PubMed] [Google Scholar]
- 12.Wilkin TJ, Lalama CM, McKinnon J, et al. A pilot trial of adding maraviroc to suppressive antiretroviral therapy for suboptimal CD4+ T-cell recovery despite sustained virologic suppression: ACTG A5256. J Infect Dis. 2012;206:534–542. doi: 10.1093/infdis/jis376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Funderburg N, Kalinowska M, Eason J, et al. Effects of maraviroc and efavirenz on markers of immune activiation and inflammation and associations with CD4+ cell rises in HIV-infected patients. PLos One. 2010;5:e13188. doi: 10.1371/journal.pone.0013188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Meyer S, Lathouwers E, Dierynck I, et al. Characterization of virologic failure patients on darunavir/ritonavir in treatment-experienced patients. AIDS. 2009;23:1829–1840. doi: 10.1097/QAD.0b013e32832cbcec. [DOI] [PubMed] [Google Scholar]
- 15.Yilmaz A, Watson V, Else L, Gisslèn M. Cerebrospinal fluid maraviroc concentrations in HIV-1 infected patients. AIDS. 2009;23:2537–2540. doi: 10.1097/QAD.0b013e328333ae0e. [DOI] [PubMed] [Google Scholar]
- 16.Croteau D, Rossi SS, Best BM, et al. Darunavir is predominantly unbound to protein in cerebrospinal fluid and concentrations exceed the wild-type HIV-1 median 90% inhibitory concentration. J Antimicrob Chemother. 2013;68:684–689. doi: 10.1093/jac/dks441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wasmuth JC, Rockstroh JK, Hardy WD. Drug safety evaluation of maraviroc for the treatment of HIV infection. Expert Opin Drug Saf. 2012;11:161–174. doi: 10.1517/14740338.2012.640670. [DOI] [PubMed] [Google Scholar]
- 18.Cooper DA, Heera J, Goodrich J, et al. Maraviroc versus efvirenz, both in combination with zidovudine-lamivudine, for the treatment of antiretroviral-naïve subjects with CCR5-tropic HIV-1 infection. J Infect Dis. 2010;201:803–813. doi: 10.1086/650697. [DOI] [PubMed] [Google Scholar]
- 19.Ortiz R, Dejesus E, Khanlou H, Voronin E, et al. Efficacy and safety of once-daily darunavir/ritonavir versus lopinavir/ritonavir in treatment-naive HIV-1-infected patients at week 48. AIDS. 2008;22:1389–1397. doi: 10.1097/QAD.0b013e32830285fb. [DOI] [PubMed] [Google Scholar]
- 20.McFadyen L, Jacqmin P, Wade J, Weatherly B. Maraviroc exposure-efficacy (<50 copies/mL) analysis in HIV-1-infected treatment-naïve subjects – ITT population (MERIT study) [abstract TUPE0053]. Abstracts of the XVII International AIDS Conference.2008. [Google Scholar]
- 21.Kakuda TN, Abel S, Davis J, et al. Pharmacokinetic interactions of maraviroc with darunavir-ritonavir, etravirine and etravirine-darunavir-ritonavir in healthy volunteers: results of two drug interaction trials. Antimicrobial Agents and Chemotherapy. 2011;55:2290–2296. doi: 10.1128/AAC.01046-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Okoli C, Siccardi M, Thomas-William S, et al. Once daily maraviroc 300 mg or 150 mg in combination with ritonavir-boosted darunavir 800/100 mg. J Antimicrob Chemother. 2012;67:671–674. doi: 10.1093/jac/dkr493. [DOI] [PubMed] [Google Scholar]
- 23.Gulick RM, Lalezari J, Goodrich J, et al. Maraviroc for previously treated patients with R5 HIV-1 infection. N Engl J Med. 2008;359:1429–1441. doi: 10.1056/NEJMoa0803152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chesney MA, Ickovics JR, Chambers DB, et al. Self-reported adherence to antiretroviral medications among participants in HIV clinical trials: the AACTG adherence instrument. Patient Care Committee and Adherence Working Group of the Outcomes Committee of the Adult AIDS Clinical Trials Group (AACTG) AIDS Care. 2000;12:255–266. doi: 10.1080/09540120050042891. [DOI] [PubMed] [Google Scholar]
- 25.Tsibris AM, Sagar M, Gulick RM, et al. In vivo emergence of vicriviroc resistance in a human immunodeficiency virus type 1 subtype C-infected subject. J Virol. 2008;82:8210–8214. doi: 10.1128/JVI.00444-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.D’Argenio DZ, Schumitzky A, Wang X. Biomedical Simulations Resource. Los Angeles: 2009. ADAPT 5 User’s guide: Pharmacokinetic/Pharmacodynamic Systems Analysis Software. [Google Scholar]
- 27.Wu H, Ding AA. Population HIV-1 dynamics in vivo: applicable models and inferential tool for virological data from AIDS clinical trials. Biometrics. 1999;55:410–418. doi: 10.1111/j.0006-341x.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 28.Haubrich RH, Riddler SA, Ribaudo H, et al. Initial viral decay to assess the relative antiretroviral potency of protease inhibitor-sparing, and nucleoside reverse transcriptase inhibitor-sparing regimens for first-line therapy of HIV infection. AIDS. 2011;25:2269–2278. doi: 10.1097/QAD.0b013e32834d0c20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kuritzkes DR, Ribaudo HJ, Squires KE, et al. Plasma HIV-1 RNA dynamics in antiretroviral-naïve subjects receiving either triple-nucleoside or efavirenz-containing regimens: ACTG A5166s. J Infect Dis. 2007;195:1169–1176. doi: 10.1086/512619. [DOI] [PubMed] [Google Scholar]
- 30.Mills A, Mildvan D, Podzamczer D, et al. Once-daily maraviroc in combination with ritonavir-boosted atazanavir in treatment-naive patients infected with CCR5-tropic HIV-1 (study A4001078): 96-week results [TUAB0102]. Presented at: XIX International AIDS Conference; Washington DC. 2012. [Google Scholar]
- 31.Best BM, Letendre SL, Brigid E, et al. Low atazanavir concentrations in cerebrospinal fluid. AIDS. 2009;23:83–87. doi: 10.1097/QAD.0b013e328317a702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mora-Peris B, Croucher A, Else L, et al. Pharmacokinetic profile of maraviroc 150 mg dosed with darunavir/ritonavir once daily with and without nucleoside analogues in HIV-infected subjects. J Antimicrobial Chemother. 2013 doi: 10.1093/jac/dkt006. E pub ahead of print. [DOI] [PubMed] [Google Scholar]
- 33.Weatherley B, McFadyen L, Chan PLS, Marshall S. Population pharmacokinetic covariate analysis of maraviroc in the MERIT study in treatment naïve studies [Poster 17B] Abstracts of the 9th International Workshop on Clinical Pharmacology of HIV Therapy. 2008 [Google Scholar]
- 34.Dahl V, Josefsson L, Palmer S. HIV reservoirs, latency, and reactivation: prospects for eradication. Antiviral Res. 2010;85:286–294. doi: 10.1016/j.antiviral.2009.09.016. [DOI] [PubMed] [Google Scholar]
- 35.Polis MA, Sidorov IA, Yoder C, et al. Correlation between reduction in plasma HIV-1 RNA concentration 1 week after start of antiretroviral treatment and long-term efficacy. Lancet. 2001;358:1760–1765. doi: 10.1016/s0140-6736(01)06802-7. [DOI] [PubMed] [Google Scholar]


