Abstract
We provide new historical evidence on the developmental origins of health and disease in a cohort of boys born between 1907 and 1922 in Wellington, New Zealand. Using a dataset of 1,523 birth records that include birth weight and length we find 852 (58%) of the adult cohort in World War II records measuring stature, body mass and blood pressure. On average, the boys weighed 3.5kg at birth, similar to Australian and American babies of the era, and nearly identical to full-term New Zealand babies in the 1990s. Using OLS regression models we estimate the effect of birth weight on adult stature and systolic blood pressure. We find an increase in birth weight of 1kg is associated with an increase in stature of 2.6cm (95% confidence interval [CI] 1.6cm to 3.6cm), and a decrease in systolic blood pressure of 2.1 mm/Hg (95% CI −5.00 to 0.67). This is the earliest cohort by fifty years for whom the fetal origins hypothesis has been examined in early adulthood. Our estimates of the effect of birth weight on blood pressure are towards the upper end of the range of published estimates in modern cohorts.
Introduction
The hypothesis that conditions in early life—social, economic, environmental, nutritional, or experiencing illness—can have long run effects on individual and population health can be traced to at least the early twentieth century (Kermack et al., 1934). Social and medical scientists have given increasing attention to this idea since the late 1980s. The work of David Barker and colleagues was particularly influential in drawing attention to the potential connections between nutrition before birth, birth weight, later adult health and subsequent cardiovascular mortality (D. Barker et al., 1989a; D. J. P. Barker & Osmond, 1986; D. J. P. Barker et al., 1989b). Barker’s influence on the field is acknowledged in the widespread use of the term “Barker hypothesis” to denote the idea that adult diseases and mortality can have fetal origins (Almond & Currie, 2011). The fetal origins hypothesis does not posit that adult health outcomes are predestined by fetal conditions, and thus closely related, but slightly broader literatures investigate the “developmental origins of health and disease” (Gluckman et al., 2010) and life course influences on health (Ben-Shlomo & Kuh, 2002).
A significant amount of the literature on the fetal origins hypothesis by Barker and others has addressed the specific question of how birth weight is associated with blood pressure in later life. Evidence from modern studies has shown that increases in birth weight of 1kg are associated with declines in systolic blood pressure of between 1–4 mm/Hg (Gamborg et al., 2007b). Conversely, increases in birth weight are associated with increases in adult stature (Sorensen et al., 1999). With lower blood pressure and increased stature both associated with lower adult mortality, there is strong evidence that increased birth weight is associated, at least indirectly, with improved health in later life even for babies well over the clinical low birth weight threshold of 2.5 kilograms.
The fetal and developmental origins literature and life course epidemiology emphasize that health conditions at any point in life are influenced by experience over a person’s life from conception on. Accumulated experience will differ among individuals within a similar cohort, and at a population level across socio-economic and environmental contexts. An important and under-recognized implication of this literature is that the relationship between early life health and later life outcomes may change over time and differ across geographic areas as different cohorts are exposed to inter alia different environmental conditions, nutritional practices, disease exposure, and medical care. Because the fetal origins literature has largely developed since the 1980s scholars have been able to measure cohorts born before World War II in later life, and only been able to measure younger cohorts born since the 1960s.
This paper provides the earliest ever evidence on the fetal origins hypothesis in young adults. Our cohort was born in Wellington, New Zealand between 1907 and 1922. We use maternity hospital records linked to military enlistment records for men enlisting in World War II to obtain information on size at birth and health in early adulthood. Combining the information from these records allows us to address several closely related questions about birth weight and early-adult health. Specifically, we measure the
Size at birth of infants in New Zealand as an indicator of child health during the New Zealand infant mortality decline,
Association of birth weight and birth length, with adult height
Association of birth weight and birth length, with adult blood pressure: the fetal origins hypothesis
While modern studies collecting data prospectively can go beyond body composition to more precise measures of nutrition, growth and health, only anthropometric data is available in historical populations. Our data combines accurately measured—rather than recalled—birth weight and reliable measures of blood pressure. Although other authors have examined the fetal origins hypothesis in cohorts born in the early twentieth century (Eriksson et al., 2004), our study is the first to test the hypothesis in these cohorts in young adulthood. Indeed, our cohort is born at least 45 years earlier than any group for whom the fetal origins hypothesis has been studied in young adults (Järvelin et al., 2004).
Evidence from New Zealand is especially interesting because of its apparently very healthy population in the early twentieth century. New Zealand, like Scandinavia, had the earliest sustained infant mortality decline in the industrialized world (Edvinsson et al., 2008; Woodbury, 1922). Contemporary commentators attributed New Zealand’s decline to the establishment in 1907 of the infant welfare group: the Plunket Society (Bryder, 2003). But the New Zealand infant mortality decline started in the 1880s, and there is no demographic evidence Plunket caused the decline. A similar trajectory of decline was seen in Scandinavia, which like New Zealand was wealthy with low population density (Edvinsson et al., 2008; Mein Smith, 1988). Thus, this paper also brings overdue attention to measured health outcomes among cohorts in the New Zealand infant mortality decline.
Results of prior studies: 1. Association of birth weight and length with adult height
The relationship between birth and adult size has interested physical anthropologists and others for several centuries (Baldwin, 1921; Bogin & Kapell, 1997; Tanner, 1981) But there were few large studies of growth from birth to maturity until the twentieth century (Young et al., 1991). Many early twentieth century studies of childhood growth recruited samples in schools, rather than at birth. Both the early twentieth century growth studies, and research since the 1970s have provided significant evidence that weight and length at birth are correlated with adult height. For example, a study of Danish conscripts born in the 1970s found that average stature of men weighing 2.5–3kg at birth was 4cm less than men who weighed 3.75 – 4kg at birth (Sorensen et al., 1999). Similar results were observed in a large study of Norwegian conscripts (Eide et al., 2005). In the 1958 British birth cohort an increase in birth weight of 1kg was associated with a 2cm increase in adult height (Li et al., 2004).
The association between size at birth and adult stature is important because stature is associated with morbidity and mortality (Engeland et al., 2003a; Engeland et al., 2003b; Karri Silventoinen, 2003; Waaler, 1984). The association between height and mortality risk reflects that adult height summarizes environmental conditions—particularly caloric intake and disease burden—experienced during the growth period. A significant amount of research has focused on the inverse relationship between height and coronary heart disease (Paajanen et al., 2010; K Silventoinen et al., 2006). Similarly, there is an inverse association between height and diabetes, with shorter men having a higher prevalence of diabetes, and greater levels of insulin resistance (Asao et al., 2006). Height is also inversely related to stroke, respiratory disease, and stomach cancers (Smith & Lynch, 2004). But if stature itself is associated with birth weight, then analyses of stature and health outcomes that do not control for birth weight will over-estimate the effect of conditions in childhood (measured by stature) compared to conditions in-utero (measured by birth weight).
Results of prior studies: 2. Association of birth weight and length with blood pressure
The relationship between birth weight and blood pressure in adulthood is central to research on the developmental or fetal origins hypothesis (Almond & Currie, 2011). Since Barker’s early work in the 1980s there has been significant medical and epidemiological research into the connection (D.J.P. Barker, 1998). Recent comprehensive summaries of modern literature by Huxley et al, and Lawlor and Davey-Smith suggest that for each one kilogram increase in birth weight systolic blood pressure decreases of 1.5 to 2 mm/Hg (Huxley et al., 2002; Lawlor & Smith, 2005). Much of the modern literature studies the relationship when subjects are aged 50 or older, significantly older than our sample aged 18–35 at enlistment. Because blood pressure changes throughout the life course it is unclear whether the association between birth weight and blood pressure changes over time (Davies et al., 2006; Huxley et al., 2002).
Data
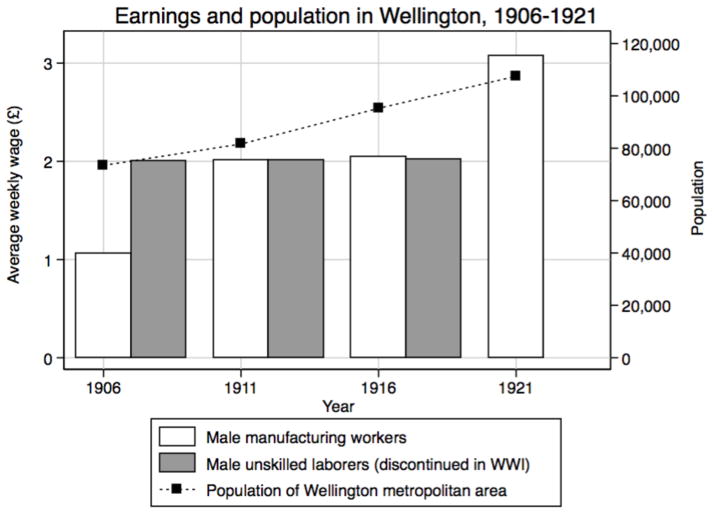
Our sample comes from births at Wellington’s St Helens Hospital between 1907 and 1922. The St Helens Hospitals were established during an era of concern about high maternal and infant mortality. Following the 1904 Midwives Act seven St Helens hospitals were established in New Zealand cities between 1905 and 1920. They provided state-subsidised maternity care for working-class women whose husbands earned less than £3 (1905–1912) or £4 (1912 on) a week, and a place for training midwives. In order to be eligible to birth at the hospital, parents contributed half a week’s wages. For most of the period covered by our data, average weekly wages for unskilled general laborers and skilled workers were significantly below these limits (Figure 1). The hospital records lack information on individual fathers’ occupations. Previously published research tracing a selection of the families into city directories that listed fathers’ occupations showed the majority of fathers had semi-skilled or skilled occupations, including laborers, trades such as carpentry and clerical workers (Wood & Foureur, 2007). However, we know nearly all families lived in Wellington. As the national capital and the country’s major port the city grew rapidly between 1907 and 1922 with growth in exports and government administration during World War I. Approximately 60% of Wellington men worked in tertiary services, and another 31% in manufacturing.
Figure 1.
Earnings and population in Wellington, 1906–1921
The first St Helens Hospital was established in Wellington in May 1905. Women were attended by midwives and the medical superintendent was only called in for complicated cases. On all indices of safe maternity care, including overall maternal mortality rates, the St Helens hospitals were the safest birthing locations in New Zealand (Parr et al., 1921; Wood & Foureur, 2007). “Indoor” casebook records survive for births between 1907 and 1922. The volumes containing births in parts of 1907, 1910 and 1911, 1922 are missing, because the books containing these records were lost when the hospital’s records were moved between buildings. While their loss is regrettable, the cause of the missing data suggests it will not bias the sample in any particular way.
The surviving casebooks provide information on 3,166 births. On admission to the hospital, the woman’s name, address, age, date of admission, parity and physical appearance were noted, as well as any comments on her general health. Ethnicity and marital status were not noted – all women were given the title “Mrs” and nearly all were married. The baby’s presentation at birth, the length of each stage of labour, the date and time of delivery, the sex, weight and length of the infant, and any complications were added after birth. The names of those attending the birth were also recorded. Midwives also commented on the mother’s and baby’s progress and care, and any complications or treatments. At discharge, the infant’s weight and mode of feeding were recorded with remarks about the mother’s condition. The St Helens records are very similar to contemporaneous records from other Anglophone countries (Dye, 1987; Morley et al., 2006; Nuttall, 2007).
Sample construction
Between 1907 and 1922 there were 3,166 births at the Wellington St Helens Hospital. Between a birth at St Helens and enlistment in World War II there were several stages at which an individual could drop from the sample.
The first stage in constructing our sample was to focus on live, singleton male births. Our record of early adult health comes from military records, limiting our ability to trace women to maturity. While several thousand New Zealand women served in auxiliary or nursing roles in World War II, they were a small fraction of women in this birth cohort. By contrast, based on overall levels of World War II service we expected approximately half the men would have surviving military medical examination records.
The early introduction of national conscription in New Zealand reduces the potential for selection bias from a sample constructed from military records. From August 1940, all New Zealand men between the ages of 19 and 45 were conscripted for military service, after an initial period of voluntary enlistment after September 1939. By mid-1942 all men in our birth cohort who had survived to early adulthood should have been registered and medically examined for service (Baker, 2000). Just 5% of men were judged “permanently incapable … [and] unfit for any military service,” but only men who served in the military have extant medical records (Table 3).
Table 3.
Characteristics of sample linked to military records
| N | Mean | SE | Min | Max | |
|---|---|---|---|---|---|
| Age | 841 | 27.98 | 0.22 | 0.00 | 46.00 |
| Parity at birth | 842 | 2.80 | 0.07 | 1.00 | 14.00 |
| Year of enlistment | 740 | 1940.48 | 0.04 | 1939 | 1945 |
| 1939 (%) | 98 | 13.24 | |||
| 1940 (%) | 354 | 47.84 | |||
| 1941 (%) | 143 | 19.32 | |||
| 1942 (%) | 134 | 18.11 | |||
| 1943 (%) | 8 | 1.08 | |||
| 1947 (%) | 1 | 0.41 | |||
| Age at enlistment | 740 | 24.10 | 0.15 | 18.00 | 35.00 |
| Educational attainment | |||||
| Some primary | 760 | 0.58 | 0.02 | 0.00 | 1.00 |
| Some high school | 760 | 0.18 | 0.01 | 0.00 | 1.00 |
| Completed high school | 760 | 0.16 | 0.01 | 0.00 | 1.00 |
| Some tertiary | 760 | 0.04 | 0.01 | 0.00 | 1.00 |
| Tertiary qualification | 760 | 0.02 | 0.01 | 0.00 | 1.00 |
| Unknown education | 760 | 0.02 | 0.01 | 0.00 | 1.00 |
| Occupation at enlistment | |||||
| Professional or manager | 852 | 0.06 | 0.01 | 0.00 | 1.00 |
| Clerical | 852 | 0.11 | 0.01 | 0.00 | 1.00 |
| Sales | 852 | 0.06 | 0.01 | 0.00 | 1.00 |
| Service | 852 | 0.03 | 0.01 | 0.00 | 1.00 |
| Skilled worker in manufacturing utilities or transport | 852 | 0.55 | 0.02 | 0.00 | 1.00 |
| Laborer (not on farm) | 852 | 0.06 | 0.01 | 0.00 | 1.00 |
| Farmer or farm manager | 852 | 0.02 | 0.01 | 0.00 | 1.00 |
| Farm laborer | 852 | 0.06 | 0.01 | 0.00 | 1.00 |
| Unknown occupation | 852 | 0.06 | 0.01 | 0.00 | 1.00 |
| Marital status | |||||
| Married | 787 | 0.31 | 0.02 | 0.00 | 1.00 |
| Divorced or separated | 787 | 0.01 | 0.00 | 0.00 | 1.00 |
| Widowed | 787 | 0.00 | 0.00 | 0.00 | 1.00 |
| Single | 787 | 0.68 | 0.02 | 0.00 | 1.00 |
| Health at birth | |||||
| Birth weight (kilograms) | 833 | 3.56 | 0.02 | 1.59 | 5.33 |
| Less than 2.5kg at birth | 852 | 0.02 | 0.01 | 0.00 | 1.00 |
| Health at enlistment | |||||
| Adult height (21 years and older) | 784 | 172.04 | 0.21 | 152.40 | 187.96 |
| Weight (kg) | 621 | 65.92 | 0.33 | 35.61 | 115.21 |
| BMI | 620 | 22.28 | 0.10 | 16.60 | 34.85 |
| Systolic blood pressure | 726 | 134.74 | 0.52 | 100.00 | 198.00 |
| Diastolic blood pressure | 725 | 78.49 | 0.41 | 17.00 | 140.00 |
| Systolic blood pressure > 140 | 726 | 0.25 | 0.01 | 0.00 | 1.00 |
We matched 58% of boys to military records in World War II (Table 1). The St Helens records recorded the mother’s full name, so we obtained children’s names using mother’s names, the known birth location (Wellington) and residence (mostly Wellington), and the exact date of birth. We matched these details to official birth registers maintained by the Registrar of Births, Deaths and Marriages. We matched 98% of target boys from hospital records to birth registers, with just 37 of 1496 singleton, live male births not found in the registers. This result confirms contemporary views that New Zealand had a highly effective civil registration system (Children’s Bureau, 1914).
Table 1.
Sample construction from records of St. Helens Hospital and World War II enlistment files
| Sample | Number | Proportion of previous line | Mean birth weight (kg) (standard deviation) | Mean maternal age (standard deviation) | ||
|---|---|---|---|---|---|---|
| Specified group | Lost from above | Specified group | Lost from above | |||
| All births, 1907–1922 | 3166 | 27.89 (6.17) | ||||
| Births involving boys | 1566 | 0.49 | 27.86 (6.28) | 27.92 (6.07) | ||
| Live births | 1523 | 0.97 | 27.82 (6.25) | 29.24 (6.89) | ||
| Singleton births | 1496 | 0.98 | 3.53 (0.54) | 27.75 (6.23) | 32.70 (5.90) | |
| Found in birth registers | 1459 | 0.98 | 3.53 (0.54) | 3.54 (0.56) | 27.78 (6.26) | 26.35 (4.74) |
| Enlisted in World War II | 852 | 0.58 | 3.56 (0.50) | 3.48 (0.58) | 27.92 (6.37) | 27.52 (6.04) |
| Variables measured | ||||||
| Height | 789 | 0.93 | 3.56 (0.51) | 3.52 (0.50) | 27.97 (6.47) | 27.40 (5.18) |
| Blood pressure | 729 | 0.93 | 3.57 (0.51) | 3.49 (0.51) | 28.06 (6.45) | 27.15 (5.85) |
| Weight | 622 | 0.85 | 3.56 (0.50) | 3.57 (0.51) | 27.93 (6.62) | 27.89 (5.67) |
| All key variables | 599 | 0.96 | 3.56 (0.50) | 3.57 (0.51) | 28.03 (6.55) | 27.67 (5.94) |
We linked individuals to World War II records by matching variables available in maternity, civil registration, and enlistment records: full given name of the subject, exact date of birth, location of birth, and full parental names. The combination of these variables uniquely identified all children within the dataset, and allowed straightforward identification of the subjects in World War II records. In a country as small as New Zealand with between 24,000 and 30,000 annual births (thus approximately half that number of male births) in any year between 1907 and 1922, this information allowed us to make exact matches with a high degree of confidence. The recording of exact dates of birth, and middle names for both parents and children, was particularly critical in distinguishing between people with common names. We traced individuals into all three branches—Army, Navy, and Air Force—of the military.
All forces collected similar information on men enlisting including socio-economic information, principally occupation and educational achievement, and an extensive medical examination. The most consistently collected medical information was on height, blood pressure, and weight. Military medical examinations were conducted by two general practitioners, assisted by a recording clerk and supervised by a military officer. Instructions for the examinations required all shoes and clothing to be removed for measuring height and weight. Blood pressure measurements were to be taken in a seated position (Watt, 1940).
Analysis
Our analysis focuses first on the characteristics of the sample at birth as an indicator of infant health in New Zealand, and then on the association between birth size and adult height, and birth size and systolic blood pressure. To facilitate comparison with modern studies we follow the convention in the literature of measuring birth weight as a continuous variable (Hardy et al., 2004a). Models of adult height and blood pressure included controls for educational attainment and occupation at enlistment where these were available in order to examine whether results were confounded by socio-economic differences. In modern studies blood pressure has been found to vary across socio-economic groups, measured by occupation and education (Schnall et al., 1994; Strike & Steptoe, 2004). However, variable non-response for education and occupation reduced sample sizes, without substantively affecting coefficient estimates for early-life health variables. Therefore, we report analyses including the larger sample without controls for education and occupation at enlistment. All analyses were conducted in Stata 12. Summary statistics for the sample at birth and enlistment are presented in Table 3.
Results
Birth weight and length in early twentieth century New Zealand
Babies born at St Helens Hospital between 1907 and 1922 averaged a healthy 3467 grams at birth. There was no time trend towards increasing or decreasing birth weight. Consistent with nearly all prior research, boys weighed more than girls. Across the 15 years of records, boys at St Helens averaged 3531 grams at birth, while girls averaged 3403 grams at births (Table 4).
Table 4.
Comparison of average birth- weight and length at St Helens and other developed countries
| Sample | Years | Female | Male | Less than 2500g | ||
|---|---|---|---|---|---|---|
| Weight (grams) | Length (cm) | Weight (grams) | Length (cm) | |||
| Wellington St Helens Standard deviation |
1907–1922 | 3403 (570) | 51.6 (3.32) | 3531 (601) | 52.2 (3.54) | 4.2% |
| United States | ||||||
| Stanford University Medical School Baby Clinic (Faber, 1920) | 1906–1919 | 3305 | 3495 | |||
| New York Lying-in Hospital (Costa, 1998) (NB: Medians, not means) | 1910–1931 | 3430 | 51 | 3500 | 51 | 5.5% |
| Minneapolis General Hospital (Brenton, 1922) | 1917 | 3282 | 3419 | |||
| United Kingdom | ||||||
| Lambeth Lying Hospital (Pearson, 1899) | c. 1900 | 3208 | 51 | 3312 | 52 | |
| Westminster Health Society, London (T. Robertson, 1916) | 1914 | 3210 | 3310 | |||
| Leeds Babies Welcome (T. Robertson, 1916) | 1914 | 3200 | 3300 | |||
| Birmingham Maternity Hospital (T. B. Robertson, 1915b) | c. 1914 | 3217 | 3257 | |||
| Australia | ||||||
| Queens Home, Adelaide, South Australia (T. Robertson, 1915a) | 1909–1913 | 3410 | 3590 | |||
| Japan | ||||||
| Japan (Misawa, 1909) | c. 1905 | 2870 | 49 | 3040 | 49 | |
Length at birth varies less than weight, and is less useful as an indicator of fetal nutrition. Under nutrition while in utero is manifested in being lighter than the potential weight, rather than being shorter. Length was also not as consistently measured at St Helens. Whereas 112 of the 3023 live, singleton births at St Helens were missing weight measurements, 263 were missing length. The St Helens babies averaged 51.9 cm at birth, with males averaging 52.2 cm and females 51.6 cm.
Selectivity of the linked sample
Selectivity of the sample of men linked to their World War II enlistment records is a key issue in how far the results from this analysis can be generalized. If there is significant selectivity by birth weight in the men we find in World War II, then the results are not as generalizable to a wider population.
The overall results of the medical examinations of the national cohort suggest that while men who served in the military were fitter than average, the majority of men who did not serve were also healthy. We expect this sample selected into the military from a working and lower-middle class background to have biases in offsetting directions. Men of working class background are likely to be slightly less healthy, while selection into the military will likely include the healthiest of these men. The men linked to World War II records averaged 172cm, identical to overall mean stature of New Zealand soldiers (K. Inwood et al., 2009), suggesting the sample is not noticeably biased. In comparison, the mean stature of American World War II soldiers was 173cm (Karpinos, 1958).
There were small differences in birth weight between men who enlisted, and those who did not. On average, men who enlisted weighed 3564 grams at birth, compared to 3487 grams for men who did not enlist in World War II. The difference is statistically significant (p=0.008), but there were not large differences in the distribution of birth weights across the groups. Kolmogorov-Smirnov tests for equality of the distribution functions cannot reject the hypothesis the two distributions were equal. Probit models of World War II service show being 1kg heavier at birth increased the chance of enlisting by 6.4%. A boy at the low birth weight margin of 2500g had a 52% chance of World War II service, while a boy of 4kg (one standard deviation larger than the mean) had a 60% chance of World War II service. Maternal age and birth weight did not differ significantly by enlistment status, suggesting the group enlisting in World War II was representative of the initial population (Table 1).
Association of size at birth with adult height
Consistent with modern research birth weight was strongly related to final adult height. For the 98 men who weighed less than 3000 grams at birth, the average height on enlistment in World War II was 169.6cm. For men between 3000 and 3500 grams average height was 1.5cm greater, and men weighing up to 4000 grams averaged 172.6cm when they finished growing. Controlling for maternal age, birth order, marital status of the mother, and year of birth we estimate that for every one-kilogram increment in birth weight, a man was 2.6cm (approximately one inch) taller in adulthood (Table 5).
Table 5.
Association of size at birth with adult height
| Coefficient | Standard error | t | P > |t| | Standardized coefficients | |
|---|---|---|---|---|---|
| Birth weight (kg) | 2.58 | 0.51 | 5.09 | 0.00 | 0.22 |
| Length at birth (cm) | 0.23 | 0.22 | 1.02 | 0.31 | 0.04 |
| Mother married | 0.73 | 4.44 | 0.16 | 0.87 | 0.01 |
| Maternal age | 0.06 | 0.15 | 0.43 | 0.67 | 0.07 |
| Square of maternal age | −0.00 | 0.00 | −0.00 | 1.00 | −0.00 |
| Number of pregnancies | −0.27 | 0.14 | −1.97 | 0.05 | −0.09 |
| Occupation Reference category: Professionals and managers | |||||
| Clerical | 0.01 | 1.18 | 0.00 | 1.00 | 0.00 |
| Sales | −0.63 | 1.36 | −0.46 | 0.64 | −0.03 |
| Service | −0.12 | 1.63 | −0.07 | 0.94 | −0.00 |
| Manufacturing workers | −1.16 | 1.07 | −1.09 | 0.28 | −0.10 |
| Laborers | −1.44 | 1.40 | −1.03 | 0.30 | −0.06 |
| Farmers | 0.31 | 1.76 | 0.18 | 0.86 | 0.01 |
| Farm managers | −0.91 | 1.38 | −0.66 | 0.51 | −0.04 |
| No occupation | −4.61 | 3.06 | −1.51 | 0.13 | −0.06 |
| Education Reference category: Some primary schooling | |||||
| Some high school | 0.71 | 0.62 | 1.15 | 0.25 | 0.05 |
| Completed high school | 1.80 | 0.72 | 2.51 | 0.01 | 0.11 |
| Some tertiary | −0.03 | 1.21 | −0.03 | 0.98 | −0.00 |
| Tertiary qualification | 1.23 | 1.91 | 0.65 | 0.52 | 0.03 |
| Unknown education | 3.84 | 1.43 | 2.68 | 0.01 | 0.10 |
| Constant (cm) | 155.61 | 6.57 | 23.68 | 0.00 | . |
| Observations | 664 | ||||
| R-squared | 0.13 | ||||
Note: Additional controls for exact year of birth omitted. Only men aged 21 or over included. Dependent variable: Adult height in centimetres recorded in World War II records
Length at birth had a smaller impact on adult height. For every 1cm increment in birth length, a man was 0.15cm taller in adulthood. A man who was one standard deviation heavier than average at birth grew up to be 1.5cm taller in adulthood. By comparison, men one standard deviation longer at birth were just 0.5 cm taller in adulthood. This finding is consistent with modern studies (Sorensen et al., 1999).
There is some evidence that men born later in the cohort were slightly taller. Coefficients for individual years of birth relative to the 1907 baseline were larger for boys born after 1915. Partitioning the sample into boys born 1907–1915 and 1916–1922, the later group was 1cm taller on average (p=0.025). Men in both groups were 21 or older when measured, but there is a much greater chance men born later were still growing since 73% of those born after 1916 were aged 21–23 at enlistment. This suggests nutritional conditions across the growth period were better for men born later in the cohort.
Relationship of birth weight to adult systolic blood pressure
Mean systolic blood pressure at enlistment was 135 (Table 3). A quarter of recruits had systolic blood pressure above 140, the conventional threshold for high blood pressure. These values are higher than modern population means for the same age group in New Zealand and North America, but similar to modern levels in Europe (Danaei et al., 2011; Metcalf et al., 2006; Williams & Poulton, 2002; Wolf-Maier et al., 2003).
We find a slightly larger association between birth weight and systolic blood pressure than most modern studies (Table 6). Without controlling for current weight, we estimate that every one kilogram increase in birth weight is associated with a decrease in systolic blood pressure of 2.4 mm Hg (Model 1, 95% CI −4.90 to 0.01). Controlling for current weight or body mass index marginally decreases the size of the inverse association to 2.3 mm Hg/kg (Model 2). Addition of further controls for education and occupation at military enlistment also attenuates the effect of birth weight on systolic blood pressure to −2.2 mm Hg/kg (Table 7, Model 4. 95% CI −5.00 to 0.67).
Table 6.
Association of size at birth with adult systolic blood pressure
| (1) No control for current BMI | (2) Controls for current BMI | (3) No control for BMI if BMI measured | (4) Controls for BMI and social status | (5) No controls for social status if measured | |
|---|---|---|---|---|---|
| Birth weight (kg) | −2.44 (−1.95) | −2.31 (−1.64) | −2.15 (−1.53) | −2.16 (−1.50) | −2.31 (−1.62) |
| Length (cm) at birth | −0.03 (−0.14) | −0.16 (−0.67) | −0.17 (−0.71) | −0.25 (−1.01) | −0.21 (−0.86) |
| Maternal age | −0.27 (−0.68) | −0.20 (−0.50) | −0.22 (−0.53) | −0.18 (−0.44) | −0.21 (−0.53) |
| Square of maternal age | 0.01 (0.92) | 0.01 (0.82) | 0.01 (0.84) | 0.01 (0.78) | 0.01 (0.83) |
| Number of pregnancies | 0.48 (1.43) | 0.18 (0.48) | 0.19 (0.52) | 0.05 (0.14) | 0.16 (0.43) |
| Mother married | 17.78 (1.67) | 16.17 (1.48) | 17.53 (1.62) | 16.40 (1.48) | 14.88 (1.37) |
| Adult BMI | 0.30 (1.14) | 0.38 (1.42) | 0.37 (1.42) | ||
| Occupation Reference category: Professionals and managers | 0.00 (.) | ||||
| Clerical | −2.62 (−1.47) | ||||
| Sales | −1.89 (−0.95) | ||||
| Service | −4.30 (−1.22) | ||||
| Manufacturing workers | −7.38 (−1.32) | ||||
| Laborers | 2.95 (0.78) | ||||
| Farmers | 0.00 (.) | ||||
| Farm managers | 2.62 (0.77) | ||||
| No occupation | 5.89 (1.52) | ||||
| Education Reference category: Some primary schooling | −2.33 (−0.47) | ||||
| Some high school | −0.58 (−0.19) | ||||
| Completed high school | −0.65 (−0.17) | ||||
| Some tertiary | 0.43 (0.09) | ||||
| Tertiary qualification | 0.50 (0.12) | ||||
| Unknown education | −6.84 (−0.89) | ||||
| Constant | 132.90 (8.41) | 132.37 (7.41) | 138.88 (8.20) | 135.29 (7.29) | 134.42 (7.43) |
| R-squared | 0.043 | 0.042 | 0.039 | 0.071 | 0.044 |
| Observations | 654 | 537 | 537 | 523 | 523 |
t statistics in parentheses. Additional controls for exact year of birth omitted
Dependent variable in all analyses: Adult systolic blood pressure measured in World War II records.
However, not all recruits measured in World War II provided all measurements of height, weight, and social standing. Therefore, we estimate the model again without controlling for current BMI on the group that did provide all measurements (Table 7, Model 3), and perform a similar analysis for social status (Table 7, Model 5). The strength of the association between birth weight and blood pressure attenuates further when we omit controls for BMI in the group that was weighed at enlistment. This result is consistent with modern studies, and shows the importance of controls for adult body composition when testing the effect of birth weight on adult outcomes.
Discussion
Using a novel sample of maternity records linked to World War II enlistment records we provide evidence on three important questions: infant health in New Zealand during the early twentieth century infant mortality decline; and the earliest historical measurements ever of the association between birth size and adult stature, and the earliest ever measurements of the fetal origins hypothesis in young adults. In this sample of children born in a hospital serving working class families we find babies averaged 3.5kg at birth. Having traced a representative sample through to World War II enlistment records we find that the association of size at birth with adult stature and systolic blood pressure was higher than in modern studies.
Infant health in early twentieth century New Zealand
Despite the great interest in New Zealand infant welfare in the early twentieth century, there has been no historical evidence on birth weight in New Zealand compared to overseas. Comparison with babies born at a similar time in similar countries shows Wellington babies were a similar size to babies born in the United States and Australia, and larger than British babies (Table 4). Slightly fewer of the New Zealand babies were less than 2500 grams than at the New York Lying-in Hospital studied by Costa for a similar time period. The St Helens babies averaged 52cm at birth, which was slightly longer than observed in other similar populations (Costa, 1998). Because we cannot know precisely how selectivity by socio-economic status varied between the various countries, it is important not to make too much of small differences. The evidence is clear that babies of working class parents in New Zealand were as well-nourished as those in similar hospitals in Australia and the United States, and that all three “new world” countries had significantly heavier babies than the United Kingdom.
Comparison of our unconditional mean birth weights with modern data is slightly complicated because modern studies report sex and gestation-specific means, while we lack information on gestation. The median birth weight of live male singletons in our study (3574g) is nearly identical to the New Zealand median male birth weight at 40 weeks gestation in 1990/91, and midway between European birth weight medians at 39 and 40 weeks gestation in the late 1990s in Auckland (McCowan & Stewart, 2004; Thompson et al., 1994). These comparisons suggest that maternal nutrition in New Zealand in the early twentieth century was similar to modern standards.
Association of size at birth with adult height
Our estimates of the association between size at birth and adult height similar to the range of published values in the modern literature. Whereas we found a 1kg increase in birth weight associated with a 2.6cm increase in adult height, modern studies have found associations between 2 and 3cm (Li et al., 2004; Sorensen et al., 1999). The effects of birth weight on stature are significant, and larger than social class differences in stature. For example, studying New Zealand soldiers Inwood et al found urban professionals and managers were approximately 1.25 cm taller than urban manufacturing workers and laborers (Kris Inwood et al., 2010).
Consistent with modern research, we also found that parity progression reduced adult stature, even after controlling for maternal age. Each subsequent pregnancy reduced stature by just under one-third of a centimeter. These effects are of a somewhat larger magnitude than modern studies (Eide et al., 2005). This is consistent with the hypothesis that as population averages for birth weight and stature increase, the relationship between these variables and later-life outcomes will be weaker. In poorer historical cohorts, families had less economic capacity to provide for subsequent children.
Association of size at birth with adult systolic blood pressure
This study investigates the fetal origins hypothesis in the earliest cohort for which it has been investigated, with the earliest previous cohorts beginning in 1910. Our study pushes back measurement of the fetal origins hypothesis in young adults at least 45 years, with previous studies of young adults being limited to men born after 1966 (Gamborg et al., 2007a). Our estimates of the effect of birth weight on adult height and blood pressure are in the same direction as modern studies. However, we tend to find slightly larger magnitudes for our estimates than in most modern studies. Our preferred estimate from a model adjusted for current BMI and social status suggests that an increase in birth weight of 1kg is associated with a decrease in systolic blood pressure of 2.1 mm Hg. In comparison, reviews of the literature suggest the association lies between −1.5 and −2 (Lawlor & Smith, 2005).
But the majority of modern studies examining the fetal origins hypothesis have studied subjects in middle age and later life. Both cross-sectional and longitudinal studies suggest the relationship between birth weight and blood pressure is amplified with age (Davies et al., 2006), even after controlling for current BMI. Thus, it is important to compare our results with other studies of men in early adulthood. Our estimates of a 2.1 to 2.4 mm/Hg increase in systolic blood pressure with a 1kg decrease in birth weight lie towards the upper end of the results from other studies of young men. The most directly comparable modern studies to ours are of Swedish conscripts aged 18–24. These studies have estimated an inverse association of around 1 mm/Hg (Bergvall et al., 2005; Nilsson et al., 1997). Our estimates lie closer to the size of the association found in adults in their late 30s and early 40s in both British and Nordic studies (Hardy et al., 2003; Hardy et al., 2006; Hardy et al., 2004b). In this context, our results lend support to the argument that the association between birth weight and systolic blood pressure has weakened in the last century (Gamborg et al., 2007b).
A further context for understanding these results is comparison to mean systolic blood pressure levels over time (Adair & Dahly, 2005). The change in systolic blood pressure of 1 or 2 mm Hg due to decreases in birth weight is small relative to historical changes in blood pressure levels. Mean systolic blood pressure in our sample was approximately 10 mm/Hg higher than men of a similar age in New Zealand or North America today. In concert with our finding that male birth weights in New Zealand have not changed significantly, this suggests that only a small fraction of population-wide changes in blood pressure levels and hypertension incidence can be due to changes in fetal nutrition and birth outcomes.
However our finding that blood pressure was more strongly related to birth weight than in modern studies is significant. It suggests that adult outcomes were more sensitive to health at birth than in modern studies, and is consistent with evidence our cohort grew up in challenging economic circumstances. The cohorts examined here grew up during a long period of economic uncertainty in New Zealand (Greasley & Oxley, 2009). In particular, growth in New Zealand was weak during the 1920s, and economists have characterized the period from the end of World War I through the Great Depression as a long depression. Only boys born later in our cohort—from about 1916 to 1922—would have experienced at least part of their adolescent growth spurt after 1934 when the New Zealand economy recovered strongly from the Great Depression. Our finding that the younger men in our sample were taller is consistent with this hypothesis.
Conclusion
The fetal origins hypothesis has attracted significant attention in the past two decades, but has not been frequently tested on historical birth cohorts. Using a unique data set of measured birth weights and health measurements in early adulthood, we examine the relationship of size at birth with adult height and blood pressure in the earliest cohort ever studied. The successful linking of maternity records to military enlistment records opens up new possibilities for historical research on early life conditions and later life health.
Consistent with modern studies we find that birth weight has a statistically significant association with adult height and blood pressure. Our estimates are consistent in direction with modern research, but are larger in magnitude for blood pressure. Overall our findings suggest early adult health was more sensitive to health at birth in an historical cohort than in modern research. This suggests that while the association between size at birth and adult health may vary over time and space with social and economic conditions, it probably does so in a biologically determined range. Being smaller at birth had significant consequences in the past, and continues to have significant consequences to the present day.
Table 2.
Fitness of cohort and survival of medical examinations for New Zealand men aged 19–45 in World War II
| Fitness grade | Service eligibility | Per cent of overall cohort | Medical exams retained after military service |
|---|---|---|---|
| I | Active service in any theater | 62.6 | Yes |
| II | Home defence or base work | 13.3 | No |
| III | Unfit for military service. Capable of essential clerical or industrial work | 19.9 | No |
| IV | Not fit enough at any time for military or essential civilian industries | 4.3 | No |
Source: (Stout & Duncan, 1958; National Service Department, 1946)
References
- Adair L, Dahly D. Developmental determinants of blood pressure in adults. Annual Review of Nutrition. 2005;25:407–434. doi: 10.1146/annurev.nutr.25.050304.092538. [DOI] [PubMed] [Google Scholar]
- Almond D, Currie J. Killing Me Softly: The Fetal Origins Hypothesis. Journal of Economic Perspectives. 2011;25:153–172. doi: 10.1257/jep.25.3.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asao K, Kao WHL, Baptiste-Roberts K, Bandeen-Roche K, Erlinger TP, Brancati FL. Short Stature and the Risk of Adiposity, Insulin Resistance, and Type 2 Diabetes in Middle Age: The Third National Health and Nutrition Examination Survey (NHANES III), 1988–1994. Diabetes Care. 2006;29:1632–1637. doi: 10.2337/dc05-1997. [DOI] [PubMed] [Google Scholar]
- Baker P. Conscription. In: McGibbon I, editor. Oxford Companion to New Zealand Military History. Melbourne: Oxford University Press; 2000. [Google Scholar]
- Baldwin BT. The physical growth of children from birth to maturity. Iowa City: University of Iowa Child Welfare Research Station; 1921. [Google Scholar]
- Barker D, Osmond C, Golding J, Kuh D, Wadsworth ME. Growth in utero, blood pressure in childhood and adult life, and mortality from cardiovascular disease. British Medical Journal. 1989a;298:564. doi: 10.1136/bmj.298.6673.564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJP. Mothers, Babies, and Health in Later Life. London: Churchill Livingstone; 1998. [Google Scholar]
- Barker DJP, Osmond C. Infant mortality, childhood nutrition, and ischaemic heart disease in England and Wales. The Lancet. 1986;327:1077–1081. doi: 10.1016/s0140-6736(86)91340-1. [DOI] [PubMed] [Google Scholar]
- Barker DJP, Osmond C, Winter PD, Margetts B, Simmonds SJ. Weight in Infancy and Death from Ischaemic Heart Disease. The Lancet. 1989b;334:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- Ben-Shlomo Y, Kuh D. A life course approach to chronic disease epidemiology: conceptual models, empirical challenges and interdisciplinary perspectives. International Journal of Epidemiology. 2002;31:285–293. [PubMed] [Google Scholar]
- Bergvall N, Iliadou A, Tuvemo T, Cnattingius S. Birth Characteristics and Risk of High Systolic Blood Pressure in Early Adulthood: Socioeconomic Factors and Familial Effects. Epidemiology. 2005;16:635–640. doi: 10.1097/01.ede.0000172134.45742.49. 610.1097/1001.ede.0000172134.0000145742.0000172149. [DOI] [PubMed] [Google Scholar]
- Bogin B, Kapell M. Growth Studies. In: Spencer F, editor. History of Physical Anthropology: An Encyclopedia. New York: Garland; 1997. pp. 461–466. [Google Scholar]
- Bryder L. A voice for mothers: the Plunket Society and infant welfare, 1907–2000. Auckland, N.Z: Auckland University Press; 2003. [Google Scholar]
- Costa DL. Unequal at Birth: A Long-Term Comparison of Income and Birth Weight. The Journal of Economic History. 1998;58:987–1009. [Google Scholar]
- Danaei G, Finucane MM, Lin JK, Singh GM, Paciorek CJ, Cowan MJ, et al. National, regional, and global trends in systolic blood pressure since 1980: systematic analysis of health examination surveys and epidemiological studies with 786 country-years and 5·4 million participants. The Lancet. 2011;377:568–577. doi: 10.1016/S0140-6736(10)62036-3. [DOI] [PubMed] [Google Scholar]
- Davies AA, Smith GD, May MT, Ben-Shlomo Y. Association Between Birth Weight and Blood Pressure Is Robust, Amplifies With Age, and May Be Underestimated. Hypertension. 2006;48:431–436. doi: 10.1161/01.HYP.0000236551.00191.61. [DOI] [PubMed] [Google Scholar]
- Dye NS. Modern Obstetrics and Working-Class Women: The New York Midwifery Dispensary, 1890–1920. Journal of Social History. 1987;20:549–564. doi: 10.1353/jsh/20.3.549. [DOI] [PubMed] [Google Scholar]
- Edvinsson S, Gar∂arsdóttir Ó, Thorvaldsen G. Infant mortality in the Nordic countries, 1780–1930. Continuity and Change. 2008;23:457–485. [Google Scholar]
- Eide M, Oyen N, Skjaerven R, Nilsen S, Bjerkedal T, Tell G. Size at Birth and Gestational Age as Predictors of Adult Height and Weight. Epidemiology. 2005;16:175. doi: 10.1097/01.ede.0000152524.89074.bf. [DOI] [PubMed] [Google Scholar]
- Engeland A, Bjorge T, Selmer RM, Tverdal A, Engeland A, Bjorge T, et al. Height and body mass index in relation to total mortality. Epidemiology. 2003a;14:293–299. [PubMed] [Google Scholar]
- Engeland A, Bjorge T, Sogaard AJ, Tverdal A, Engeland A, Bjorge T, et al. Body mass index in adolescence in relation to total mortality: 32-year follow-up of 227,000 Norwegian boys and girls. American Journal of Epidemiology. 2003b;157:517–523. doi: 10.1093/aje/kwf219. [DOI] [PubMed] [Google Scholar]
- Eriksson M, Wallander MA, Krakau I, Wedel H, Svärdsudd K. Birth weight and cardiovascular risk factors in a cohort followed until 80 years of age: the study of men born in 1913. Journal of Internal Medicine. 2004;255:236–246. doi: 10.1046/j.1365-2796.2003.01289.x. [DOI] [PubMed] [Google Scholar]
- Gamborg M, Byberg L, Rasmussen F, Andersen PK, Baker JL, Bengtsson C, et al. Birth Weight and Systolic Blood Pressure in Adolescence and Adulthood: Meta-Regression Analysis of Sex- and Age-specific Results from 20 Nordic Studies. American Journal of Epidemiology. 2007a;166:634–645. doi: 10.1093/aje/kwm042. [DOI] [PubMed] [Google Scholar]
- Gamborg M, Byberg L, Rasmussen F, Andersen PK, Baker JL, Bengtsson C, et al. Birth Weight and Systolic Blood Pressure in Adolescence and Adulthood: Meta-Regression Analysis of Sex- and Age-specific Results from 20 Nordic Studies. American Journal of Epidemiology. 2007b;166:634–645. doi: 10.1093/aje/kwm042. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Buklijas T. A conceptual framework for the developmental origins of health and disease. Journal of Developmental Origins of Health and Disease. 2010;1:6–18. doi: 10.1017/S2040174409990171. [DOI] [PubMed] [Google Scholar]
- Greasley D, Oxley L. The pastoral boom, the rural land market, and long swings in New Zealand economic growth, 1873–1939. Economic History Review. 2009;62:324–349. [Google Scholar]
- Hardy R, Kuh D, Langenberg C, Wadsworth MEJ. Birthweight, childhood social class, and change in adult blood pressure in the 1946 British birth cohort. The Lancet. 2003;362:1178–1183. doi: 10.1016/S0140-6736(03)14539-4. [DOI] [PubMed] [Google Scholar]
- Hardy R, Sovio U, King VJ, Skidmore PML, Helmsdal G, Olsen SF, et al. Birthweight and blood pressure in five European birth cohort studies: an investigation of confounding factors. The European Journal of Public Health. 2006;16:21–30. doi: 10.1093/eurpub/cki171. [DOI] [PubMed] [Google Scholar]
- Hardy R, Wadsworth ME, Langenberg C, Kuh D. Birthweight, childhood growth, and blood pressure at 43 years in a British birth cohort. International Journal of Epidemiology. 2004a;33:121–129. doi: 10.1093/ije/dyh027. [DOI] [PubMed] [Google Scholar]
- Hardy R, Wadsworth MEJ, Langenberg C, Kuh D. Birthweight, childhood growth, and blood pressure at 43 years in a British birth cohort. International Journal of Epidemiology. 2004b;33:121–129. doi: 10.1093/ije/dyh027. [DOI] [PubMed] [Google Scholar]
- Huxley R, Neil A, Collins R. Unravelling the fetal origins hypothesis: is there really an inverse association between birthweight and subsequent blood pressure? The Lancet. 2002;360:659–665. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- Inwood K, Oxley L, Roberts E. Rather above than under the common size? Stature and Living Standards in New Zealand. World Economic History Congress; Utrecht. 2009. [Google Scholar]
- Inwood K, Oxley L, Roberts E. Physical stature in nineteenth century New Zealand—a preliminary interpretation. Australian Economic History Review. 2010;50:262–283. [Google Scholar]
- Järvelin MR, Sovio U, King V, Lauren L, Xu B, McCarthy MI, et al. Early life factors and blood pressure at age 31 years in the 1966 northern Finland birth cohort. Hypertension. 2004;44:838–846. doi: 10.1161/01.HYP.0000148304.33869.ee. [DOI] [PubMed] [Google Scholar]
- Karpinos B. Height and weight of selective service registrants processed for military service WWII. Human Biology. 1958;30:292–321. [PubMed] [Google Scholar]
- Kermack W, McKendrick A, McKinlay P. Death-rates in Great Britain and Sweden some general regularities and their significance. The Lancet. 1934;223:698–703. doi: 10.1093/ije/30.4.678. [DOI] [PubMed] [Google Scholar]
- Lawlor DA, Smith GD. Early life determinants of adult blood pressure. Current Opinion in Nephrology and Hypertension. 2005;14:259–264. doi: 10.1097/01.mnh.0000165893.13620.2b. [DOI] [PubMed] [Google Scholar]
- Li L, Manor O, Power C. Early environment and child-to-adult growth trajectories in the 1958 British birth cohort. American Journal of Clinical Nutrition. 2004;80:185–192. doi: 10.1093/ajcn/80.1.185. [DOI] [PubMed] [Google Scholar]
- McCowan L, Stewart AW. Term birthweight centiles for babies from New Zealand’s main ethnic groups. Australian and New Zealand Journal of Obstetrics and Gynaecology. 2004;44:432–435. doi: 10.1111/j.1479-828X.2004.00273.x. [DOI] [PubMed] [Google Scholar]
- Mein Smith P. Truby King in Australia: A Revisionist View of Reduced Infant Mortality. New Zealand Journal of History. 1988;22:23–43. [PubMed] [Google Scholar]
- Metcalf P, Scragg R, Schaaf D, Dyall L, Black P, Jackson R. Trends in major cardiovascular risk factors in Auckland, New Zealand: 1982 to 2002–2003. New Zealand Medical Journal. 2006:119. [PubMed] [Google Scholar]
- Morley R, McCalman J, Carlin JB. Birthweight and coronary heart disease in a cohort born 1857–1900 in Melbourne, Australia. International Journal of Epidemiology. 2006:35. doi: 10.1093/ije/dyl032. [DOI] [PubMed] [Google Scholar]
- Nilsson PM, Östergren PO, Nyberg P, Söderström M, Allebeck P. Low birth weight is associated with elevated systolic blood pressure in adolescence: a prospective study of a birth cohort of 149 378 Swedish boys. Journal of Hypertension. 1997;15:1627–1631. doi: 10.1097/00004872-199715120-00064. [DOI] [PubMed] [Google Scholar]
- Nuttall A. ‘Because of Poverty brought into Hospital: …’ A Casenote-Based Analysis of the Changing Role of the Edinburgh Royal Maternity Hospital, 1850–1912. Social History of Medicine. 2007;20:263–280. doi: 10.1093/shm/hkm042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paajanen TA, Oksala NKJ, Kuukasjärvi P, Karhunen PJ. Short stature is associated with coronary heart disease: a systematic review of the literature and a meta-analysis. European heart journal. 2010;31:1802–1809. doi: 10.1093/eurheartj/ehq155. [DOI] [PubMed] [Google Scholar]
- Parr CJ, McGavin D, Elliott JS. Maternal Mortality in New Zealand. Appendix to the Journal of the House of Representatives, Session I–II. 1921:H-31B. [Google Scholar]
- Schnall PL, Landsbergis PA, Baker D. Job strain and cardiovascular disease. Annual Review of Public Health. 1994;15:381–411. doi: 10.1146/annurev.pu.15.050194.002121. [DOI] [PubMed] [Google Scholar]
- Silventoinen K. Determinants of variation in adult body height. Journal of Biosocial Science. 2003;35:263–285. doi: 10.1017/s0021932003002633. [DOI] [PubMed] [Google Scholar]
- Silventoinen K, Zdravkovic S, Skytthe A, McCarron P, Herskind A, Koskenvuo M, et al. Association between height and coronary heart disease mortality: a prospective study of 35,000 twin pairs. American Journal of Epidemiology. 2006;163:615–621. doi: 10.1093/aje/kwj081. [DOI] [PubMed] [Google Scholar]
- Smith GD, Lynch JW. Life course approaches to socioeconomic differentials in health. In: Kuh D, Ben-Shlomo Y, editors. A life course approach to chronic disease epidemiology. Oxford: Oxford University Press; 2004. pp. 77–96. [Google Scholar]
- Sorensen H, Sabroe S, Rothman K, Gillman M, Steffensen F, Fischer P, et al. Birth weight and length as predictors for adult height. American Journal of Epidemiology. 1999;149:726–729. doi: 10.1093/oxfordjournals.aje.a009881. [DOI] [PubMed] [Google Scholar]
- Strike PC, Steptoe A. Psychosocial factors in the development of coronary artery disease. Progress in Cardiovascular Diseases. 2004;46:337–347. doi: 10.1016/j.pcad.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Tanner JM. A history of the study of human growth. Cambridge: Cambridge University Press; 1981. [Google Scholar]
- Thompson JM, Mitchell EA, Borman B. Sex specific birthweight percentiles by gestational age for New Zealand. The New Zealand Medical Journal. 1994;107:1. [PubMed] [Google Scholar]
- Waaler H. Height, weight and mortality. The Norwegian experience. Acta Medica Scandinavica, Supplementum. 1984;679:1–56. doi: 10.1111/j.0954-6820.1984.tb12901.x. [DOI] [PubMed] [Google Scholar]
- Watt MH. Instructions for Conduct of Medical Exmination under the National Service Emergency Regulations. Wellington: National Medical Committee: Archives New Zealand; 1940. AD11252/271/18/2, Part 2. [Google Scholar]
- Williams S, Poulton R. Birth size, growth, and blood pressure between the ages of 7 and 26 years: failure to support the fetal origins hypothesis. American Journal of Epidemiology. 2002;155:849–852. doi: 10.1093/aje/155.9.849. [DOI] [PubMed] [Google Scholar]
- Wolf-Maier K, Cooper RS, Banegas JR, Giampaoli S, Hense HW, Joffres M, et al. Hypertension prevalence and blood pressure levels in 6 European countries, Canada, and the United states. JAMA. 2003;289:2363–2369. doi: 10.1001/jama.289.18.2363. [DOI] [PubMed] [Google Scholar]
- Wood P, Foureur M. A clean front passage: dirt, douches and disinfectants at St Helens Hospital, Wellington, New Zealand, 1907–1922. In: Kirkman M, editor. Exploring the dirty side of women’s health. Milton Park: Routledge; 2007. pp. 30–43. [Google Scholar]
- Woodbury RM. Infant mortality and preventive work in New Zealand. Washington: United States Children’s Bureau; 1922. [Google Scholar]
- Young CH, Savola KL, Phelps E. Inventory of longitudinal studies in the social sciences. Newbury Park: Sage Publications; 1991. [Google Scholar]