Abstract
Ascending aortic constriction is the most common and successful surgical model for creating pressure overload induced cardiac hypertrophy and heart failure. Here, we describe a detailed surgical procedure for creating pressure overload and cardiac hypertrophy in rats by constriction of the ascending aorta using a small metallic clip. After anesthesia, the trachea is intubated by inserting a cannula through a half way incision made between two cartilage rings of trachea. Then a skin incision is made at the level of the second intercostal space on the left chest wall and muscle layers are cleared to locate the ascending portion of aorta. The ascending aorta is constricted to 50–60% of its original diameter by application of a small sized titanium clip. Following aortic constriction, the second and third ribs are approximated with prolene sutures. The tracheal cannula is removed once spontaneous breathing was re-established. The animal is allowed to recover on the heating pad by gradually lowering anesthesia. The intensity of pressure overload created by constriction of the ascending aorta is determined by recording the pressure gradient using trans-thoracic two dimensional Doppler-echocardiography. Overall this protocol is useful to study the remodeling events and contractile properties of the heart during the gradual onset and progression from compensated cardiac hypertrophy to heart failure stage.
Keywords: Medicine, Issue 88, ascending aorta, cardiac hypertrophy, pressure overload, aortic constriction, thoracotomy, surgical model.
Introduction
Small animal models are the most preferred tools for the study of chronic changes in the development of cardiac hypertrophy and its progression to heart failure. Heart failure models by surgical methods were originally developed in rats as these animals are most suitable for open chest surgical procedures, echocardiography and evaluation of hemodynamic parameters. They also provide adequate post mortem samples for tissue, cellular and molecular level studies1. Constriction of the ascending aorta is one of the most common and successful surgical models for creating pressure overload heart failure2,3. This model is well suited to study the cellular and sub cellular remodeling events and contractile properties of the heart during the transition from hypertrophy to heart failure4.
A major advantage of this model is the gradual onset of pressure overload on the heart5,6. This model is clinically relevant because of its slow but steady progression from compensated cardiac hypertrophy to decompensated phase and finally to the heart failure stage. The duration for progression of cardiac hypertrophy to heart failure and the associated physiological changes are mainly influenced by the degree of stenosis produced7. Ascending aortic constriction can be performed in two ways, either by using sutures or by application of metallic clips8,9. The main advantage of using a metallic clip for aortic constriction is that the surgical procedures are less complicated. In this method one has to locate the supra valvular ascending aorta and insert the clip around the aorta to obtain desired level of constriction. A chest retractor is not necessary in this procedure. Aortic banding by suturing requires more surgical manipulations for placing the gauge needle beside the ascending aorta and suturing around it to produce the desired level of aortic constriction. The latter technique is more time consuming when compared with the use of metallic clips for aortic constriction. The method described in this video demonstrates the surgical procedure for ascending aortic constriction in rats that allows creation of pressure overloaded left ventricular hypertrophy model.
Protocol
Animal experiments were performed after obtaining approval from the Institutional Animal Ethics Committee. Wistar rats were housed under a 12 hr dark to light cycle, constant room temperature (24 ± 2 °C) with food and water ad libitum (as per the CPCSEA guidelines).
1) Pre-operative Care and Anesthesia
Keep rat (approximately 200 g body weight) under antibiotic umbrella 24 hr before surgery by administering amoxicillin orally (dose: 500 mg/L of water).
Sterilize all surgical tools (such as scissors, forceps, clip applicator, suture needles, and needle holder) by autoclaving at 121 °C for 15 min.
Use a heating pad to maintain the animal body temperature around 37 °C to avoid a rapid decrease in heart rate.
Take care to avoid dehydration of the animal before and during surgery.
Anesthetize the rat in an induction chamber with 3% isoflurane mixed with 0.5-1.0 L/min 100% oxygen; maintain anesthesia with 1.5% isoflurane.
Check the pedal reflex to confirm successful anesthesia.
2) Preparation of the Surgical Site
Keep the rat on top of a temperature controlled heating pad in the supine position.
Position a rubber band over the rat’s front teeth to keep the animal in position while maintaining anesthesia with a nose cone.
Remove fur from the neckline and left chest region of the rat (preferably using an electric shaver).
Disinfect surgical site with povidone-iodine solution and then with 70% ethyl alcohol.
Use a sterile drape to expose only the surgical site during the operative procedure. Note: As an additional option for the animal care, 0.25% Bupivicaine ID (2.5 mg/kg) can be incorporated prior to incising at each incision site for local anesthesia/analgesia.
3) Tracheal Intubation in Rat
Make a small incision on the skin parallel to the trachea using a sterile surgical blade and carefully separate the underlying tissue in order to expose the trachea.
Apply a tie of 4-0 silk suture thread underneath the trachea and gently raise the tie upward.
Make a half way incision between two cartilage rings and insert the tracheal cannula into the trachea of the rat.
Connect the tracheal canula to a rodent ventilator for maintaining a respiratory rate of 50 breaths/min, tidal volume of 1.70 ml and inspiration time of 0.60 sec (for rat with 200 g body weight).
4) Constriction of Ascending Aorta
After sensing the position of the ribs with fingers, make a skin incision of about 2 cm on the left chest wall between the second and third ribs.
Separate the intercostal muscles layer by layer and make an incision about 1.5 cm long between the second and third ribs. (Care should be taken to avoid any injury to the lungs while making incision between the ribs)
Retract the ribs and locate the ascending portion of the aorta.
Place a small sized titanium clip around the ascending aorta with the help of an applicator whereby constricting the aorta to 50–60% of the original diameter.
Following the aortic constriction, approximate the second and third ribs with 3.0 prolene in an interrupted suture pattern. Simultaneously, pause the ventilator for about 2-4 sec in order to re-inflate the lungs.
Appose the muscle layers with 3.0 prolene sutures and skin with 4.0 silk sutures in an interrupted suture pattern. Synthetic non absorbable sutures can be used instead of silk for suturing the skin.
Remove tracheal cannula from the rat once spontaneous breathing is re-established.
Close the tracheal opening with 3.0 prolene sutures and the skin layer with 4.0 silk sutures.
Allow the animal to recover on the heating pad by gradually lowering the anesthesia and ventilator assisted respiration.
In age-matched sham control animals, perform tracheotomy and thoracotomy without constriction of ascending aorta.
5) Post-operative Care
Disinfect surgical area with povidone-iodine solution.
As post-operative analgesia, inject tramadol intra-peritoneally (10 mg/kg body weight/day) for 1 week.
Inject sterile normal saline intra-peritoneally if signs of dehydration appear after surgery (approximately 1 ml).
Administer amoxycillin orally, mixed with drinking water, for seven days (dose: 500 mg/L of water).
Remove any remaining silk sutures from the skin post 10 days of surgery under inhalation anesthesia.
6) Confirmation of Successful Constriction of Ascending Aorta
Perform a trans-thoracic two dimensional Doppler- echocardiography to determine the intensity of the pressure overload produced by the constriction of ascending aorta.
Anesthetize the rats one week post-surgery as described earlier (step 1.5)
Place the ultrasound transducer at the supra sternal position to record the pressure gradient across the constricted portion of ascending aorta. Note: Rats with a pressure gradient of about 60 mm of Hg at the aortic constricted site will develop severe cardiac hypertrophy by about 8-10 weeks post surgery.
Representative Results
In our experiments we are able to achieve more than 80% survival rates. The effectiveness of aortic constriction was confirmed by performing Doppler echocardiography. Rats (n=6) with a pressure gradient of about 60 mm of Hg at the aortic constricted site were observed for 8 weeks and sacrificed to analyze for the development of cardiac hypertrophy (Figure 1). After 8 weeks post-surgery echocardiographic evaluation revealed a significant increase in left ventricular parameters such as inter ventricular septal thickness, left ventricular posterior wall thickness and decrease in left ventricular internal diameter at diastolic state, in animals which underwent aortic constriction, compared to sham operated controls (Table 1, Figure 2). A significant increase in heart weight/body weight ratios and left ventricular weight/body weight ratios were observed in rats with aortic constriction (Table 2, Figure 3). These data suggest that 8 weeks post-surgery rats with aortic constriction develop a significant level of cardiac hypertrophy, compared to sham operated controls.
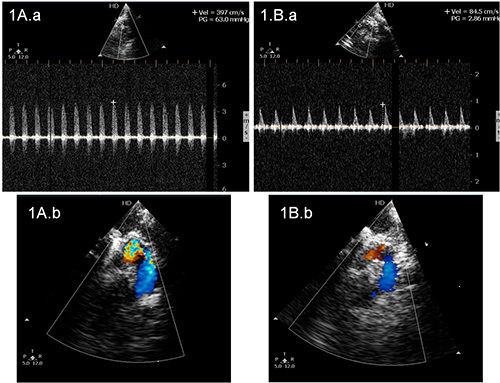 Figure 1. Echocardiogram in rats representing the pressure gradient across the ascending aorta. A.a) The representative echocardiogram from a rat with constriction of aorta and having a pressure gradient of 63.0 mm of Hg and A.b represents color Doppler echo of the site across the aorta where pressure gradient was measured. B.a) The representative echocardiogram from a sham operated control rat having a pressure gradient of 2.86 mm of Hg and B.b represents color Doppler echo of the site across the aorta where the pressure gradient was measured. This increase in pressure gradient indicates successful banding of the aorta in rats which underwent partial ascending aortic constriction.
Figure 1. Echocardiogram in rats representing the pressure gradient across the ascending aorta. A.a) The representative echocardiogram from a rat with constriction of aorta and having a pressure gradient of 63.0 mm of Hg and A.b represents color Doppler echo of the site across the aorta where pressure gradient was measured. B.a) The representative echocardiogram from a sham operated control rat having a pressure gradient of 2.86 mm of Hg and B.b represents color Doppler echo of the site across the aorta where the pressure gradient was measured. This increase in pressure gradient indicates successful banding of the aorta in rats which underwent partial ascending aortic constriction.
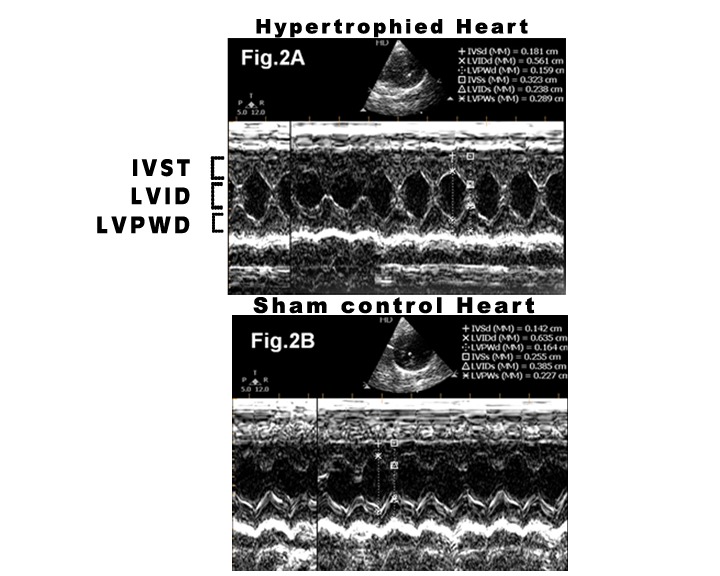 Figure 2. M- mode echocardiogram of the left ventricle in rats with aortic constriction (A) and control rats (B) depicting left ventricular dimensions at the time of sacrifice 8 weeks post-surgery. IVST= inter ventricular septal thickness; LVPWT= left ventricular posterior wall thickness; LVID= left ventricular internal diameter in diastole. In rats with aortic constriction a significant increase in inter ventricular septal thickness, left ventricular posterior wall thickness and decrease in left ventricular internal diameter at diastolic state can be observed.
Figure 2. M- mode echocardiogram of the left ventricle in rats with aortic constriction (A) and control rats (B) depicting left ventricular dimensions at the time of sacrifice 8 weeks post-surgery. IVST= inter ventricular septal thickness; LVPWT= left ventricular posterior wall thickness; LVID= left ventricular internal diameter in diastole. In rats with aortic constriction a significant increase in inter ventricular septal thickness, left ventricular posterior wall thickness and decrease in left ventricular internal diameter at diastolic state can be observed.
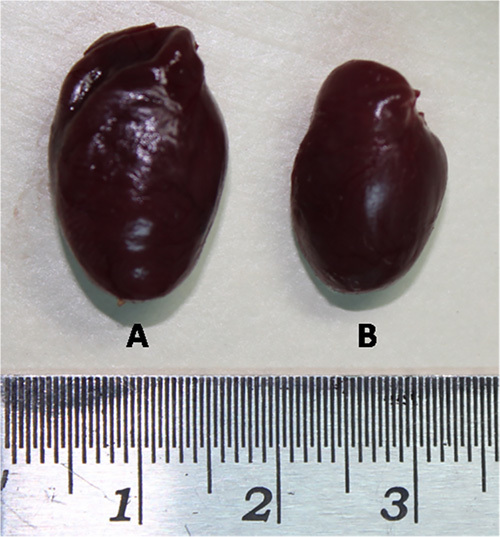 Figure 3. Images of the whole hearts of a rat which underwent aortic constriction (A) and a sham operated control rat (B) 8 weeks post-surgery. The rat with aortic constriction has significant cardiac hypertrophy compared to sham operated control.
Figure 3. Images of the whole hearts of a rat which underwent aortic constriction (A) and a sham operated control rat (B) 8 weeks post-surgery. The rat with aortic constriction has significant cardiac hypertrophy compared to sham operated control.
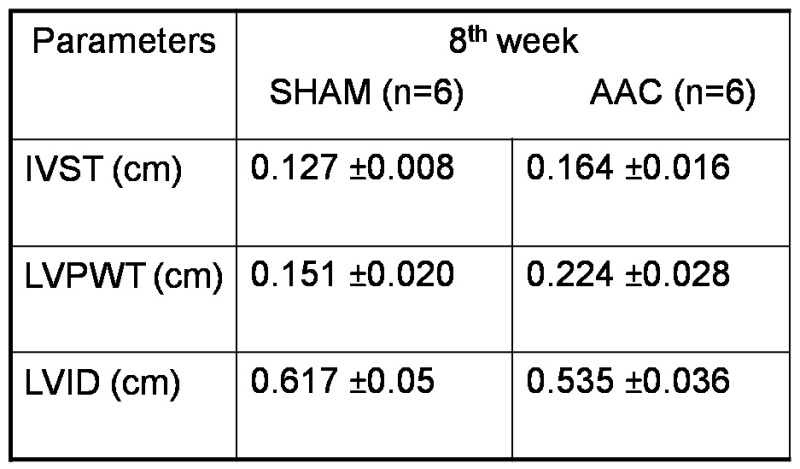 Table 1. M- mode echocardiographic parameters of cardiac left ventricle at the time of sacrifice, 8 weeks post-surgery. IVST= inter ventricular septal thickness; LVPWT= left ventricular posterior wall thickness; LVID= left ventricular internal diameter at diastolic state; AAC= ascending aortic constriction.
Table 1. M- mode echocardiographic parameters of cardiac left ventricle at the time of sacrifice, 8 weeks post-surgery. IVST= inter ventricular septal thickness; LVPWT= left ventricular posterior wall thickness; LVID= left ventricular internal diameter at diastolic state; AAC= ascending aortic constriction.
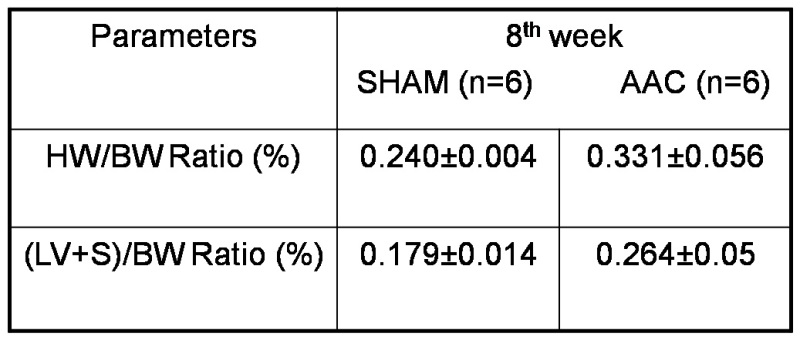 Table 2. Heart weight: Body weight ratio and left ventricular weight: body weight ratio in aortic constricted and sham control rats sacrificed 8 weeks post-surgery. Hw= Heart weight; Bw= Body weight; LV+S= Left ventricle+ inter ventricular septum; AAC= ascending aortic constriction.
Table 2. Heart weight: Body weight ratio and left ventricular weight: body weight ratio in aortic constricted and sham control rats sacrificed 8 weeks post-surgery. Hw= Heart weight; Bw= Body weight; LV+S= Left ventricle+ inter ventricular septum; AAC= ascending aortic constriction.
Discussion
There are several surgical models for the induction of pressure overload as well as cardiac hypertrophy and heart failure. In small animals, Lorell and colleagues developed the model we have described here and they reported the occurrence of compensated cardiac hypertrophy 8 weeks post aortic banding5. Cardiac changes gradually progress to a decompensated state and then result in heart failure. The main advantage of this model is the gradual onset of increase in left ventricular pressure and thus similar to clinical situations in chronic hypertension. It is also a suitable animal model for studying left heart failure associated pulmonary hypertension10.
Other animal models for pressure overload heart failure, such as Dahl sensitive rats, spontaneously hypertensive rats and rats with constriction of abdominal aorta, also develop chronic pressure overload. They, however, take extended time periods for progression from cardiac hypertrophy to heart failure. The maintenance cost of experimental rats is therefore also higher1. deAlmeida and colleagues employed transverse aortic constriction for inducing left ventricular hypertrophy in mice11. The technique involves median sternotomy and retraction of sternum and hence is more challenging.
Ensure a swift change of anesthesia from nose cone to tracheal intubation before thoracotomy and from tracheal intubation to nose cone after the operative procedures. An alternate method for tracheal intubation in rats is by using polyethylene tube for endotracheal intubation instead of tracheotomy. However, this technique requires adequate skill. Care should be taken to avoid any injury to the lungs while making incision between the ribs. Application of titanium clips sufficient to produce critical aortic constriction for left ventricular stress, but not severe enough to produce acute left ventricular failure and pulmonary oedema. Use of prolene sutures for the approximation of ribs is critical for successful recovery of the animal. During the approximation of ribs, pause the ventilator for about 2-4 sec in order to re-inflate the lungs.
We had only 20% operative mortality in the animals and there were no post-operative complications. The technique we described here is thus an efficient and suitable tool for studying chronic patho-physiological changes in cardiac hypertrophy and progressive heart failure.
Disclosures
The authors have nothing to disclose.
Acknowledgments
The authors acknowledge Department of Biotechnology, Government of India for financial support of this project. Ajith Kumar G S was supported with senior research fellowship from Council of Scientific and Industrial Research, Government of India and Binil Raj with senior research fellowship from Indian Council of Medical Research, Government of India.
References
- Patten RD, Hall-Porter MR. Small animal models of heart failure: development of novel therapies, past and present. Circ Heart Fail. 2009;2:138–144. doi: 10.1161/CIRCHEARTFAILURE.108.839761. [DOI] [PubMed] [Google Scholar]
- Weinberg EO, et al. Angiotensin-converting enzyme inhibition prolongs survival and modifies the transition to heart failure in rats with pressure overload hypertrophy due to ascending aortic stenosis. Circulation. 1994;90:1410–1422. doi: 10.1161/01.cir.90.3.1410. [DOI] [PubMed] [Google Scholar]
- Schunkert H, et al. Increased rat cardiac angiotensin converting enzyme activity and mRNA expression in pressure overload left ventricular hypertrophy. Effects on coronary resistance, contractility, and relaxation. J Clin Invest. 1990;86:1913–1920. doi: 10.1172/JCI114924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenfuss G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc Res. 1998;39:60–76. doi: 10.1016/s0008-6363(98)00110-2. [DOI] [PubMed] [Google Scholar]
- Litwin SE, et al. Serial echocardiographic-Doppler assessment of left ventricular geometry and function in rats with pressure-overload hypertrophy. Chronic angiotensin-converting enzyme inhibition attenuates the transition to heart failure. Circulation. 1995;91:2642–2654. doi: 10.1161/01.cir.91.10.2642. [DOI] [PubMed] [Google Scholar]
- Miyamoto MI, et al. Adenoviral gene transfer of SERCA2a improves left-ventricular function in aortic-banded rats in transition to heart failure. Proc Natl Acad Sci U S A. 2000;97:793–798. doi: 10.1073/pnas.97.2.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagaya Y, Hajjar RJ, Gwathmey JK, Barry WH, Lorell BH. Long-term angiotensin-converting enzyme inhibition with fosinopril improves depressed responsiveness to Ca2+ in myocytes from aortic-banded rats. Circulation. 1996;94:2915–2922. doi: 10.1161/01.cir.94.11.2915. [DOI] [PubMed] [Google Scholar]
- Del Monte F, Butler K, Boecker W, Gwathmey JK, Hajjar RJ. Novel technique of aortic banding followed by gene transfer during hypertrophy and heart failure. Physiol Genomics. 2002;9:49–56. doi: 10.1152/physiolgenomics.00035.2001. [DOI] [PubMed] [Google Scholar]
- Turcani M, Rupp H. Heart failure development in rats with ascending aortic constriction and angiotensin-converting enzyme inhibition. Br J Pharmacol. 2000;130:1671–1677. doi: 10.1038/sj.bjp.0703467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerem A, et al. Lung endothelial dysfunction in congestive heart failure: role of impaired Ca2+ signaling and cytoskeletal reorganization. Circ Res. 2010;106:1103–1116. doi: 10.1161/CIRCRESAHA.109.210542. [DOI] [PubMed] [Google Scholar]
- Almeida AC, van Oort RJ, Wehrens XH. Transverse aortic constriction in mice. J Vis Exp. 2010. [DOI] [PMC free article] [PubMed]


