Abstract
Context:
Few authors have assessed neuromuscular knee-stabilization strategies in individuals with chronic ankle instability (CAI) during functional activities.
Objective:
To investigate the influence of CAI on neuromuscular characteristics around the knee during a stop-jump task.
Design:
Case-control study.
Setting:
Research laboratory.
Participants or Other Participants:
A total of 19 participants with self-reported unilateral CAI and 19 healthy control participants volunteered for this study.
Intervention(s):
Participants performed double-legged, vertical stop-jump tasks onto a force plate, and we measured muscle activation around the knee of each limb.
Main Outcome Measure(s):
We calculated the integrated electromyography for the vastus medialis oblique, vastus lateralis, medial hamstrings, and lateral hamstrings muscles during the 100 ms before and after initial foot contacts with the force plate and normalized by the ensemble peak electromyographic value. Knee sagittal-plane kinematics were also analyzed during a stop-jump task.
Results:
Compared with control participants, the CAI group demonstrated greater prelanding integrated electromyographic activity of the vastus medialis oblique (CAI = 52.28 ± 11.25%·ms, control = 43.90 ± 10.13%·ms, t36 = 2.41, P = .021, effect size = 0.78, 95% confidence interval = 0.11, 1.43) and less knee-flexion angle at the point of initial foot contact (CAI = 7.81° ± 8.27°, control = 14.09° ± 8.7°, t36 = −2.28, P = .029, effect size = −0.74, 95% confidence interval = −1.38, −0.07) and at 100 ms post–initial foot contact (CAI = 51.36° ± 5.29°, control = 58.66° ± 7.66°, t36 = −3.42, P = .002, effect size = −1.11, 95% confidence interval = −1.77, −0.40). No significant results were noted for the other electromyographic measures.
Conclusions:
We found altered feed-forward patterns of the vastus medialis oblique and altered postlanding knee sagittal-plane kinematics in the CAI group. These observations may provide insight regarding sensorimotor characteristics that may be associated with CAI.
Key Words: feed-forward pattern, feedback, sensorimotor control
Key Points
Increased preparatory vastus medialis oblique muscle activation and decreased postlanding knee-flexion angle were seen in participants with chronic ankle instability compared with the control group during a vertical stop jump.
Feed-forward sensorimotor control around the knee should be addressed during therapeutic interventions for chronic ankle instability.
Chronic ankle instability (CAI) is common after an acute lateral ankle sprain (LAS) in physically active individuals,1–3 leading to self-assessed disability and decreased quality of life.4 It has been reported that CAI is a leading factor for the development of posttraumatic osteoarthritis in the ankle,1,5 requiring costly medical diagnostic techniques and extensive treatments. With a high rate of recurrence plus the complications of prolonged functional impairments after ankle sprains, there is an increased need for evidence-based practice to develop and implement more effective intervention programs for CAI.
One important step in reducing the recurrence rate is to understand the underlying sensorimotor mechanisms for CAI. After LAS, altered afferent inputs from the somatosensory system around the ankle and central changes in sensorimotor control may result in proximal joint adaptations to compensate for residual symptoms and functional impairments.6–13 Previous researchers6,10 have indirectly assessed sensorimotor control strategies in CAI patients at the knee using sagittal-plane kinematics. Gribble and Robinson6 found greater knee extension before and at the point of ground impact7 during a vertical jump-landing task, whereas Caulfield and Garrett10 observed greater knee flexion before and after landing during a drop-jump task. These contradictory findings may be attributable to differences between these studies in the demands of the jump-landing tasks; however, neither group quantified electromyography (EMG) of the knee musculature during the landing tasks. Thus, we still do not know how activation of the knee flexors and extensors influences sagittal-plane kinematics during jump-landing tasks in individuals with a history of ankle injury.
Although several EMG investigations have demonstrated altered preparatory muscle-activation patterns in the ankle during jump landings in CAI patients,9,11–14 few investigators have examined preparatory EMG measures in the knee during a dynamic task within this population, which may limit our estimation of relative preparatory activity of muscles in the knee. Delahunt et al11 observed an increase in rectus femoris activation before ground impact during a lateral-hop task in individuals with CAI, supporting modified preprogrammed muscle-activation patterns via feed-forward motor control to prepare for ground impact. Additionally, Delahunt et al14 reported that those with CAI exhibited no differences in preparatory rectus femoris muscle activation during a drop-landing task. However, a drop-jump task is not a sport-related functional task and does not necessarily replicate the potential mechanism of injury.
Whereas a relationship between CAI and altered biomechanical patterns at the knee has been established, few authors have quantitatively assessed sensorimotor control strategies at the knee using EMG while measuring sagittal-plane kinematics in individuals with CAI during other high-risk and sport-related functional activities, such as a vertical stop jump. It is important to examine knee muscle activation and sagittal-plane kinematic patterns to determine the potential effect of CAI on the sensorimotor control mechanism, especially pre-event decisions and postevent reactions, during a stop-jump maneuver. Researchers6,10 have speculated that adapted feed-forward sensorimotor control strategies at the knee associated with CAI could be a way to protect the unstable ankle by controlling the position of the ankle and center of mass on ground impact. However, a diminished level of dynamic stability and energy dissipation of the knee musculature after landing has been observed in individuals with CAI.6,7,15 Therefore, this proposed protective response to the unstable ankle may not be an efficient response. Although information is limited, there is a potential link between a history of ankle sprain and risk of injury at the knee joint.16–18 The potential sensorimotor adaptations associated with CAI may not necessarily be protective for other joints. Identifying an underlying relationship between CAI and compensatory sensorimotor control in the knee may help clinicians and researchers to develop more comprehensive interventions to enhance global coordination in those with CAI and prevent future injury. Thus, the purpose of our study was to examine individuals with and without unilateral CAI for the presence of altered neuromuscular control in the knee during a vertical stop jump. Previous investigators observed a smaller knee-flexion angle before and after ground impact during a jump-landing task in participants with CAI compared with healthy controls.6,7,19 Furthermore, increased preparatory muscle activation in the ankle during a stop-jump task has been reported in those with CAI.12 Our hypotheses were that, during a stop-jump task, participants with CAI would demonstrate (1) increased quadriceps muscle activation before and after landing; (2) increased hamstrings muscle activation before landing; (3) decreased hamstrings muscle activation after landing; and (4) reduced knee-flexion angle before and after landing, as compared with participants without CAI.
METHODS
Experimental Design
The study was a case-control design that assessed muscle activation and sagittal-plane kinematics at the knee in both CAI and healthy participants.
Participants
We recruited 38 physically active participants from the university community. Physically active was defined as an individual engaging in at least 20 minutes of vigorous activity, 3 or more days per week.20 All participants were free of any diagnosed balance or vestibular disorders. The participants were categorized as healthy (control) or CAI. Age and anthropometric characteristics of the healthy and CAI participants are shown in Table 1. All participants read and signed the informed consent forms approved by the University of Toledo Institutional Review Board (which also approved the study) at the beginning of testing.
Table 1.
Demographic Information and Questionnaire Scores for the Chronic Ankle Instability and Control Groups (Mean ± SD)
| Variables |
Chronic Ankle Instability Group |
Control Group |
P Value |
| n | 19 (10 men, 9 women) | 19 (10 men, 9 women) | – |
| Age, y | 20.11 ± 1.63 | 21.32 ± 4.04 | .30 |
| Height, cm | 177.06 ± 9.38 | 171.27 ± 9.02 | .19 |
| Body mass, kg | 75.90 ± 17.04 | 71.25 ± 14.92 | .14 |
| Foot and Ankle Disability Index, % | 83.15 ± 8.83 | 100.00 ± 0.00 | <.001a |
| Foot and Ankle Disability Index Sports Scale, % | 65.79 ± 10.90 | 100.00 ± 0.00 | <.001a |
| Ankle Instability Instrument | 5.58 ± 1.54 | 0.00 ± 0.00 | <.001a |
Differences between groups in all questionnaire scores.
The control group consisted of 19 participants with no history of any self-reported musculoskeletal or neurovascular injury or disorder in the lower extremity, no history of low back pain in the last 6 months, and no history of surgery in the lower extremity.
The unilateral CAI group consisted of 19 participants who self-reported (1) a previous history of at least 1 acute unilateral ankle sprain that caused swelling, pain, and temporary loss of function but no significant injury to the ankle in the last 6 months,7 (2) a history of at least 2 self-reported episodes of “giving way” in the last 3 months,7 (3) no previous history of any musculoskeletal and neurovascular injury in the lower extremity other than the ankle in the last 2 years, (4) no previous history of low back pain in the last 6 months, and (5) no previous fractures or surgery in the lower extremity in the last 2 years.
To determine additional inclusion criteria, participants completed 2 questionnaires related to ankle instability: the Foot and Ankle Instability Disability Index (FADI), including the FADI Sports Subscale, and the Ankle Instability Instrument (AII). The FADI and AII have been shown as reliable and valid in assessing functional limitations in those with CAI.21,22 To be classified into the CAI group, the participant was required to self-report functional disability with a score of ≤90% on the FADI and ≤80% on the FADI Sport Subscale7,23 as well as a score of at least 3 on the AII.24 Group means for the FADI, FADI Sport, and AII are shown in Table 2.
Table 2.
Prelanding Integrated Electromyography of Knee Muscles and Knee Sagittal-Plane Kinematics for the Chronic Ankle Instability and Control Groups
| Variable |
Chronic Ankle Instability Group |
Control Group |
t36 |
P Value |
Power |
| Integrated electromyography, %·ms | Mean ± SD (95% Confidence Interval) | ||||
| Vastus medialis oblique | 52.28 ± 11.25 (47.30, 57.26) | 43.90 ± 10.13 (38.92, 48.88) | 2.41 | .021a | 0.78 |
| Vastus lateralis | 46.51 ± 10.99 (41.76, 51.27) | 45.85 ± 9.30 (41.10, 50.60) | 0.20 | .843 | 0.07 |
| Medial hamstrings | 42.80 ± 8.25 (37.94, 47.40) | 41.16 ± 12.60 (36.70, 46.16) | 0.47 | .639 | 0.12 |
| Lateral hamstrings | 43.11 ± 13.04 (37.53, 48.70) | 50.50 ± 10.87 (44.91, 56.08) | −1.90 | .066 | 0.57 |
| Kinematics, ° | |||||
| At 100 ms pre–ground impact | 4.84 ± 10.58 (9.60, 0.08) | 8.88 ± 7.31 (12.17, 5.59) | −0.72 | .477 | 0.39 |
Difference between groups in prelanding integrated electromyography of the vastus medialis oblique (P < .05).
Instrumentation
An 8-channel telemeterized EMG system (Noraxon USA, Inc, Scottsdale, AZ) and electromagnetic tracking system (Ascension Technology Corporation, Burlington, VT) synchronized with a nonconductive force plate (model 4060NC; Bertec Inc, Columbus, OH) were integrated with MotionMonitor software (version 7.0; Innovative Sports Training, Inc, Chicago, IL) to quantify the sensorimotor control variables during the stop-jump task. A Vertec vertical jump tester (Sports Imports, Columbus, OH) was used to assess participants' jump height.
Procedures
Participants reported to the Musculoskeletal Health and Movement Science Laboratory for 1 testing session. At the beginning of the session, we assessed maximum vertical jump height (Vertmax) so that maximum and submaximum targets (50% of Vertmax) could be designated for each participant during the vertical stop-jump trials.25
Participant Preparation
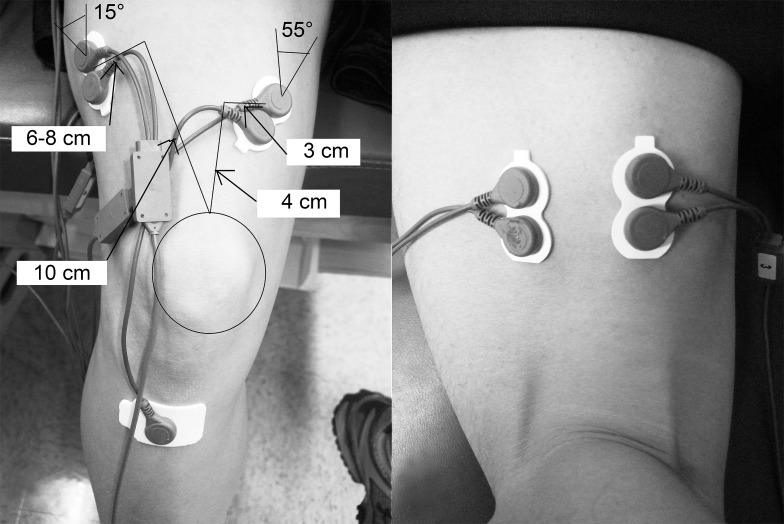
We placed a pair of self-adhesive and disposable dual circular silver/silver chloride surface electrodes (0.8-cm diameter, 1.5-cm center-to-center interelectrode distance; Noraxon USA, Inc) over the vastus medialis oblique (VMO),26 vastus lateralis (VL),26 lateral hamstrings (LH),27,28 and medial hamstrings muscles (MH)27,28 (Figure 1), as identified during submaximal isometric contraction against manual resistance. The common reference electrode was placed over the tibial tuberosity.26 The skin was prepared by shaving the hair of each electrode site, if necessary, and cleaning it with alcohol preparation pads. We placed electromagnetic sensors over the sacrum, lateral midthigh, lateral midshank, and dorsal surface of the foot of the testing leg and secured them to the skin using double-sided tape, nonadhesive elastic tape, and white adhesive tape.6 A fifth sensor was attached to a plastic stylus and used for digitizing the body segments in the software.6
Figure 1.
Electrode placement on the vastus medialis oblique, vastus lateralis, lateral hamstrings, and medial hamstrings muscles.
After completing the preparation, participants performed a vertical stop jump while we assessed muscle-activation patterns of each designated muscle and sagittal-plane knee kinematics.
Vertical Stop-Jump Task
Participants performed a vertical stop-jump task described previously29 that was modified for this study. Each participant stood on a line that was set up at a distance from the center of the force plate equal to his or her height (Figure 2A) and took a step forward with the testing limb to a line measured as 50% of the participant's height from the center of the force plate (Figure 2B). He or she took off on the testing limb immediately after the testing foot made contact with the ground on the line (Figure 2C), reached up to touch a marker indicated as 50% of Vertmax on the Vertec, landed with both feet at the same time (and only the testing limb in the middle of the force plate; Figure 2D), and then performed a maximum 2-legged vertical jump while reaching up to touch a marker indicated for Vertmax on the Vertec (Figure 2E). The participant landed in approximately the same position after the maximum vertical jump. The tasks of the vertical stop-jump were first explained and demonstrated by the investigator. Each person was allowed to practice this task until he or she felt comfortable. No instructions about jumping techniques were provided to participants in order to minimize a coaching effect on the natural performances of the task. Finally, each participant performed 5 testing trials on the testing leg, with approximately 30 seconds of rest between trials to prevent fatigue. Trials were discarded and repeated if the participant failed to reach Vertmax or to make contact with the nontesting leg on the force plate.
Figure 2.
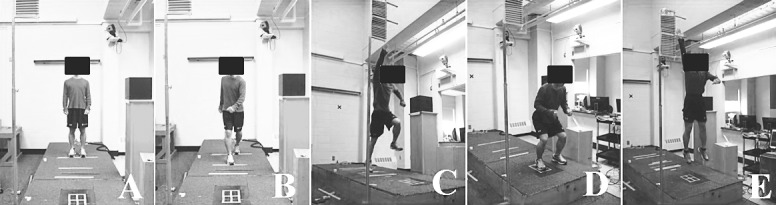
Modified vertical stop-jump procedure. A, Starting position. B, Step forward with the testing leg. C, A single-limb forward jump to target (50% Vertmax). D, A double-limb landing. E, An immediate two-legged vertical jump to target (Vertmax).
Data Collection and Reduction
We collected the prelanding and postlanding EMG variables at a sampling rate of 1000 Hz. Raw analog EMG signals were full-wave rectified and filtered by a bandpass Butterworth filter at a frequency between 10 and 500 Hz. The common mode ratio was >100 dB, the input impedance was >100 MΩ, and baseline noise was <1 μV. Ground reaction force was sampled at 1000 Hz from the nonconductive force plate. The integrated EMG (IEMG) was calculated for each muscle during the prelanding period, defined here as the 100 ms immediately before ground impact.30 The IEMG was defined as the area under the voltage curve of the EMG signal and measured in V·ms.31 Use of a 100-ms prelanding phase allowed us to examine muscular preactivation in preparation for landing. This time frame was consistent with that used in previous studies.6,10,32
Additionally, the EMG measures for each muscle were integrated over the time period from the initial ground impact (the point at which the vertical ground reaction forces exceeded 10 N) to the point of 100 ms post–ground impact during the stop jump.
We normalized the IEMG variables for each muscle with the ensemble peak (ie, the highest-amplitude) EMG values that were recorded during each landing phase of the stop-jump trials33,34 and calculated from the average of the 5 trials. To produce the ensemble EMG signals, the amplitudes of the rectified EMG signals were averaged for each participant separately at the same prelanding and postlanding time periods for each muscle individually across the 5 trials. Normalized IEMG values were expressed as %·ms. After IEMG calculation, the means of the 5 trials during each prelanding and postlanding phase were determined. All EMG data analyses were performed using Excel (version 2007; Microsoft Corporation, Redmond, WA).
We collected sagittal-plane kinematics data at a sampling rate of 100 Hz and filtered by the MotionMonitor software with a low-pass, third-order Butterworth filter set at a cutoff frequency of 20 Hz.35 The lower extremity model was generated by digitizing the ankle-, knee-, and hip-joint centers. The representations of the ankle, knee, and hip joints were created by using the proximal segment as the reference frame in the software setup. The ankle-joint center was defined as the midpoint between the digitized medial and lateral malleoli, and the knee-joint center was defined as the midpoint between the digitized medial and lateral femoral condyles. The Davis method was used to estimate the hip-joint center.36 The segment axis systems of the foot, shank, thigh, and sacrum were established with a right-hand coordinate system, with the x-axis designated as positive leftward-medial, the y-axis as positive forward-anterior, and the z-axis as positive upward-superior.37 The Grood-Suntay angle-orientation function in the software was used to determine knee sagittal-plane kinematics at initial foot contact with the force plate and at 100 ms pre–ground impact and post–ground impact.
Statistical Analysis
Using an independent-samples t test, we compared demographic variables and self-reported measures between groups to verify group inclusion. Independent t tests were conducted to compare the means and standard deviations of the EMG and kinematic dependent variables between the CAI and control groups during the jump-landing task. The level of significance was set a priori at P < .05 using SPSS (version 17.0 for Windows; SPSS Inc, Chicago, IL). Cohen d effect sizes using the pooled standard deviations were calculated,38 along with 95% confidence intervals (CIs) to determine the magnitude of difference in dependent variables between the CAI and control groups. The strength of the effect sizes was interpreted as weak (d < 0.4), moderate (0.4 ≤ d < 0.8), or strong (0.8 ≤ d).39
RESULTS
We found no differences in age, height, or mass between groups (Table 1). The CAI group scored significantly lower on the FADI and FADI Sport instruments as well as higher on the AII, verifying the presence of the targeted condition (Table 1).
Prelanding IEMG and Kinematics
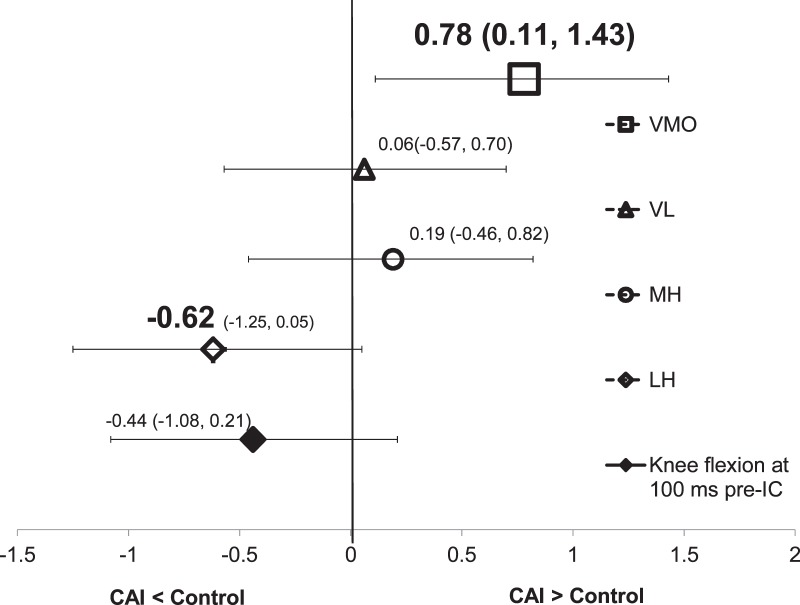
Means and standard deviations for all prelanding IEMG and sagittal-plane kinematic variables are found in Table 2. Participants with CAI demonstrated greater VMO activity 100 ms before initial foot contact compared with the control group (t36 = 2.41, P = .021). The effect size was strong for the prelanding IEMG of VMO, and the 95% CI did not cross zero (Figure 3).
Figure 3.
Effect sizes and associated 95% confidence intervals for prelanding integrated electromyography of vastus medialis oblique (VMO), vastus lateralis (VL), medial hamstrings (MH), and lateral hamstrings (LH) as well as knee sagittal-plane kinematics. Abbreviations: CAI, chronic ankle instability; IC, initial contact.
The group difference in the prelanding IEMG of the LH approached significance (t36 = −1.90, P = .066), and we found a moderate effect size (d = −0.62). The LH activity was less in the CAI group than in the control group. No statistically significant differences were noted in the IEMG of the VL and MH or the knee sagittal-plane kinematics during the prelanding period (Table 2 and Figure 3).
Postlanding IEMG
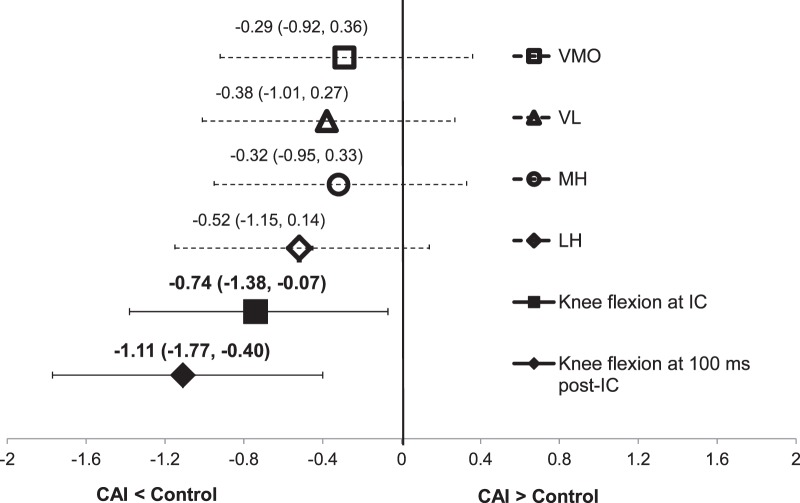
Group means and standard deviations for postlanding IEMG and kinematic variables are detailed in Table 3. Participants with CAI exhibited a smaller knee-flexion angle at initial foot contact (t36 = −2.28, P = .029) and 100 ms after initial contact (t36 = −3.42, P = .002) during the stop-jump task compared with healthy controls (Table 3). The range of effect sizes was moderate to large for postlanding knee kinematics with a 95% CI that did not cross zero (Figure 4). We found no statistically significant group differences in IEMG for any of the tested muscles over the time period from the moment of landing to the point of 100 ms after landing (Table 3). The effect sizes and 95% CIs around the effect sizes are shown in Figure 4.
Table 3.
Postlanding Integrated Electromyography of Knee Muscles and Knee Sagittal-Plane Kinematics for the Chronic Ankle Instability and Control Groups
| Variable |
Chronic Ankle Instability Group |
Control Group |
t36 |
P Value |
Power |
| Integrated electromyography, %·ms | Mean ± SD (95% Confidence Interval) | ||||
| Vastus medialis oblique | 35.36 ± 10.05 (30.60, 40.07) | 38.30 ± 10.45 (33.36, 42.82) | −0.89 | .382 | 0.22 |
| Vastus lateralis | 34.96 ± 10.41 (24.17, 45.40) | 43.99 ± 31.86 (32.78, 54.01) | −1.17 | .248 | 0.32 |
| Medial hamstrings | 47.39 ± 9.49 (35.39, 59.50) | 55.46 ± 34.44 (43.20, 67.30) | −0.98 | .332 | 0.26 |
| Lateral hamstrings | 45.66 ± 9.57 (41.39, 49.62) | 50.40 ± 8.74 (46.46, 54.70) | −1.60 | .119 | 0.48 |
| Kinematics, ° | |||||
| At ground impact | 7.81 ± 8.27 (11.53, 4.09) | 14.09 ± 8.72 (18.01, 10.17) | −2.28 | .029a | 0.74 |
| At 100 ms post–ground impact | 51.36 ± 5.29 (53.74, 48.98) | 58.66 ± 7.66 (62.10, 55.22) | −3.42 | .002a | 0.96 |
Difference in knee sagittal-plane kinematics after landing (P < .05).
Figure 4.
Effect sizes and associated 95% confidence intervals for postlanding integrated electromyography of vastus medialis oblique (VMO), vastus lateralis (VL), medial hamstrings (MH), and lateral hamstrings (LH) as well as knee sagittal-plane kinematics. Abbreviations: CAI, chronic ankle instability; IC, initial contact.
DISCUSSION
The objective of our study was to examine the influence of CAI on muscle activation and sagittal-plane kinematics at the knee joint during a functional task. The most important finding was that the CAI group exhibited greater preparatory VMO activity and reduced knee sagittal-plane kinematics after landing during the stop-jump task compared with the control group. It has been thought that the presence of CAI influences sensorimotor control at the supraspinal level.40 The proximal joint alterations we observed may be caused by centrally mediated changes to sensorimotor control. Whereas other investigators6–14 have examined knee kinematics and muscle-activation patterns of the rectus femoris and various ankle muscles during the preparatory prelanding phase and after ground impact with a variety of jump-landing tasks, our results support their reports that preprogrammed, feed-forward mechanisms of motor control may be modified in participants with CAI. Therefore, our findings of increased IEMG of the VMO before ground impact and decreased knee-flexion angle after landing during a stop-jump task may contribute to the understanding of the sensorimotor alterations associated with CAI. It is important for future authors to determine whether rehabilitation targeting neuromuscular control around the knee in addition to the ankle can promote global coordination in patients with CAI and decrease the incidence of giving way and reinjury.
Feed-forward motor control involves anticipation and pre-event decisions to plan movements based on previous experience with the tasks. McKinley and Pedotti30 suggested that prelanding muscle activities may be responsible for control of the early deceleration phase associated with landing to maintain balance and minimize impact forces. We observed an increase in prelanding IEMG of the VMO in the CAI group but no differences in postlanding IEMG activity of all muscles between CAI and control groups. The increased prelanding VMO activity in the CAI group may be a manifestation of a modified feed-forward mechanism to prepare for joint loading after ground impact.
The amount of quadriceps activation is a major factor controlling knee-flexion angle and dissipating the external loading experienced during the descent phase of the jump-landing task to prevent the knee from collapsing in the sagittal plane.41,42 We found no differences in postlanding quadriceps muscle activation between the CAI and control group; however, the CAI group demonstrated a decreased knee-flexion angle after landing during the stop-jump task compared with the control group. This may indicate that greater quadriceps activation after landing was not necessary to control sagittal-plane kinematics at the knee joint because participants with CAI landed with the knee in a more extended position. Increased knee flexion during landing requires greater activation from the quadriceps owing to the greater external torque on the knee.41 If external knee flexion was not countered by the quadriceps that provide the internal knee-extension moment, the lower extremity would be prone to collapse during landing.43 However, with an extended knee at landing, the quadriceps muscles are in a less advantageous position to dissipate the external loading experienced after ground impact. These postlanding knee kinematics and quadriceps-activation patterns demonstrated by CAI participants could influence energy-dissipation capability around the knee and perhaps alter the kinetic chain relationship. Less energy-dissipation capability around the knee has been observed in individuals with CAI during a stop-jump task.15 Therefore, it is possible that the increased VMO activation before ground impact results from central changes in sensorimotor control aimed at anticipating impact on the landing as preparation for dissipating the external loading upon ground impact.
However, if one assumes that the increase in preparatory VMO activation may be protective in nature, it still may not provide enough sensorimotor adaptation to influence the ankle joint, given that participants with CAI have exhibited decreases in knee-flexion angle after landing.6,7 The extended knee, which presents with less potential energy-attenuation capability, may result in placing greater demands on the ankle joint to store elastic energy as preparation for generating force for the final double-leg vertical jump immediately following the landing. Greater energy-dissipation capability around the ankle has been observed in individuals with CAI during a stop-jump task.15 Placing greater demands on the ankle joint to control energy may lead to rapid fatigability of the muscles around the ankle. These findings of altered knee biomechanics after ground impact suggest that the presence of CAI may alter the distal-to-proximal linkage that provides an efficient and effective system to transfer force up the kinetic chain. Altered kinetic chain relationships in the lower extremity during landing tasks may be an important factor in the recurrent nature of CAI. With only retrospective relationship data available to date, clearly there is a need for prospective investigations to verify these speculations.
We did not observe differences in prelanding IEMG of the VL or sagittal-plane kinematics between the CAI and control groups, which does not support our hypotheses. However, the moderate effect size for knee sagittal-plane kinematics suggests that knee flexion may be reduced before initial foot contact with the ground in the CAI group compared with the control group. The increase in prelanding IEMG of the VMO without differences in prelanding IEMG of VL could be explained plausibly by its role as a medial stabilizer of the patella. During closed kinetic chain activities, such as landing, increased external rotation of the knee can occur as a combination of increased internal femoral rotation accelerated by LH contraction and external tibial rotation.41 This position could lead to the relative lateral displacement of the patella.41 The mechanism of ankle sprain is rear-foot inversion and internal rotation coupled with excessive external rotation of the lower leg.44–46 It has been shown that CAI is associated with altered knee transverse-plane kinematics8 and an altered joint-coupling relationship between the lower leg rotation and the rear-foot frontal-plane kinematics.47 We also found that differences in prelanding IEMG of the LH between the CAI and control groups trended toward statistical significance, with an associated moderate effect size. This indicates that prelanding LH activity was likely dampened less in the CAI group than in the control group. Therefore, increased prelanding VMO activation (but not VL activation), coupled with potentially decreased LH activation, may be an attempt to provide medial stability to the patella from excessive tibial external rotation as preparation by the feed-forward system for upcoming events after landing. However, the findings should be interpreted with caution because we are unaware of any studies in which the investigators assessed EMG variables of the VMO and LH with knee transverse-plane kinematics or a joint-coupling relationship in individuals with CAI during jump-landing tasks. Future researchers should incorporate lower extremity joint coupling and transverse-plane kinematic variables with EMG assessments to fully illustrate the potential relationship between the prelanding muscle activation and lower extremity movement coordination.
Limitations
Post hoc power analyses showed that all of our nonsignificant findings were associated with low to moderate statistical power (observed powers = 0.07 to 0.57), increasing the risk of a type II error. However, the effect sizes reported were low for most nonsignificant group differences with associated 95% CIs crossing zero, indicating that these differences may not be clinically significant. Some of the nonsignificant differences were associated with moderate effect sizes, indicating that these relationships are likely associated with a moderate clinical difference. In cases of moderate effect sizes with a 95% CI that crossed zero, these relationships may be associated with statistical error and could perhaps be strengthened with an expanded sample size.
The presence of CAI appears to influence a feed-forward control in the sensorimotor system, manifesting as increased VMO activation before landing and reduced knee-flexion angle after landing. We speculate that the demonstrated VMO activation level is perhaps an effort by the sensorimotor system to protect the extremity by preparing for upcoming events. However, the retrospective design does not permit us to establish a causal link between CAI and the identified alterations in neuromuscular control. Therefore, it remains unknown whether the altered feed-forward sensorimotor mechanisms we observed are helpful to protect the ankle or predispose individuals with CAI to their self-reported injuries. Clearly, prospective studies are needed to fully address these questions. It may also be interesting in the future to explore long-term sensorimotor consequences after an initial lateral ankle sprain.
Chronic ankle instability is associated with the development of joint somatosensory deficits after LAS,48,49 which would be implicated in a loss of feedback efficiency. Although alterations in knee sagittal-plane kinematics after landing could be explained by the centrally mediated changes in sensorimotor control, some researchers50,51 have argued that proximal joint alterations in neuromuscular control associated with CAI may be occurring through feedback sensorimotor mechanisms. With this experimental procedure, it is difficult to examine whether the reductions in knee sagittal-plane kinematics after landing resulted from altered feedback sensorimotor control in the CAI group in this study, because it is likely a mixed response of preprogrammed and reactive neuromuscular control. Therefore, a separate examination of these 2 mechanisms is needed to determine whether feedback sensorimotor mechanisms related to the CAI influence proximal joint neuromuscular control during a jump-landing task.
Finally, in our current investigation we reported our EMG measures only during a period from 100 ms prelanding to 100 ms postlanding. We selected a time point that would capture information to determine whether differences in preparatory or reactive muscle-activation pattern during the tasks would be observed between our CAI and healthy control participants. Altered kinematic patterns during a similar time frame of the preparatory phase have been reported by previous investigators.6,10 The time interval of the postlanding phase was used to quantify the response of the quadriceps and hamstrings muscles after ground contact because it has been demonstrated that an LAS occurs quickly after the foot makes contact with the ground.45 Although reporting only our selected time points may be a limitation of the study, we are unaware whether extending the time frame to 200 ms or more on either side of the landing event would change our findings. Therefore, further quantification of muscle-activation patterns during additional time periods of the prelanding and postlanding phases of the task is needed.
Clinical Implications
The information from this study may help clinicians and researchers develop a better understanding of CAI and how the sensorimotor system prepares for a predicted event during dynamic activity. Our findings confirm previous reports that CAI is associated with altered knee sagittal-plane kinematics after ground impact and provides additional data that CAI may influence prelanding VMO activation.
Although speculative for now, this may be an illustration of central nervous system changes in the CAI patients we observed; however, we do not yet know if this is a positive or negative adaptation. Our results suggest the need to consider the influence of CAI on global coordination during functional tasks. Therefore, it is important for clinicians to (1) treat CAI as a global and not just a local injury; (2) evaluate the contributions of movement coordination at the knee to functional performance in individuals with CAI; and (3) address feed-forward sensorimotor control around the knee during ankle rehabilitation.
Additionally, our data support the suggestion that the LH was possibly less active during the stop-jump task in the CAI group compared with the control group. Therefore, the postlanding muscle response in these selected muscles may be less in the CAI group than in the control group; however, this relationship needs further exploration. These findings may have important clinical implications regarding an association between CAI and future proximal joint injury because (1) less muscular effort after ground impact increases the compressive impact force at the knee joint and stresses the capsuloligamentous structures; and (2) reduced LH activity coupled with a reduced knee-flexion angle might be associated with an increased risk for anterior cruciate ligament injury.
CONCLUSIONS
We demonstrated an increase in preparatory VMO activation in the CAI group compared with the control group during a vertical stop jump. This observation of a potential reorganized feed-forward mechanism may provide additional insight into sensorimotor adaptations in the CAI population. We also found decreases in knee-flexion angle after initial ground contact with the force plate in participants with CAI compared with the control group, supporting the idea that centrally mediated changes to sensorimotor control may exist. Within the scope of our results, it is still unclear whether the observed preparatory VMO activation and postlanding knee sagittal-plane kinematics in the CAI group increase the risk for resprains or protect the ankle joint. Thus, this question should be addressed in a future prospective investigation. Last, data on additional biomechanical and EMG variables are needed to determine whether the relationships among these variables provide insight regarding ankle-injury mechanisms.
ACKNOWLEDGMENTS
This study was supported by the National Athletic Trainers' Association Research and Education Foundation (Dallas, TX) through its Osternig Master's Grant Program. We thank Dr Charles Armstrong and Dr Naoko Aminaka for their contribution to the electromyography analysis.
REFERENCES
- 1.Anandacoomarasamy A, Barnsley L. Long term outcomes of inversion ankle injuries. Br J Sports Med. 2005;39(3):e14. doi: 10.1136/bjsm.2004.011676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Konradsen L, Bech L, Ehrenbjerg M, Nickelsen T. Seven years follow-up after ankle inversion trauma. Scand J Med Sci Sports. 2002;12(3):129–135. doi: 10.1034/j.1600-0838.2002.02104.x. [DOI] [PubMed] [Google Scholar]
- 3.Yeung MS, Chan KM, So CH, Yuan WY. An epidemiological survey on ankle sprain. Br J Sports Med. 1994;28(2):112–116. doi: 10.1136/bjsm.28.2.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arnold BL, Wright CJ, Ross SE. Functional ankle instability and health-related quality of life. J Athl Train. 2011;46(6):634–641. doi: 10.4085/1062-6050-46.6.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Valderrabano V, Hintermann B, Horisberger M, Fung TS. Ligamentous posttraumatic ankle osteoarthritis. Am J Sports Med. 2006;34(4):612–620. doi: 10.1177/0363546505281813. [DOI] [PubMed] [Google Scholar]
- 6.Gribble P, Robinson R. Differences in spatiotemporal landing variables during a dynamic stability task in subjects with CAI. Scand J Med Sci Sports. 2010;20(1):e63–e71. doi: 10.1111/j.1600-0838.2009.00899.x. [DOI] [PubMed] [Google Scholar]
- 7.Gribble PA, Robinson RH. Alterations in knee kinematics and dynamic stability associated with chronic ankle instability. J Athl Train. 2009;44(4):350–355. doi: 10.4085/1062-6050-44.4.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brown C, Bowser B, Simpson KJ. Movement variability during single leg jump landings in individuals with and without chronic ankle instability. Clin Biomech (Bristol, Avon) 2012;27(1):52–63. doi: 10.1016/j.clinbiomech.2011.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Caulfield B, Crammond T, O'Sullivan A, Reynolds S, Ward T. Altered ankle-muscle activation during jump landing in participants with functional instability of the ankle joint. J Sport Rehabil. 2004;13(3):189–200. [Google Scholar]
- 10.Caulfield BM, Garrett M. Functional instability of the ankle: differences in patterns of ankle and knee movement prior to and post landing in a single leg jump. Int J Sports Med. 2002;23(1):64–68. doi: 10.1055/s-2002-19272. [DOI] [PubMed] [Google Scholar]
- 11.Delahunt E, Monaghan K, Caulfield B. Ankle function during hopping in subjects with functional instability of the ankle joint. Scand J Med Sci Sports. 2007;17(6):641–648. doi: 10.1111/j.1600-0838.2006.00612.x. [DOI] [PubMed] [Google Scholar]
- 12.Gutierrez GM, Knight CA, Swanik CB, et al. Examining neuromuscular control during landings on a supinating platform in persons with and without ankle instability. Am J Sports Med. 2012;40(1):193–201. doi: 10.1177/0363546511422323. [DOI] [PubMed] [Google Scholar]
- 13.Lin CF, Chen CY, Lin CW. Dynamic ankle control in athletes with ankle instability during sports maneuvers. Am J Sports Med. 2011;39(9):2007–2015. doi: 10.1177/0363546511406868. [DOI] [PubMed] [Google Scholar]
- 14.Delahunt E, Monaghan K, Caulfield B. Changes in lower limb kinematics, kinetics, and muscle activity in subjects with functional instability of the ankle joint during a single leg drop jump. J Orthop Res. 2006;24(10):1991–2000. doi: 10.1002/jor.20235. [DOI] [PubMed] [Google Scholar]
- 15.Terada M, Pfile KR, Pietrosimone BG, Gribble PA. Effects of chronic ankle instability on energy dissipation in the lower extremity. J Athl Train. 2012;47((suppl 3)):S56. doi: 10.1249/MSS.0b013e31829a3d0b. [DOI] [PubMed] [Google Scholar]
- 16.Backman LJ, Danielson P. Low range of ankle dorsiflexion predisposes for patellar tendinopathy in junior elite basketball players: a 1-year prospective study. Am J Sports Med. 2011;39(12):2626–2633. doi: 10.1177/0363546511420552. [DOI] [PubMed] [Google Scholar]
- 17.Kramer LC, Denegar CR, Buckley WE, Hertel J. Factors associated with anterior cruciate ligament injury: history in female athletes. J Sports Med Phys Fitness. 2007;47(4):446–454. [PubMed] [Google Scholar]
- 18.Nadler SF, Wu KD, Galski T, Feinberg JH. Low back pain in college athletes. A prospective study correlating lower extremity overuse or acquired ligamentous laxity with low back pain. Spine (Phila Pa 1976) 1998;23(7):828–833. doi: 10.1097/00007632-199804010-00018. [DOI] [PubMed] [Google Scholar]
- 19.Terada M, Pietrosimone BP, Armstrong CW, Gribble PA. Examining biomechanical factors related to anterior cruciate ligament injury in individuals with chronic ankle instability. Med Sci Sports Exerc. 2012;44((suppl 5)):S88. [Google Scholar]
- 20.US Department of Health and Human Services. Healthy People 2010. Washington, DC: US Dept of Health and Human Services;; 2000. [Google Scholar]
- 21.Docherty CL, Gansneder BM, Arnold BL, Hurwitz SR. Development and reliability of the ankle instability instrument. J Athl Train. 2006;41(2):154–158. [PMC free article] [PubMed] [Google Scholar]
- 22.Hale SA, Hertel J. Reliability and sensitivity of the Foot and Ankle Disability Index in subjects with chronic ankle instability. J Athl Train. 2005;40(1):35–40. [PMC free article] [PubMed] [Google Scholar]
- 23.McKeon PO, Hertel J. Spatiotemporal postural control deficits are present in those with chronic ankle instability. BMC Musculoskelet Disord. 2008;9:76. doi: 10.1186/1471-2474-9-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grindstaff TL, Beazell JR, Sauer LD, Magrum EM, Ingersoll CD, Hertel J. Immediate effects of a tibiofibular joint manipulation on lower extremity H-reflex measurements in individuals with chronic ankle instability. J Electromyogr Kinesiol. 2011;21(4):652–658. doi: 10.1016/j.jelekin.2011.03.011. [DOI] [PubMed] [Google Scholar]
- 25.Shaw MY, Gribble PA, Frye JL. Ankle bracing, fatigue, and time to stabilization in collegiate volleyball athletes. J Athl Train. 2008;43(2):164–171. doi: 10.4085/1062-6050-43.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cowan SM, Bennell KL, Hodges PW, Crossley KM, McConnell J. Delayed onset of electromyographic activity of vastus medialis obliquus relative to vastus lateralis in subjects with patellofemoral pain syndrome. Arch Phys Med Rehabil. 2001;82(2):183–189. doi: 10.1053/apmr.2001.19022. [DOI] [PubMed] [Google Scholar]
- 27.De Luca CJ. The use of surface electromyography in biomechanics. J Appl Biomech. 1997;13(2):135–163. [Google Scholar]
- 28.Lynn SK, Costigan PA. Effect of foot rotation on knee kinetics and hamstring activation in older adults with and without signs of knee osteoarthritis. Clin Biomech (Bristol, Avon) 2008;23(6):779–786. doi: 10.1016/j.clinbiomech.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 29.Chappell JD, Creighton RA, Giuliani C, Yu B, Garrett WE. Kinematics and electromyography of landing preparation in vertical stop-jump: risks for noncontact anterior cruciate ligament injury. Am J Sports Med. 2007;35(2):235–241. doi: 10.1177/0363546506294077. [DOI] [PubMed] [Google Scholar]
- 30.McKinley P, Pedotti A. Motor strategies in landing from a jump: the role of skill in task execution. Exp Brain Res. 1992;90(2):427–440. doi: 10.1007/BF00227257. [DOI] [PubMed] [Google Scholar]
- 31.Merletti R, Torino P. Standards for reporting EMG data. J Electromyogr Kinesiol. 1999;9(1):iii–iv. [Google Scholar]
- 32.Kipp K, Palmieri-Smith RM. Principal component based analysis of biomechanical inter-trial variability in individuals with chronic ankle instability. Clin Biomech (Bristol, Avon) 2012;27(7):706–710. doi: 10.1016/j.clinbiomech.2012.02.005. [DOI] [PubMed] [Google Scholar]
- 33.Fu SN, Hui-Chan CW. Modulation of prelanding lower-limb muscle responses in athletes with multiple ankle sprains. Med Sci Sports Exerc. 2007;39(10):1774–1783. doi: 10.1249/mss.0b013e3181343629. [DOI] [PubMed] [Google Scholar]
- 34.Yang JF, Winter DA. Electromyographic amplitude normalization methods: improving their sensitivity as diagnostic tools in gait analysis. Arch Phys Med Rehabil. 1984;65(9):517–521. [PubMed] [Google Scholar]
- 35.Winter DA. Biomechanics and Motor Control of Human Movement. 2nd ed. New York, NY: Wiley Inter-Science;; 1990. pp. 34–52. [Google Scholar]
- 36.Davis RB, Ounpuu S, Tyburski D, Gage JR. A gait analysis data-collection and reduction technique. Hum Mov Sci. 1991;10(5):575–587. [Google Scholar]
- 37.Grood ES, Suntay WJ. A joint coordinate system for the clinical description of three-dimensional motions: application to the knee. J Biomech Eng. 1983;105(2):136–144. doi: 10.1115/1.3138397. [DOI] [PubMed] [Google Scholar]
- 38.Rosnow RL, Rosenthal R, Rubin DB. Contrasts and correlations in effect-size estimation. Psychol Sci. 2000;11(6):446–453. doi: 10.1111/1467-9280.00287. [DOI] [PubMed] [Google Scholar]
- 39.Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2nd ed. Hillsdale, NJ: Erlbaum Associates;; 1988. [Google Scholar]
- 40.Hass CJ, Bishop MD, Doidge D, Wikstrom EA. Chronic ankle instability alters central organization of movement. Am J Sports Med. 2010;38(4):829–834. doi: 10.1177/0363546509351562. [DOI] [PubMed] [Google Scholar]
- 41.Neumann DA. Kinesiology of the Musculoskeletal System: Foundations for Rehabilitation. 2nd ed. St Louis, MO: Mosby/Elsevier;; 2010. [Google Scholar]
- 42.Palmieri-Smith RM, Thomas AC. A neuromuscular mechanism of posttraumatic osteoarthritis associated with ACL injury. Exerc Sport Sci Rev. 2009;37(3):147–153. doi: 10.1097/JES.0b013e3181aa6669. [DOI] [PubMed] [Google Scholar]
- 43.Blackburn JT, Padua DA. Sagittal-plane trunk position, landing forces, and quadriceps electromyographic activity. J Athl Train. 2009;44(2):174–179. doi: 10.4085/1062-6050-44.2.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kristianslund E, Bahr R, Krosshaug T. Kinematics and kinetics of an accidental lateral ankle sprain. J Biomech. 2011;44(14):2576–2578. doi: 10.1016/j.jbiomech.2011.07.014. [DOI] [PubMed] [Google Scholar]
- 45.Mok KM, Fong DT, Krosshaug T, et al. Kinematics analysis of ankle inversion ligamentous sprain injuries in sports: 2 cases during the 2008 Beijing Olympics. Am J Sports Med. 2011;39(7):1548–1552. doi: 10.1177/0363546511399384. [DOI] [PubMed] [Google Scholar]
- 46.Fong DT, Ha SC, Mok KM, Chan CW, Chan KM. Kinematics analysis of ankle inversion ligamentous sprain injuries in sports: five cases from televised tennis competitions. Am J Sports Med. 2012;40(11):2627–2632. doi: 10.1177/0363546512458259. [DOI] [PubMed] [Google Scholar]
- 47.Drewes LK, McKeon PO, Paolini G, et al. Altered ankle kinematics and shank-rear-foot coupling in those with chronic ankle instability. J Sport Rehabil. 2009;18(3):375–388. doi: 10.1123/jsr.18.3.375. [DOI] [PubMed] [Google Scholar]
- 48.Hertel J. Functional anatomy, pathomechanics, and pathophysiology of lateral ankle instability. J Athl Train. 2002;37(4):364–375. [PMC free article] [PubMed] [Google Scholar]
- 49.Willems T, Witvrouw E, Verstuyft J, Vaes P, De Clercq D. Proprioception and muscle strength in subjects with a history of ankle sprains and chronic instability. J Athl Train. 2002;37(4):487–493. [PMC free article] [PubMed] [Google Scholar]
- 50.Bullock-Saxton JE, Janda V, Bullock MI. The influence of ankle sprain injury on muscle activation during hip extension. Int J Sports Med. 1994;15(6):330–334. doi: 10.1055/s-2007-1021069. [DOI] [PubMed] [Google Scholar]
- 51.Van Deun S, Staes FF, Stappaerts KH, Janssens L, Levin O, Peers KK. Relationship of chronic ankle instability to muscle activation patterns during the transition from double-leg to single-leg stance. Am J Sports Med. 2007;35(2):274–281. doi: 10.1177/0363546506294470. [DOI] [PubMed] [Google Scholar]