Abstract
Rationale and Objectives
To test the ability of quantitative measures from preoperative Dynamic Contrast Enhanced MRI (DCE-MRI) to predict, independently and/or with the Katz pathologic nomogram, which breast cancer patients with a positive sentinel lymph node biopsy will have ≥ 4 positive axillary lymph nodes upon completion axillary dissection.
Methods and Materials
A retrospective review was conducted to identify clinically node-negative invasive breast cancer patients who underwent preoperative DCE-MRI, followed by sentinel node biopsy with positive findings and complete axillary dissection (6/2005 – 1/2010). Clinical/pathologic factors, primary lesion size and quantitative DCE-MRI kinetics were collected from clinical records and prospective databases. DCE-MRI parameters with univariate significance (p < 0.05) to predict ≥ 4 positive axillary nodes were modeled with stepwise regression and compared to the Katz nomogram alone and to a combined MRI-Katz nomogram model.
Results
Ninety-eight patients with 99 positive sentinel biopsies met study criteria. Stepwise regression identified DCE-MRI total persistent enhancement and volume adjusted peak enhancement as significant predictors of ≥4 metastatic nodes. Receiver operating characteristic (ROC) curves demonstrated an area under the curve (AUC) of 0.78 for the Katz nomogram, 0.79 for the DCE-MRI multivariate model, and 0.87 for the combined MRI-Katz model. The combined model was significantly more predictive than the Katz nomogram alone (p = 0.003).
Conclusion
Integration of DCE-MRI primary lesion kinetics significantly improved the Katz pathologic nomogram accuracy to predict presence of metastases in ≥ 4 nodes. DCE-MRI may help identify sentinel node positive patients requiring further localregional therapy.
Keywords: breast cancerc, radiation, MRI, sentinel lymph node, axilla
INTRODUCTION
For breast cancer patients without clinical axillary lymph node involvement, sentinel lymph node biopsy has become the standard of care for assessing local-regional extent of disease. It is well agreed upon that patients with negative sentinel sampling need no further axillary surgery (1). However, which patients may avoid completion axillary dissection after positive sentinel sampling is an evolving consideration, especially in the setting of adjuvant radiotherapy. Further, optimal design of radiotherapy fields with regard to regional lymphatic coverage is not known.
Very low rates of local-regional recurrence in select sentinel node positive patients who forego axillary dissection in the setting of planned radiotherapy supports a movement away from completion axillary dissection in low risk patients. However, low risk criteria have not been precisely defined (2–7). The rationale behind the increasing tendency to avoid additional surgery is clear – completion axillary dissection carries a 10 to 40% risk of lymphedema (8–10), and excellent local control of the axilla has been demonstrated in previous studies with radiation therapy alone (7, 11).
In our current era of practice, patients are increasingly foregoing completion axillary dissection after a positive sentinel lymph node biopsy. The presence of four or more total positive axillary lymph nodes has conferred a higher risk of regional nodal relapse thus warranting adjuvant radiotherapy to the high axillary and supraclavicular lymph nodes (12). However, without complete pathologic staging information from axillary dissection, the identification of individuals who would benefit from high axilla/supraclavicalular lymph node irradiation is less clear. Presently physicians assess risk of occult disease in the high axilla (and therefore target this area with radiotherapy) using clinical-pathologic models such as the Katz nomogram (13). This nomogram is a validated tool to identify patients with a high likelihood of harboring metastatic disease in four or more axillary lymph nodes following a positive sentinel lymph node biopsy. The Katz nomogram incorporates tumor histology, primary tumor size, lymphovascular space invasion, extranodal extension, the number of involved sentinel nodes, the number of uninvolved sentinel nodes, and the size of the largest sentinel node metastasis.
Based on findings that Dynamic Contrast Enhanced MRI (DCE-MRI) kinetics of primary breast cancers are correlated with axillary lymph node status (14–16), we hypothesized that DCE-MRI will improve our ability to predict the presence of extensive additional axillary disease after a positive sentinel lymph node biopsy and add predictive value to the Katz nomogram. To our knowledge, no published studies have investigated the approach of combining DCE-MRI tumor kinetics features with the Katz nomogram to improve prediction of axillary node involvement. We tested our hypothesis in a retrospective analysis of patients at our center who underwent DCE-MRI, positive sentinel lymph node biopsy, and completion axillary lymph node dissection.
MATERIALS AND METHODS
Following institutional review board approval, a retrospective review was performed of consecutive breast cancer patients with clinically negative axillae who underwent sentinel lymph node biopsy at the University of Washington June 2005 through January 2010. Subjects were identified through the University of Washington Sentinel Lymph Node Registry, a prospective research database. Patients with biopsy-proven invasive breast cancer who had a positive sentinel node biopsy and completion axillary dissection were eligible for inclusion in this study. Patients were excluded if they received preoperative systemic therapy (chemotherapy, hormonal therapy, or therapy with biologically targeted agents) because of known effects on lesion enhancement (17, 18) or did not undergo completion axillary dissection after the positive sentinel node biopsy. All patients were at least 18 years of age and female.
Patients who underwent preoperative/pre-sentinel node DCE-MRI at our institution were then identified through cross-reference with the Consortium Oncology Data Integration (CODI) project database, a solid-tumor clinical research database developed and maintained by the Fred Hutchinson Cancer Research Center in collaboration with the University of Washington. DCE-MRI data for study cases were extracted from the CODI database. Clinical and pathologic features of each case were extracted from the medical record and pathology database.
Pathology
For patients undergoing treatment during the study period, sentinel lymph nodes were routinely sectioned at 2mm intervals and entirely submitted for histologic examination. Three hematoxylin and eosin (H&E) stained levels were performed and examined on each sentinel lymph node tissue block.
Immunohistochemistry with pan-cytokeratin antibodies was performed only in cases with no histologic evidence of metastatic disease on H&E in patients with primary invasive lobular carcinoma.
DCE-MRI Acquisition
All MR examinations were performed on a 1.5T LX scanner (GE Healthcare, Waukesha, WI) using a dedicated breast coil between June 2005 and December 2009 as previously described (19–22). Scanning protocols follow guidelines established by the American College of Radiology breast MRI accreditation program (23). Imaging sequences included one pre- and at least two post-contrast T1- weighted 3D fast spoiled gradient recalled series. Prior to October 2005, scans were performed in the sagittal plane with TR/TE, 6.7/4.2 ms; flip angle 10°; field of view (FOV) 18–22 cm; slice thickness 3 mm; and matrix, 256 × 192. From October 2005 through June 2006, scans were performed in the axial plane with TR/TE, 6.2/3 ms; flip angle 10°; FOV 32–38 cm; slice thickness 2.2 mm; an d matrix, 350 × 350. Scan time was 90 seconds per acquisition. One pre-contrast and five post-contrast acquisitions centered at 90, 180, 270, 360 and 450 seconds after injection were obtained. From July 2006, scans were performed in the axial plane with TR/TE, 5.5/2.7 ms; flip angle 10°; FOV 32–38 cm; slice thickness 1.6 mm; and matrix, 420 × 420. Scan time was 180 seconds per acquisition. One pre-contrast and three post-contrast sequences (centered at 90, 270, and 450 seconds after injection) were obtained. All studies were interpreted by a radiologist fellowship trained in breast imaging.
DCE-MRI Data Analysis
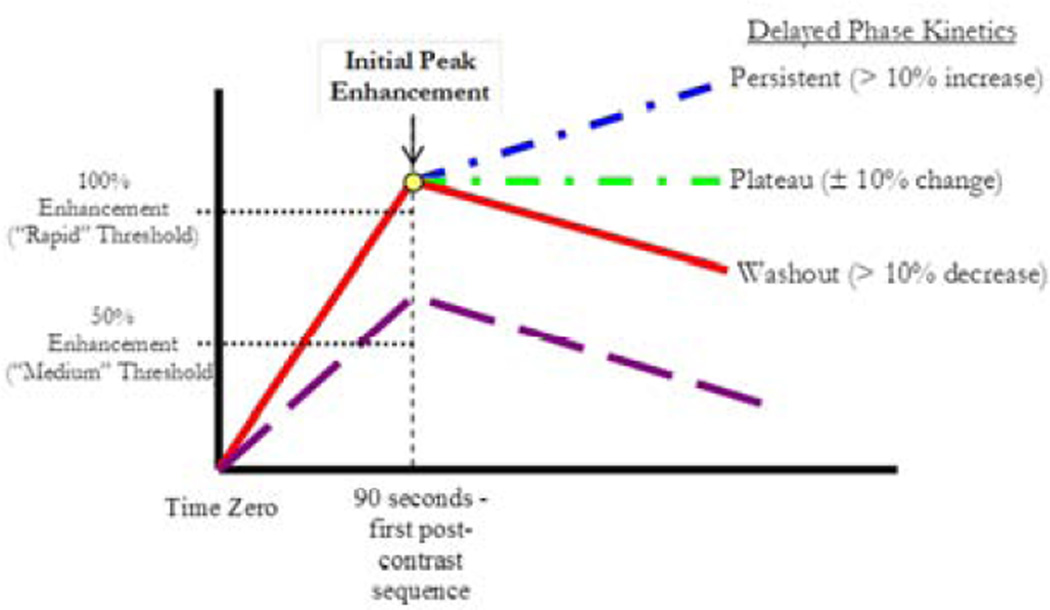
DCE-MRI kinetic parameters were prospectively measured for the primary tumor in each patient at the time of MRI interpretation using a computer aided evaluation (CAE) program (CADstream, Confirma, Bellevue, WA). The CAE program compares pixel signal intensity values on the pre-contrast and initial postcontrast scans (centered at 90 seconds). For pixels meeting the initial enhancement threshold, early and delayed-phase enhancement characteristics are calculated as described below. Contiguous areas of enhancement are summed and constitute a lesion, and a detailed synopsis of the initial and delayed kinetic enhancement is automatically generated. For any patient with multiple lesions on MRI, the analysis was performed on the largest one. Figure 1 demonstrates characterization of the primary lesion enhancement kinetic curve.
Figure 1.
DCE-MRI early and delayed enhancement profiles were characterized for each primary cancer. Early Phase: The change in signal intensity immediately after contrast injection. Maximal single voxel enhancement within the primary breast cancer centered at 90 seconds is recorded as initial peak enhancement. Percentages of voxels enhancing greater than 50% and 100% over baseline are respectively designated as percent medium and percent rapid enhancement. Delayed Phase: Enhancement in subsequent post-contrast series was stratified as persistent if enhancement increased by more than 10%, washout if enhancement decreased by more than 10% and otherwise as plateau.
Early phase kinetics
For each primary cancer, kinetic values for initial peak enhancement (PE), percent medium enhancement (ME), and percent rapid enhancement (RE) were measured (Figure 1). PE was defined as the maximum enhancement over baseline measured in any single voxel of the primary lesion volume at the initial post-contrast sequence (90 seconds post-contrast injection). ME and RE are calculated as the percentage of voxels within the lesion meeting threshold criteria defined as enhancement greater than 50% over baseline and 100% over baseline, respectively.
Delayed phase kinetics
Changes in enhancement between the initial post-contrast series and the delayed post-contrast series were characterized as persistent, plateau, or washout (Figure 1). Voxel enhancement profiles were designated as persistent if signal intensity increased by more than 10%, plateau if intensity did not increase or decrease by more than 10% and washout if signal intensity decreased by more than 10%. Total percent persistent, total percent plateau, and total percent washout variables were calculated, respectively, by summation of persistent, washout, and plateau enhancement in rapid and medium enhancing voxels. For example, a given lesion might have 13% medium plateau enhancement and 8% rapid plateau enhancement for a total of 21% plateau enhancement.
Lesion dimensions in three cross-sectional planes (superior-inferior, anteriorposterior, and medial-lateral) were recorded by the radiologist at the time the diagnostic scan was performed. Lesion volume was approximated by calculating the volume of the equivalent sphere with radius one half of the average cross sectional measurement. The clinical radiology report for each DCE-MRI was reviewed and the radiologic interpretation of the ipsilateral axilla recorded.
Statistical Analysis
The probability for patients to have four or more positive axillary lymph nodes based on pathologic data (including primary tumor histology, tumor size, lymphovascular space invasion, extranodal extension, number of involved and uninvolved sentinel nodes, and the size of the largest sentinel node metastasis) was calculated for each case according to the nomogram described by Katz et al. (13). Univariate logistic regression was performed to assess the correlations of individual DCE-MRI kinetic parameters and lesion volume with presence of four or more total pathologically positive lymph nodes. In the analysis, we also tested for interaction effects between lesion volume and DCE-MRI kinetics variables by constructing and assessing interaction terms (product of the volume and each kinetics variable).. All variables with a trend toward univariate significance (p<0.05) were evaluated in a multivariate stepwise analysis to determine the most significant predictors of four or more total positive axillary lymph nodes.
For receiver operating characteristic (ROC) curve analysis, the Katz nomogram probability was transformed via the logit function to invert the logistic probability calculation and put the Katz results on an appropriate scale for comparison with the model developed using DCE-MRI parameters. ROC curves were calculated for the DCE-MRI model alone, the Katz nomogram alone, and for a model combining DCE-MRI parameters with the logit-transformed Katz nomogram probability. Logistic regression models were compared using a likelihood ratio test.
RESULTS
Ninety-nine positive sentinel lymph node biopsy cases in 98 patients met study criteria. Patient characteristics and pathology results are shown in Table 1. One patient presented with synchronous bilateral primary breast cancers and had bilateral positive sentinel lymph node biopsies with subsequent bilateral axillary dissections. Forty-four cases had additional disease upon completion dissection. Fifteen of the positive sentinel lymph nodes contained micrometastases only, of which 3 cases had additional positive axillary lymph nodes. In one case, the sentinel lymph node metastasis was identified by immunohistochemistry only – that patient had a negative completion axillary dissection. Twenty-eight cases had four or more total positive axillary lymph nodes. Ten cases had 10 or more total positive axillary lymph nodes.
Table 1.
Patient and breast cancer pathologic characteristics.
| Mean +/− standard deviation | Range | |
|---|---|---|
| Patient Age | 55.5 +/− 10.4 years | 31.5 to 75.4 years |
| Pathologic primary cancer size at time of lumpectomy | 3.2 +/− 2.1 cm | 0.0 to 10.0 cm |
| Number of positive sentinel lymph nodes | 1.7 +/− 1.3 nodes | 1 to 11 nodes |
| Number of positive lymph nodes at completion dissection in patients with positive completion dissections | 6.1 +/− 7.3 nodes | 1 to 29 nodes |
| Proportion | ||
| Lobular Histology | 33/99 = 33% | |
| Estrogen Receptor Positive | 94/99 = 95% | |
| Progesterone Receptor Positive | 87/99 = 88% | |
| Her-2 Positive | 8/99 = 8% | |
| Nottingham Grade 1 2 3 |
24/99 = 24% 50/99 = 51% 25/99 = 25% |
|
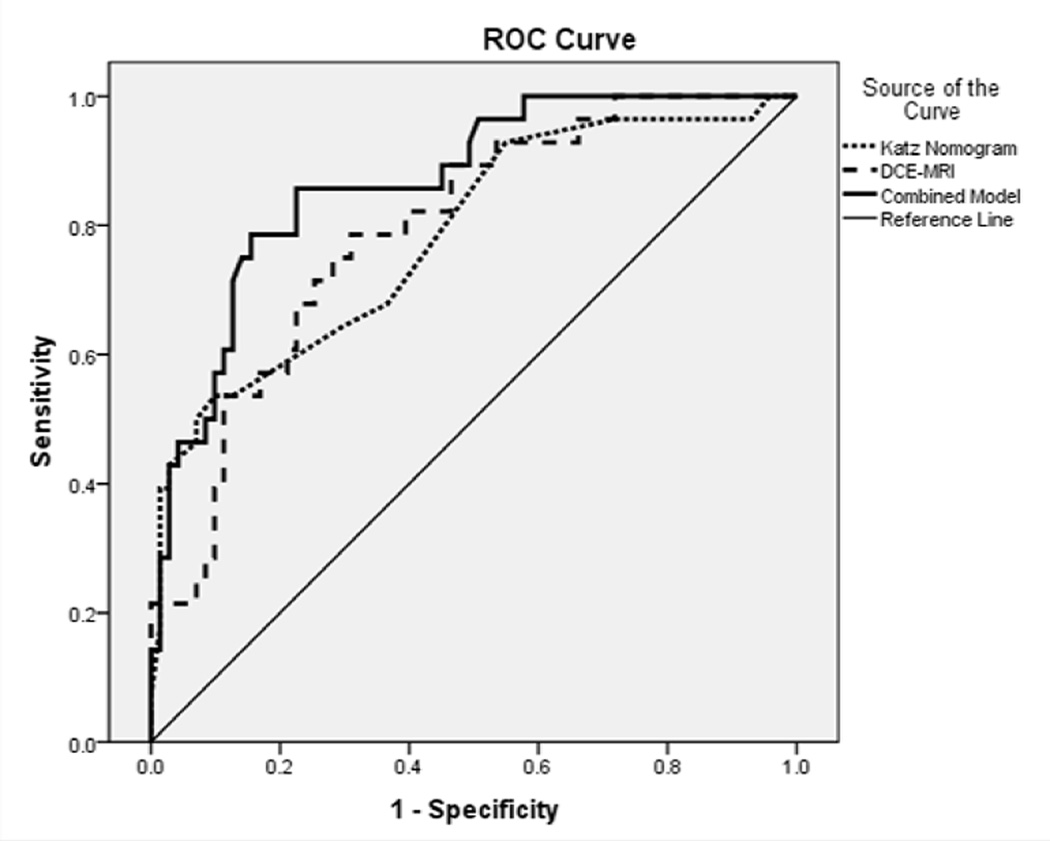
Applying the Katz nomogram to our study population yielded an ROC area under the curve (AUC) of 0.78. For DCE-MRI, significant univariate predictors (p<0.05) of four or more total axillary lymph nodes were lesion volume, the PE-volume interaction term (PE*volume), percent rapid washout, percent medium enhancement, percent medium persistent enhancement, total percent persistent enhancement, and total percent washout. Table 2 demonstrates univariate odds ratios and respective p-values for each tested parameter. Multivariate stepwise regression of the DCE-MRI parameters identified only PE*volume (OR=2.58, range 1.2–5.5, p=0.007) and total percent persistent enhancement (OR=1.55, range 1.2–1.9), p=0.0007) to hold significance, with an AUC of 0.79. The combined model incorporating the logit-transformed Katz probability, PE*volume, and total percent persistent enhancement demonstrated an AUC of 0.87. Figure 2 demonstrates the AUC curves for the Katz nomogram, DCE-MRI, and the combined model. The combined model was significantly more predictive than the Katz nomogram alone (p = 0.003).
Table 2.
Univariate analysis – correlations with presence of four or more positive axillary lymph nodes.
| Parameter | Metric | OR (95% CI) | p-value |
|---|---|---|---|
| Primary Lesion Volume | per log increase |
2.20 (1.1–4.2) | 0.009 |
| Initial Peak Enhancement | 2.12 (0.4–11.3) | 0.32 | |
| Volume*Peak | 2.02 (1.1–3.7) | 0.01 | |
| Percent Rapid Enhancement | per 10% increase |
0.87 (0.7–1.0) | 0.10 |
| Percent Rapid Persistent Enhancement | 1.24 (0.9–1.7) | 0.19 | |
| Percent Rapid Plateau Enhancement | 0.78 (0.5–1.1) | 0.19 | |
| Percent Rapid Washout | 0.66 (0.5–0.9) | 0.005 | |
| Percent Medium Enhancement | 1.21 (1.0–1.4) | 0.02 | |
| Percent Medium Persistent Enhancement | 1.39 (1.2–1.7) | 0.0003 | |
| Percent Medium Plateau Enhancement | 0.82 (0.5–1.3) | 0.35 | |
| Percent Medium Washout | 0.59 (0.3–1.2) | 0.09 | |
| Total Percent Persistent Enhancement | 1.49 (1.2–1.8) | <0.0001 | |
| Total Percent Plateau Enhancement | 0.77 (0.6–1.1) | 0.09 | |
| Total Percent Washout | 0.69 (0.5–0.9) | 0.002 |
Figure 2.
ROC curve depicting accuracy of Katz nomogram, DCE-MRI, and combination model to predict 4 or more total positive axillary nodes in patients with a positive sentinel lymph node and axillary dissection. Area under the curve for Katz Nomogram = 0.78, DCE-MRI = 0.79, and Combined Model = 0.87 (p = 0.003).
The ipsilateral axilla was commented upon as part of the breast MRI exam by the interpreting radiologist in 88 of the 99 cases. In 11 cases, axillary abnormality concerning for malignancy was noted by the radiologist. In the remaining 77 cases, the radiologist clearly stated that ipsilateral axillary lymph nodes had a normal appearance on DCE-MRI. In nine of the 11 radiographically abnormal cases, patients had additional positive non-sentinel axillary nodes upon completion dissection. Total number of positive lymph nodes in these cases identified as abnormal by the radiologist averaged 9.3 – five cases had four or more involved lymph nodes. One patient noted to have a radiographically abnormal appearing ipsilateral axilla had 31 positive lymph nodes (Figure 3). Of the 77 cases with radiographically normal ipsilateral axillae, 32 had additional positive axillary nodes upon completion dissection. Of these 32 cases, 30 were macrometastases and two were micrometastasis. The average total number of positive axillary nodes in these radiographically occult cases was 7.7 – twenty cases had four or more lymph nodes and one patient had 32 total positive lymph nodes.
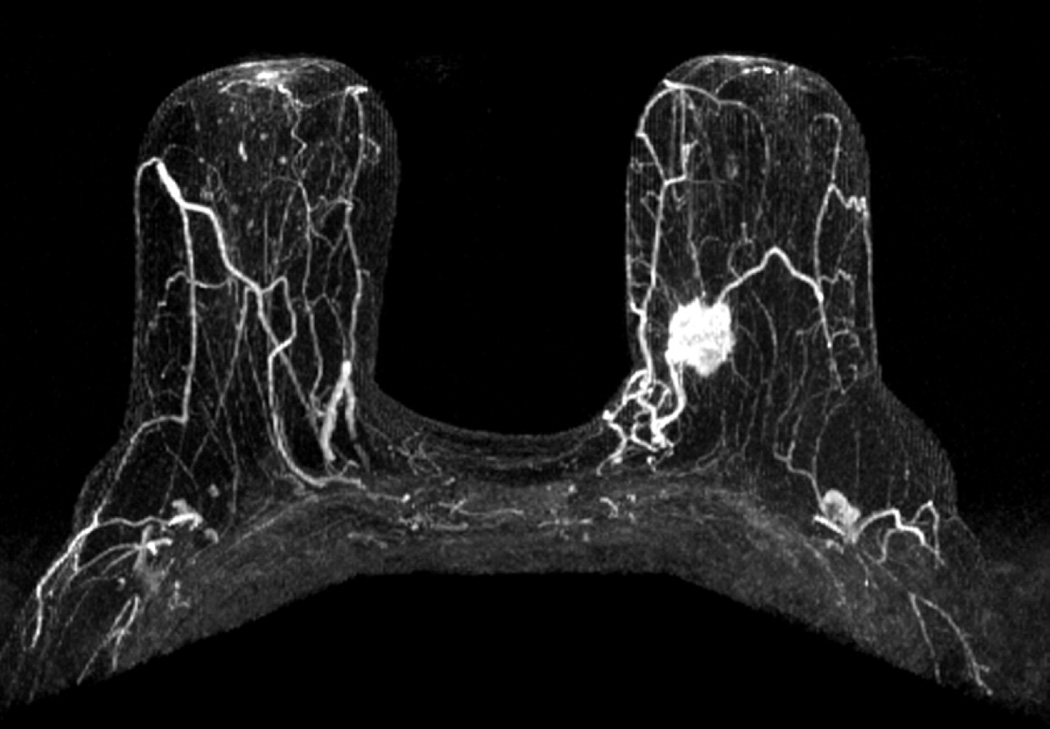
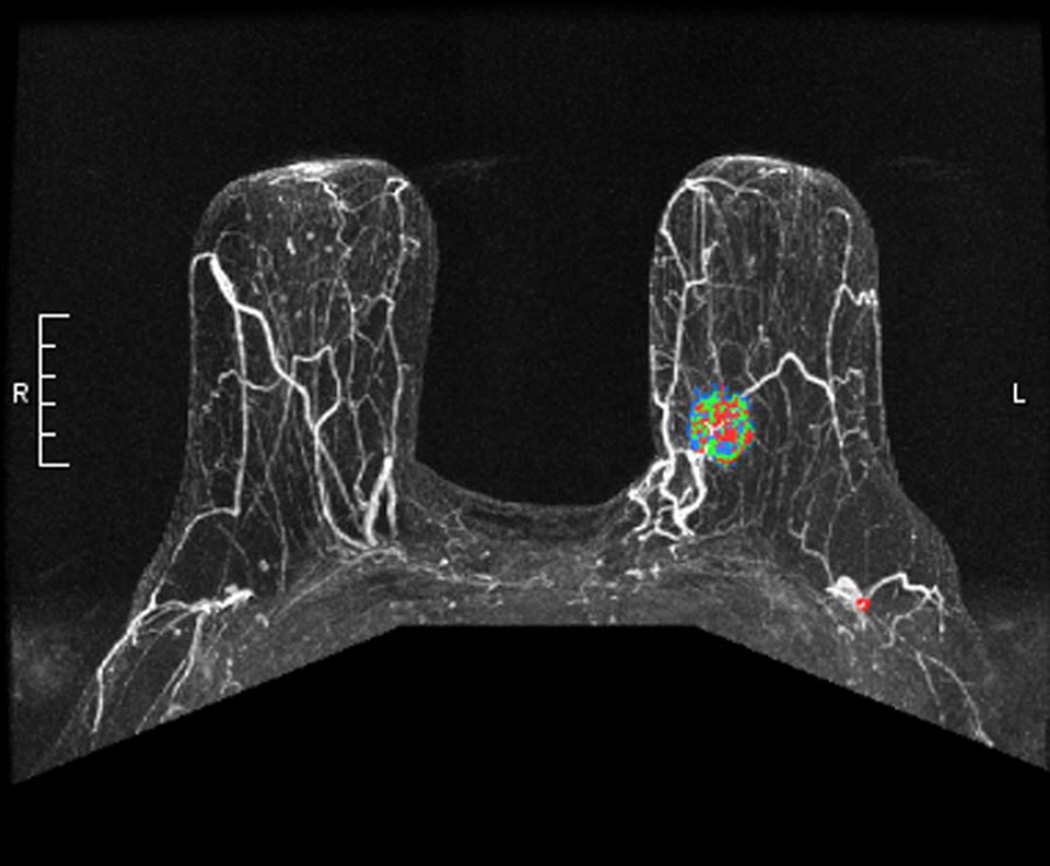
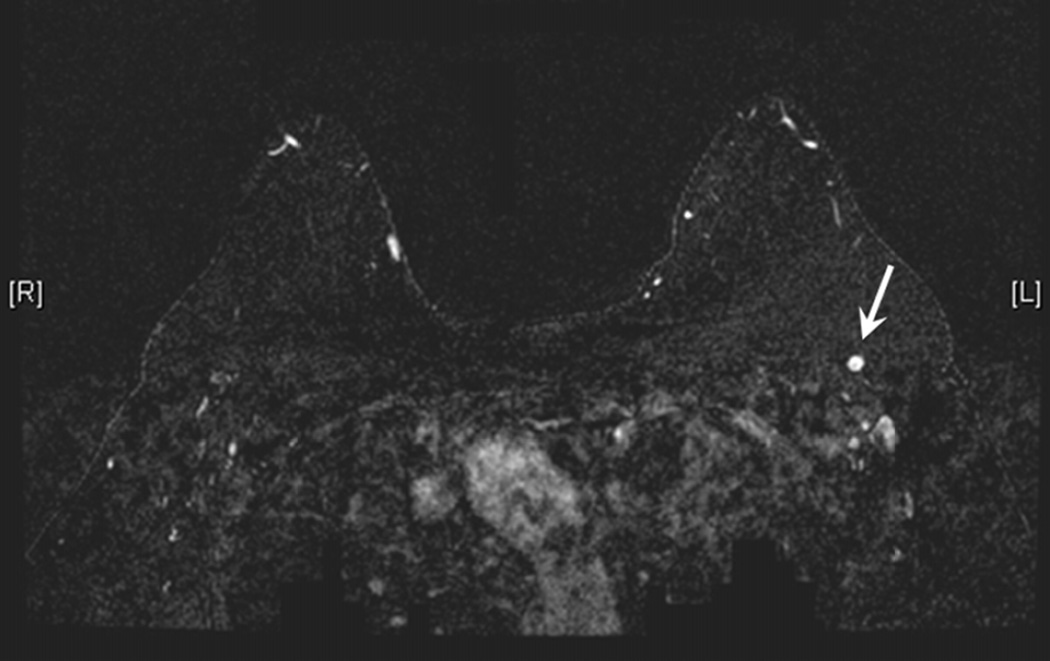
Figure 3.
a) Maximum Intensity Projection image demonstrating enhancing left breast primary breast cancer. b) Maximum Intensity Projection image with color overlay demonstrating regions of persistent (blue), plateau (green), and washout (red) enhancement – as depicted in Figure 1. Tumor volume was calculated as 6.4 cm3, initial peak enhancement (PE) was 211%, and percentages of persistent, plateau and washout enhancement were 56%, 18% and 26%, respectively. (c) Axial T1-weighted fat-saturated post gadolinium MR image revealing a suspicious left axillary lymph node (white arrow). This patient had two positive sentinel lymph nodes and 29 additional positive axillary lymph nodes upon completion axillary dissection. Pathologic tumor size was 2.2 cm.
DISCUSSION
The principal finding of our study is that DCE-MRI adds significantly to the Katz nomogram in identifying sentinel node positive breast cancer patients likely to have extensive occult metastases in the axilla. This finding is highly relevant to current local-regional treatment of breast cancer patients, because many patients now forego axillary dissection after a positive sentinel lymph node biopsy (6). Without complete staging information, physicians must rely on surrogate markers indicating the risk for high axillary disease and thereby choose appropriate targeted therapy. The Katz nomogram, and other peer-reviewed published nomograms, model traditional clinical and pathologic features of breast cancers to predict the presence of four or more total positive axillary nodes after a positive sentinel node biopsy (24). Our results suggest that DCE-MRI in conjunction with the Katz nomogram can more accurately predict which patients will have extensive occult adenopathy compared to clinical-pathologic data alone.
ACOSOG Z-0011 is a recent randomized trial that has had an immediate, dramatic impact upon management of select, favorable risk breast cancer patients undergoing conservative breast surgery, standard fractionation whole breast irradiation, and systemic therapy, who are determined to have one or two positive sentinel lymph nodes (without matted adenopathy or gross extranodal extension) (2, 3). The nearly equivalent local-regional control and overall survival of patients with or without completion axillary dissection supports omission of axillary dissection among patients meeting these specific entry criteria. However, universally accepted criteria identifying low risk sentinel node positive patients appropriate to forego axillary dissection are not well agreed upon and identifying patients with significantly more occult adenopathy for optimal local-regional therapy remains an important unmet clinical need. In the ACOSOG Z-0011 study the median number of positive lymph nodes in both the dissection and non-dissection arms was one (that is, a single positive lymph node). The patient population in our current study includes 46 patients treated with breast conserving therapy – of those, 40 patients would have met Z-0011 entry criteria. Notably, the patient whose MRI is demonstrated in Figure 3 had two positive sentinel nodes and a 2.2 cm primary tumor – meeting the pathologic entry criteria for Z-0011. However, upon completion dissection this patient had 29 additional positive nodes with metastatic cancer. DCE-MRI appears to be helpful in identifying such patients that meet entry criteria but are not well-represented in Z-0011 who would likely benefit from more aggressive local-regional therapy.
This study also indicates that interpretation of the axillary findings on breast MRI by the radiologist, based on lymph node morphology alone is insensitive for clinically occult nodal disease. While new approaches for evaluating the axilla directly on MRI may hold promise to assess presence of nodal metastases (25–27), our results show MRI imaging of the axilla is currently of limited value for predicting extensive nodal disease in patients who are candidates for sentinel lymph node biopsy. However, enhancement data from the primary tumor appear to hold predictive value for the presence of additional nodal disease beyond the sentinel lymph node.
Primary breast cancer DCE-MRI kinetics depend on peri-tumoral microvessl architecture, perfusion, vascular permeability, and lymphatic space composition (28). Breast cancer MRI characteristics are correlated with microvessel density (29–32) and with vascular growth factors such as VEGF (33). Bridging our biological and clinical knowledge, microvessel density has been shown to predict axillary lymph node and distant metastases. Thus, changes in tumor vascularity and the microvessel environment seems a plausible mechanism for the association between primary lesion kinetics and lymph node metastases. Advanced MRI techniques such as diffusion-weighted MRI and MR spectroscopy have shown potential to improve diagnostic accuracy and may provide further value in predicting lymph node involvement (19, 34)
Our study has potential limitations. Tumor volume was calculated using a manual measurement approach, which could have lower reproducibility between radiologists than more automated volume measurement techniques. However, the manual approach is practical as it is commonly employed across multiple imaging modalities for any lesion and can be performed by any radiologist. While automated methods have the advantage of potentially being more reproducible, they can fail to properly select the boundaries of an abnormality thereby over or under estimating the extent. In addition, automated methods may not be available to all radiologists. This is especially true when exams are transferred from one institution to another for consultation without raw and/or kinetic data. The retrospective study design was exploratory in nature and results must be confirmed in an independent dataset and in a multi-institutional trial across different systems to test the generalizability of the model and to determine a meaningful numeric threshold for predicting axillary node status in clinical practice.
To our knowledge, this is the first study to examine the application of pre-operative DCE-MRI to guide regional therapy for sentinel lymph node positive breast cancer patients. Many breast cancer patients currently undergo DCE-MRI as part of their initial preoperative workup. For these patients, integrating the information already gathered with regard to primary lesion kinetics and occult axillary lymph node risk will come at minimal additional cost. Although preoperative breast MRI has not been shown to be cost effective to date with regard to surgical planning alone (35, 36), use of preoperative scans in operative as well as postoperative treatment planning likely increases the benefit of MRI without significantly increasing its cost. The capability of primary lesion DCE-MRI kinetics to predict additional axillary lymph node status is highly relevant for care of sentinel lymph node positive patients and a promising area for future study and validation in a prospective trial.
Acknowledgments
This study supported by Funding from the Roger E. Moe Fellowship in Multidisciplinary Breast Cancer Care, University of Washington School of Medicine.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised phase 3 trial. Lancet Oncol. 2010;11:927–933. doi: 10.1016/S1470-2045(10)70207-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Giuliano AE, McCall L, Beitsch P, et al. Locoregional recurrence after sentinel lymph node dissection with or without axillary dissection in patients with sentinel lymph node metastases: the American College of Surgeons Oncology Group Z0011 randomized trial. Ann Surg. 2010;252:426–432. doi: 10.1097/SLA.0b013e3181f08f32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305:569–575. doi: 10.1001/jama.2011.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Giuliano AE, Chung AP. Long-term follow-up confirms the oncologic safety of sentinel node biopsy without axillary dissection in node-negative breast cancer patients. Ann Surg. 2010;251:601–603. doi: 10.1097/SLA.0b013e3181d6115f. [DOI] [PubMed] [Google Scholar]
- 5.Van Zee KJ, Manasseh DM, Bevilacqua JL, et al. A nomogram for predicting the likelihood of additional nodal metastases in breast cancer patients with a positive sentinel node biopsy. Ann Surg Oncol. 2003;10:1140–1151. doi: 10.1245/aso.2003.03.015. [DOI] [PubMed] [Google Scholar]
- 6.Park J, Fey JV, Naik AM, et al. A declining rate of completion axillary dissection in sentinel lymph node-positive breast cancer patients is associated with the use of a multivariate nomogram. Ann Surg. 2007;245:462–468. doi: 10.1097/01.sla.0000250439.86020.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Veronesi U, Orecchia R, Zurrida S, et al. Avoiding axillary dissection in breast cancer surgery: a randomized trial to assess the role of axillary radiotherapy. Ann Oncol. 2005;16:383–388. doi: 10.1093/annonc/mdi089. [DOI] [PubMed] [Google Scholar]
- 8.Kuehn T, Klauss W, Darsow M, et al. Long-term morbidity following axillary dissection in breast cancer patients--clinical assessment, significance for life quality and the impact of demographic, oncologic and therapeutic factors. Breast Cancer Res Treat. 2000;64:275–286. doi: 10.1023/a:1026564723698. [DOI] [PubMed] [Google Scholar]
- 9.Schijven MP, Vingerhoets AJ, Rutten HJ, et al. Comparison of morbidity between axillary lymph node dissection and sentinel node biopsy. Eur J Surg Oncol. 2003;29:341–350. doi: 10.1053/ejso.2002.1385. [DOI] [PubMed] [Google Scholar]
- 10.Norman SA, Localio AR, Potashnik SL, et al. Lymphedema in breast cancer survivors: incidence, degree, time course, treatment, and symptoms. J Clin Oncol. 2009;27:390–397. doi: 10.1200/JCO.2008.17.9291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fisher B, Jeong JH, Anderson S, et al. Twenty-five-year follow-up of a randomized trial comparing radical mastectomy, total mastectomy, and total mastectomy followed by irradiation. N Engl J Med. 2002;347:567–575. doi: 10.1056/NEJMoa020128. [DOI] [PubMed] [Google Scholar]
- 12.Fowble B, Gray R, Gilchrist K, et al. Identification of a subgroup of patients with breast cancer and histologically positive axillary nodes receiving adjuvant chemotherapy who may benefit from postoperative radiotherapy. J Clin Oncol. 1988;6:1107–1117. doi: 10.1200/JCO.1988.6.7.1107. [DOI] [PubMed] [Google Scholar]
- 13.Katz A, Smith BL, Golshan M, et al. Nomogram for the prediction of having four or more involved nodes for sentinel lymph node-positive breast cancer. J Clin Oncol. 2008;26:2093–2098. doi: 10.1200/JCO.2007.11.9479. [DOI] [PubMed] [Google Scholar]
- 14.Loiselle CR, Eby PR, DeMartini WB, et al. Dynamic contrast-enhanced MRI kinetics of invasive breast cancer: a potential prognostic marker for radiation therapy. Int J Radiat Oncol Biol Phys. 2010;76:1314–1319. doi: 10.1016/j.ijrobp.2009.03.053. [DOI] [PubMed] [Google Scholar]
- 15.Tuncbilek N, Karakas HM, Okten OO. Dynamic magnetic resonance imaging in determining histopathological prognostic factors of invasive breast cancers. Eur J Radiol. 2005;53:199–205. doi: 10.1016/j.ejrad.2003.11.004. [DOI] [PubMed] [Google Scholar]
- 16.Mussurakis S, Buckley DL, Horsman A. Prediction of axillary lymph node status in invasive breast cancer with dynamic contrast-enhanced MR imaging. Radiology. 1997;203:317–321. doi: 10.1148/radiology.203.2.9114081. [DOI] [PubMed] [Google Scholar]
- 17.Partridge SC, Gibbs JE, Lu Y, et al. MRI measurements of breast tumor volume predict response to neoadjuvant chemotherapy and recurrence-free survival. AJR Am J Roentgenol. 2005;184:1774–1781. doi: 10.2214/ajr.184.6.01841774. [DOI] [PubMed] [Google Scholar]
- 18.Marinovich ML, Sardanelli F, Ciatto S, et al. Early prediction of pathologic response to neoadjuvant therapy in breast cancer: systematic review of the accuracy of MRI. Breast. 2012;21:669–677. doi: 10.1016/j.breast.2012.07.006. [DOI] [PubMed] [Google Scholar]
- 19.Partridge SC, DeMartini WB, Kurland BF, et al. Quantitative diffusion-weighted imaging as an adjunct to conventional breast MRI for improved positive predictive value. AJR Am J Roentgenol. 2009;193:1716–1722. doi: 10.2214/AJR.08.2139. [DOI] [PubMed] [Google Scholar]
- 20.Wang LC, DeMartini WB, Partridge SC, et al. MRI-detected suspicious breast lesions: predictive values of kinetic features measured by computer-aided evaluation. AJR Am J Roentgenol. 2009;193:826–831. doi: 10.2214/AJR.08.1335. [DOI] [PubMed] [Google Scholar]
- 21.Eby PR, Partridge SC, White SW, et al. Metabolic and vascular features of dynamic contrast-enhanced breast magnetic resonance imaging and (15)O-water positron emission tomography blood flow in breast cancer. Acad Radiol. 2008;15:1246–1254. doi: 10.1016/j.acra.2008.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Eby PR, DeMartini WB, Gutierrez RL, et al. Characteristics of probably benign breast MRI lesions. AJR Am J Roentgenol. 2009;193:861–867. doi: 10.2214/AJR.08.2096. [DOI] [PubMed] [Google Scholar]
- 23.American College of Radiology Breast Magnetic Resonance Imaging (MRI) Accreditation Program. [Accessed 2011]; Available at: http://www.acr.org/accreditation/Breast-MRI. [Google Scholar]
- 24.Werkoff G, Lambaudie E, Fondrinier E, et al. Prospective multicenter comparison of models to predict four or more involved axillary lymph nodes in patients with breast cancer with one to three metastatic sentinel lymph nodes. J Clin Oncol. 2009;27:5707–5712. doi: 10.1200/JCO.2009.21.9139. [DOI] [PubMed] [Google Scholar]
- 25.Kvistad KA, Rydland J, Smethurst HB, et al. Axillary lymph node metastases in breast cancer: preoperative detection with dynamic contrast-enhanced MRI. Eur Radiol. 2000;10:1464–1471. doi: 10.1007/s003300000370. [DOI] [PubMed] [Google Scholar]
- 26.Scaranelo AM, Eiada R, Jacks LM, et al. Accuracy of unenhanced MR imaging in the detection of axillary lymph node metastasis: study of reproducibility and reliability. Radiology. 2012;262:425–434. doi: 10.1148/radiol.11110639. [DOI] [PubMed] [Google Scholar]
- 27.He N, Xie C, Wei W, et al. A new, preoperative, MRI-based scoring system for diagnosing malignant axillary lymph nodes in women evaluated for breast cancer. Eur J Radiol. 2012;81:2602–2612. doi: 10.1016/j.ejrad.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 28.Zahra MA, Hollingsworth KG, Sala E, et al. Dynamic contrast-enhanced MRI as a predictor of tumour response to radiotherapy. Lancet Oncol. 2007;8:63–74. doi: 10.1016/S1470-2045(06)71012-9. [DOI] [PubMed] [Google Scholar]
- 29.Esserman L, Hylton N, George T, et al. Contrast-Enhanced Magnetic Resonance Imaging to Assess Tumor Histopathology and Angiogenesis in Breast Carcinoma. Breast J. 1999;5:13–21. doi: 10.1046/j.1524-4741.1999.005001013.x. [DOI] [PubMed] [Google Scholar]
- 30.Buadu LD, Murakami J, Murayama S, et al. Breast lesions: correlation of contrast medium enhancement patterns on MR images with histopathologic findings and tumor angiogenesis. Radiology. 1996;200:639–649. doi: 10.1148/radiology.200.3.8756909. [DOI] [PubMed] [Google Scholar]
- 31.Stomper PC, Winston JS, Herman S, et al. Angiogenesis and dynamic MR imaging gadolinium enhancement of malignant and benign breast lesions. Breast Cancer Res Treat. 1997;45:39–46. doi: 10.1023/a:1005897227030. [DOI] [PubMed] [Google Scholar]
- 32.Hulka CA, Edmister WB, Smith BL, et al. Dynamic echo-planar imaging of the breast: experience in diagnosing breast carcinoma and correlation with tumor angiogenesis. Radiology. 1997;205:837–842. doi: 10.1148/radiology.205.3.9393545. [DOI] [PubMed] [Google Scholar]
- 33.Matsubayashi R, Matsuo Y, Edakuni G, et al. Breast masses with peripheral rim enhancement on dynamic contrast-enhanced MR images: correlation of MR findings with histologic features and expression of growth factors. Radiology. 2000;217:841–848. doi: 10.1148/radiology.217.3.r00dc07841. [DOI] [PubMed] [Google Scholar]
- 34.Bartella L, Morris EA, Dershaw DD, et al. Proton MR spectroscopy with choline peak as malignancy marker improves positive predictive value for breast cancer diagnosis: preliminary study. Radiology. 2006;239:686–692. doi: 10.1148/radiol.2393051046. [DOI] [PubMed] [Google Scholar]
- 35.Turnbull L, Brown S, Harvey I, et al. Comparative effectiveness of MRI in breast cancer (COMICE) trial: a randomised controlled trial. Lancet. 2010;375:563–571. doi: 10.1016/S0140-6736(09)62070-5. [DOI] [PubMed] [Google Scholar]
- 36.Turnbull LW, Brown SR, Olivier C, et al. Multicentre randomised controlled trial examining the cost-effectiveness of contrast-enhanced high field magnetic resonance imaging in women with primary breast cancer scheduled for wide local excision (COMICE) Health Technol Assess. 2010;14:1–182. doi: 10.3310/hta14010. [DOI] [PubMed] [Google Scholar]