Abstract
Background:
Parastomal hernias (PSHs) are a frequent complication and remain a surgical challenge. We present a new option for single-port PSH repair with equilateral stoma relocation using preshaped, prosthetic 3-dimensional implants and flat mesh insertion in intraperitoneal onlay placement for additional augmentation of the abdominal wall.
Methods:
We describe our novel technique in detail and performed an analysis of prospectively collected data from patients who underwent single-port PSH repair, focusing on feasibility, conversions, and complications.
Results:
From September 2013 to January 2014, 9 patients with symptomatic PSHs were included. Two conversions to reduced-port laparoscopy using a second 3-mm trocar were required because of difficult adhesiolysis, dissection, and reduction of the hernia sac content. No major intra- or postoperative complications or reoperations were encountered. One patient incurred a peristomal wound healing defect that could be treated conservatively.
Conclusion:
We found that single-port PSH repair using preshaped, elastic 3-dimensional devices and additional flat mesh repair of the abdominal wall is feasible, safe, and beneficial, relating to optimal coverage of unstable stoma edges with wide overlap to all sides and simultaneous augmentation of the midline in the IPOM technique. The stoma relocation enables prolapse treatment and prevention. The features of a modular and rotatable multichannel port system offer benefits in clear dissection ongoing from a single port. Long-term follow-up data on an adequate number of patients are awaited to examine efficacy.
Keywords: Parastomal hernia, Single port, Single site, Single incision, Prevention, Laparoscopic repair
INTRODUCTION
Parastomal hernias (PSHs) are a common and frequent complication after permanent stoma construction and continue to be a distressing problem for patients.1 PSHs remain a surgical challenge despite progress in various techniques for surgical repair.2 They are still associated with high rates of morbidity, mortality, and recurrence,3,4 but the high rate of complications and above all incarcerations owing to PSHs seems to justify early repair.3 The first laparoscopic repair with different approaches using expanded polytetrafluoroethylene mesh was reported in 2005.5 Laparoscopic mesh repair is associated with shorter lengths of hospital stay and lower risk for overall morbidity compared with open techniques.6 Laparoscopic techniques can usually be performed with flat-slit or nonslit meshes in intraperitoneal onlay placement. The “keyhole technique,” which involves place a slit mesh around the stoma, results in a high recurrence rate because the edge areas of the stoma cannot be adequately covered.7,8 This led to a shift in technique to modified “Sugarbaker repair.” The concept is a laparoscopically flat mesh–supported lateralization of the ostomy that leads to lower recurrence rates.9,10 In 2007, Berger and Bientzle11 introduced a combination of keyhole and Sugarbaker repair, the so-called sandwich technique, and reported excellent results with low complication and recurrence rates. However, a concern with the sandwich technique is the occasional sharp edges of the keyhole mesh, which may lead to local erosion, and the potential functional change of the lateralized stoma bowel referred to Sugarbaker technique, which is difficult to predict and sometimes may lead to obstruction. Another drawback is that the commonly occurring concomitant stoma prolapse cannot be removed.
The high prevalence of PSH, clinical impairment, and disappointing results of PSH repair imply that prevention would be the better choice. The only effective way to prevent PSH and fascial dehiscence is by using a mesh to augment the abdominal wall around the stoma because of primary stoma formation.12 With regard to excellent results of PSH prevention with a 3-dimensional (3D) funnel device13 to keep the bowel with reinforcement of the surrounding tissues, the use of this device may be also advantageous for repair of a PSH, if combined with a satisfying reinforcement of the midline using an additional flat intraperitoneal onlay mesh. This hybrid technique for PSH repair can be performed using single-port access with reostomy with a small localized approach at the stoma site. In the present report, we describe our early experience with a novel technique for single-port laparoscopic PSH repair using preshaped 3-D funnel devices and additional intraperitoneal onlay flat mesh placement for covering the midline.
METHODS
We performed prospective data collection and retrospective analysis of consecutive patients who underwent single-port repair of symptomatic PSH using a 3D funnel device between September 2013 and January 2014 in the Department of General and Visceral Surgery at Sisters of Charity Hospital in Linz, Austria. The preoperative imaging diagnosis was composed chiefly of an abdominal computed tomographic scan. This also included sequences during an abdominal press to better visualize the extent and content of PSHs. All hernias were graded according to the classification of Moreno-Matias and the new Endo Hernia Society classification of PSH.14,15 Patients' demographics and disease characteristics were documented, including concomitant incisional hernias, graded in accordance with the Endo Hernia Society classification.16 The presence of coexisting stoma prolapse and its clinical relevance were noticed.
Technique Description
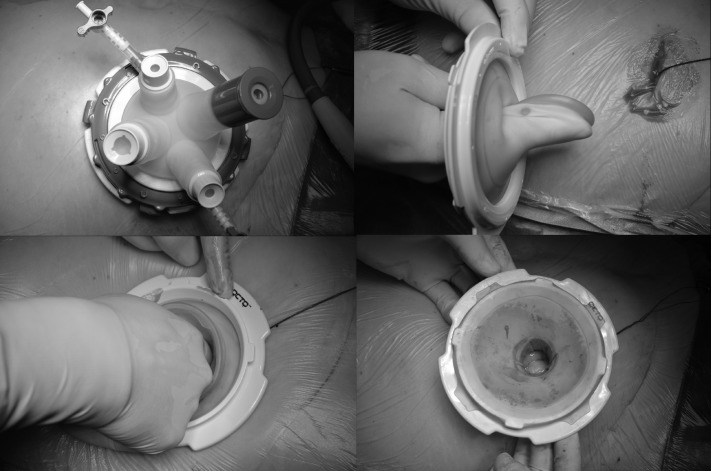
All patients are catheterized after the induction of general anesthesia and receive a single dose of antibiotic prophylaxis (sultamicillin 3 g [Unasyn; Pfizer Inc, New York, NY]) half an hour before skin incision and bowel preparation with 2 L saline solution for oral use on the day before the operation. The patient is in the supine position and mild hyperlordosis, and the stoma is covered by a translucent adhesive film. All operations were performed by a single surgeon standing together with an assistant on the right side of the patient, with the side chosen to be opposite to the PSH. The operative technique is performed starting with a 2-cm transverse skin incision and entering the peritoneum under direct vision for the insertion of a single-port access using a modular, multitasking, flexible, ambidextrous, rotatable, and detachable multichannel single-port system (OCTO Port V2-A [DalimsurgNET, Seoul, Korea], 15–30 mm; Figure 1a). The port insertion must be far lateral at the abdominal wall on the level of the umbilicus and on the contralateral side of the ostomy. It is helpful to shape the silicone ring of the port system between 2 fingers, as depicted in Figures 1b and 1c for intraperitoneal insertion, which can otherwise be challenging. Then the silicone ring must be stretched and fixed at the plastic ring (Figure 1d). Subsequently, the plastic cover is applied and locked. The insertion also of large meshes without demolition is facilitated by the removable cover of the single-port system. The use of a 10 mm, 30° angle laparoscope and a combination of straight and angulated flexible instruments enables enough range of motion for adhesiolysis and dissection so far as is safely possible and makes it possible to go around the ostomy at the back side. The port system can be rotated, and the positions of laparoscope and instruments can be chosen freely to achieve most efficient dissection. It is important to dissect the midline completely to expose any incidental incisional hernias. To divide bowel adhesions, we perform sharp dissection. If the dissection and reduction of hernia sac content is very difficult because of PSH recurrences, adhesions, scarring, and bowel adherence, laparoscopic preparation is stopped, because a localized approach at the stoma side is required in any case. On the basis of this small open access, additional dissection in the region of the stoma is easily possible. For this purpose, the skin close to the stoma is incised circularly, and a localized open approach on the stoma position is performed. Then we preliminarily close the ostomy with sutures and perform an open adhesiolysis, hernia sac excision, dissection of adherent and scarring tissue, and reduction of hernia sac content. Afterward, the colon is reduced to a suitable length, and the shortened bowel is brought out through a quadratic 3-D funnel mesh with 15- or 16-cm side length and 2- or 3-cm funnel diameter, which must be directed to the abdominal cavity. Subsequently, the ostomy is relocated equilaterally through the narrowed preexisting hernia hole after closure of the fascial gap. No additional skin incisions are required, and the new ostomy is brought out identically equal to a primary stoma creation. Therefore, the hybrid procedure does not stray from the single-port technique concept. The Dynamesh IPST implant (FEG Textiltechnik, Aachen, Germany) is a 3-D, preshaped, specially designed open-pore and monofilament mesh consisting of polyvinylidene fluoride (PVDF) and polypropylene. The PVDF side of the 2-component filament structure with the funnel is oriented to the visceral side of the abdomen. No polypropylene is exposed to the abdominal content. Eventually, the mesh is spread out laparoscopically and placed using the intraperitoneal onlay technique. The fixation takes place with absorbable strap devices (SECURESTRAP; Ethicon Endo-Surgery, Blue Ash, OH) in a double-crown technique at the edges of the flat part of the mesh and around the stoma (Figures 2a and 2b). An additional reinforcement of the median abdominal wall is done if patients had undergone previous midline laparotomy with or without concomitant incisional hernia. Flat PVDF meshes (Dynamesh IPOM; FEG Textiltechnik) are placed in intraperitoneal onlay technique and fixed with absorbable strap devices and nonabsorbable transfascial sutures. On the area where parts of the 2 inserted meshes were lying on top of each other, nonabsorbable fixation is recommended to provide a permanent linkage. Finally, the new ostomy is fixed with everting mucocutaneous sutures.
Figure 1.
(a) Flexible, multichannel single-port system. (b, c) Insertion technique. (d) Fixation of the silicone ring at the plastic ring.
Figure 2.
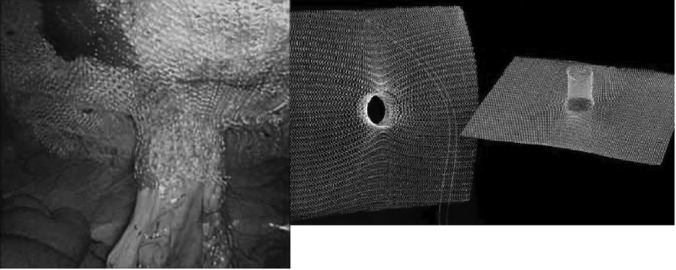
(a) Final condition of the intraperitoneal onlay placed and fixed 3-D funnel mesh. (b) The original implant.
All patients provided written informed consent allowing anonymous data collection, analysis, and publishing by authorized persons having access privileges. Data were collected from the prospective Herniamed GmbH database17 and the institution's medical records and medical files. The protocol for the research project was approved by the ethics committee of the institution within which the work was undertaken, and it conformed to the provisions of the Declaration of Helsinki (as revised in Seoul in 2008).
End points of the study were the number of single-port completions of the scheduled operations without conversions to multiport laparoscopy or open surgery, as well as intra- and postoperative complications according to the Clavien-Dindo classification18 and unplanned reoperations. Routine follow-up was done or is scheduled after 3 months by anamnesis, clinical examination, and sonography. After 6 and 12 months, additional follow-up is performed using multislice computed tomographic scanning, including sequences during abdominal press. Only descriptive statistics were used.
RESULTS
Nine patients (5 women and 4 men) with a mean age of 63.2 years and a mean body mass index of 25.4 kg/m2, all with permanent terminal left-sided colostomies, underwent elective single-port 3-D mesh repair of symptomatic PSHs. All simultaneously underwent intraperitoneal flat mesh augmentation of the abdominal wall because of preexisting midline laparotomies. Four patients had concomitant median incisional hernias, and the remaining 5 patients received prophylactic mesh for midline coverage and hernia prevention. The mean duration of the operations was 126.6 minutes. Relevant disease characteristics and outcome parameters are described in Table 1.
Table 1.
Disease Characteristics, Surgical Details and Outcome Parameters
| Patient | Parastomal Hernia |
Coincident Incisional Hernia (EHS)§ | Intraoperative Complications | Postoperative Complications | Additional Port | Length of Hospital Stay (d) | Follow-Up (wk) | ||
|---|---|---|---|---|---|---|---|---|---|
| Primary/Recurrence | Moreno-Matias Classification† | EHS Classification‡ | |||||||
| A | P | II | Type II | M2/4 W2 | No | No | No | 7 | 17 |
| B | P | II | Type I | No | No | No | 7 | 17 | |
| C | P | I | Type I | No | No | No | 6 | 13 | |
| D | R* | III | Type II | M2/3 W1 | Serosal bowel damage | No | One 3-mm port | 12 | 12 |
| E | P | II | Type III | No | No | No | 8 | 12 | |
| F | P | I | Type I | No | No | No | 7 | 9 | |
| G | R* | III | Type IV | M3 W2 | No | Parastomal wound healing defect | One 3-mm port | 18 | 8 |
| H | P | III | Type I | No | No | No | 6 | 5 | |
| I | P | II | Type IV | M4/5 W1 | No | No | No | 6 | 2 |
cIH, concomitant incisional hernia; EHS, Endo Hernia Society; P, primary; R, recurrence.
Recurrence occurred after keyhole repair.
Moreno-Matias classification of parastomal hernias: 0 = normal, I = hernial sac containing stoma loop, II = sac containing omentum, III = sac containing a loop other than stoma.
EHS classification of parastomal hernias: type I = small (≤5 cm) PSH without cIH, type II = small (≤5 cm) PSH with cIH, type III = large PSH without cIH, type IV = large PSH with cIH.
EHS classification of median incisional hernias: M1 = subxiphoidal, M2 = epigastric, M3 = umbilical, M4 = infraumbilical, M5 = suprapubical, W = width (W1, <4 cm; W2, 4–10 cm; W3, >10 cm).
One superficial postoperative parastomal wound healing defect occurred and constituted the overall morbidity. It was treated with local wound management without anesthesia and healed completely within 2 weeks (Clavien-Dindo class 3a).18 No mesh infections or mesh-related complications occurred, and no reoperations were required.
Two patients required intraoperatively an additional 3-mm port insertion because of dense adhesions and scarring with small bowel adherence to previously applied intraperitoneal onlay meshes. Both were redo operations due to recurrences after previous keyhole PSH repair. The additional port insertion offered better triangulation, and the use of an atraumatic grasper for soft tissue handling provided gentle tissue tension and dissection. Timely additional port insertion is strongly recommended for patient safety in difficult cases with poor overview. Maximal efforts should be undertaken to prevent visceral injuries, bleeding, and full-thickness enterotomy. These complications were avoided in all cases. One instance of intraoperative serosal damage of the stoma bowel close to the skin occurred because of tensile stress, but this part of the stoma bowel must in any case be subsequently resected, because the bowel had to be reduced in all cases before creating a new appropriate ostomy. Seven of 9 patients had clinically relevant stoma prolapse before relocation, and even in the 2 patients without prolapse, the mobilized stoma bowel was shortened by 10 cm up to a suitable length. No further conversion to multiport laparoscopy or open surgery was required. No mortality occurred, no redo surgery was required, and no early recurrence, prolapse reoccurrence, or late-term complications were documented during short-term follow-up (mean, 10.6 weeks).
DISCUSSION
Various techniques have been advocated for surgical repair of PSHs. The advantages of mesh repair combined with minimally invasive surgery have led to the development of different laparoscopic techniques.1,2,5 Laparoendoscopic single-site surgery made its debut in 2010.19 Data concerning single-port PSH repair are scarce.20 We do not recommend this approach chiefly for cosmetic reasons in patients who have existing ostomies but rather because of the possibility to start dividing adhesions under direct visual control ongoing from the first port. Thereafter, additional ports can safely be inserted as far as they are required. Our hybrid technique is suitable for both single-port and multiport laparoscopy. Patients with PSHs due to permanent ostomies had undergone as a rule previous abdominal surgery with subsequently more rather than less extensive adhesion formation and consequent poor laparoscopic overview. That is why the safe insertion of an additional port apart from the camera port can initially be disabled in multiport laparoscopy. The modular and rotatable OCTO Port system offers 4 flexible portals (5–12 mm) and enables accessibility of all abdominal quadrants. The skin and fascial incision can be minimized to 2 cm, which exceeds no other visually controlled laparoscopic port insertion, which is in any case recommended to avoid injuries due to potential adhesions. If a large flat mesh for covering midline incisions and/or hernias is required, we prefer to take down the removable cover gasket of the port system for smooth intraperitoneal insertion of the mesh without deformation and demolition. This would not be easy through a common 10- to 12-mm port in multiport laparoscopy.
The high incidence of PSH and the controversy surrounding its repair make its prevention an area of intense research.12 It has been experimentally shown that elastic PVDF mesh material grows inward, prevents intestinal adhesions, and shows less shrinkage tendency.21,22 On the basis of these results, we chose to use this implant not only for prevention but also for PSH repair. It offers the following advantages: 3-D funnel meshes can be used either in laparoscopic or open surgery. The PSH defect can be locally covered, with wide overlap to all sides. When a new ostomy is created, the bowel can easily be brought out through the elastic funnel of the mesh. The edges of the stoma opening are prevented as the fibers of the dome are bent to a perpendicular configuration that runs parallel to the bowel. The local fascia close to the stoma bowel does not need to be approximated with close contact to the bowel, though this is the precarious spot at which PSHs occur. It is possible to make an incision into the anterior or posterior fascia that is large enough to easily bring even a bulky stoma out through the abdominal wall, while the fascial defect remains well covered with sufficient overlap. The ostomy can be brought out through the lateral abdominal wall if necessary. It is not imperative any longer that the bowel be brought out through the rectus muscle. The rationale for applying the stoma through the rectus muscle is to avoid stomal prolapse and PSH formation, which are in any case hardly influenced. Both can essentially be decreased using a 3-D funnel mesh. Wound complications such as hematoma and infections might potentially be decreased, because the abdominal wall layers need not be separated. The implant can be easily and quickly placed. By using a second flat mesh, a preexisting midline incision can be well covered to treat concomitant incisional hernias or avoid their occurrence. The positive effect on prolapse prevention arises from the dome of the mesh, which is directed toward the abdominal cavity and fits tightly to the bowel.13
Our technique ensures that in cases of large PSHs, large hernia sacs can easily be excised using the localized approach at the stoma site to prevent seroma formation, which might always be a problem in laparoscopic hernia repair without hernia sac excision and without fascial closure. The fascial defect can be dissected and mobilized before narrowing it for partial closure, who prevents seroma formation and also provides a better locating surface (“landing plane”) for the underlying flat part of the 3-D mesh. Moreover, safe dissection in cases of parastomal dense bowel adherence and scarring is much easier to provide using a localized approach under direct visual and digital control with spare cut-out of the stoma and its preliminary closure. Purely laparoscopic dissection all around the stoma can be difficult because of adhesions, scarring, bowel adherence with poor overview, and insufficient accessibility around the stoma. Purely laparoscopic dissection of a large hernia sac and the reduction of its intestinal content without damaging the stoma bowel and its blood supply are also very challenging. These facts might theoretically argue for our technique, because a hybrid method facilitates reduction and dissection endogenously and from the outside. However, a concern with our technique is the potential risk for stoma-associated complications due to reostomy creation. Parastomal skin irritation, wound infections, bleeding, stenosis, necrosis, and stoma retraction can rarely occur. Mesh infections due to simultaneous colonic resections did not occur and should not be dreaded.23–25There is published evidence available that does not support the use of biologic grafts.26
The original funnel implant, with its seamless transition into the intestinal cuff, offers superb elasticity and flexibility at preparation of the stoma plasty. It may lead to a renaissance in broad fields of application such as equilateral stoma relocation or contralateral translocation, which were broadly abandoned because of very high local PSH recurrence rates, impairment of a further quadrant, the need for laparotomy, and the risk for occurrence of midline incisional hernias or hernias at the stoma site.5,27
In conclusion, we are convinced that PSH repair in the laparoscopic single-port technique using synthetic, preshaped, 3-D funnel implants and reostomy is not only feasible and safe but offers great advantages of practice under various elective and emergent conditions compared with traditional techniques. This is a hybrid method that combines the benefits of the laparoscopic and open techniques and the capabilities of a modular, flexible, and rotatable single-port system as well as the features of an elastic, preshaped, 3-D funnel mesh, with its seamless transition into the intestinal cuff for stoma size protection. The frequently coexisting stoma prolapse can sufficiently be removed by relocation of the shortened bowel, and prolapse reoccurrence can effectively be prevented by the tightly fitting dome of the 3-D mesh. The technique can easily be combined with an additional flat mesh repair of the median abdominal wall. Further randomized controlled trials are warranted for comparison of flat meshes and 3-D devices in traditional laparoscopic and single-port PSH repair.
Contributor Information
Gernot Köhler, Department of General and Visceral Surgery, Sisters of Charity Hospital, Linz, Austria; Academic Teaching Hospital of the Medical Universities Graz and Innsbruck.
Klaus Emmanuel, Department of General and Visceral Surgery, Sisters of Charity Hospital, Linz, Austria; Academic Teaching Hospital of the Medical Universities Graz and Innsbruck.
Rudolf Schrittwieser, Department of General Surgery, LKH, Bruck an der Mur, Austria..
References:
- 1. Hotouras A, Murphy J, Thaha M, Chan CL. The persistent challenge of parastomal herniation: a review of the literature and future developments. Colorectal Dis. 2013;15(5):e202–e214 [DOI] [PubMed] [Google Scholar]
- 2. Hansson BM, Slater NJ, van der Velden AS, et al. Surgical techniques for parastomal hernia repair: a systematic review of the literature. Ann Surg. 2012;255(4):685–695 [DOI] [PubMed] [Google Scholar]
- 3. Helgstrand F, Rosenberg J, Kehlet H, Jorgensen LN, Wara P, Bisgaard T. Risk of morbidity, mortality, and recurrence after parastomal hernia repair: a nationwide study. Dis Colon Rectum. 2013;56(11):1265–1272 [DOI] [PubMed] [Google Scholar]
- 4. Martin L, Foster G. Parastomal hernia. Ann R Coll Surg Engl. 1996;78(2):81–84 [PMC free article] [PubMed] [Google Scholar]
- 5. LeBlanc KA, Bellanger DE, Whitaker JM, Hausmann MG. Laparoscopic parastomal hernia repair. Hernia. 2005;9(2):140–144 [DOI] [PubMed] [Google Scholar]
- 6. Halabi WJ, Jafari MD, Carmichael JC, et al. Laparoscopic versus open repair of parastomal hernias: an ACS-NSQIP analysis of short-term outcomes. Surg Endosc. 2013;27(11):4067–4072 [DOI] [PubMed] [Google Scholar]
- 7. Hansson BM, Bleichrodt RP, de Hingh ICH. Laparoscopic parastomal hernia repair using a keyhole technique results in a high recurrence rate. Surg Endosc. 2009;23(7):1456–1459 [DOI] [PubMed] [Google Scholar]
- 8. Berger D. Laparoscopic repair of parastomal hernia [article in German]. Chirurg. 2010;81(11):988–992 [DOI] [PubMed] [Google Scholar]
- 9. Muysoms EE, Hauters PJ, Van Nieuwenhove Y, Huten N, Claeys DA. Laparoscopic repair of parastomal hernias: a multi-centre retrospective review and shift in technique. Acta Chir Belg. 2008;108(4):400–404 [DOI] [PubMed] [Google Scholar]
- 10. Hansson BM, Morales-Conde S, Mussack T, Valdes J, Muysoms FE, Bleichrodt RP. The laparoscopic modified Sugarbaker technique is safe and has a low recurrence rate: a multicenter cohort study. Surg Endosc. 2013;27(2):494–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Berger D, Bientzle M. Laparoscopic repair of parastomal hernias: a single surgeon's experience in 66 patients. Dis Colon Rectum. 2007;50(10):1668–1673 [DOI] [PubMed] [Google Scholar]
- 12. Tam KW, Wei PL, Kuo LJ, Wu CH. Systematic review of the use of a mesh to prevent parastomal hernia. World J Surg. 2010;34(11):2723–2729 [DOI] [PubMed] [Google Scholar]
- 13. Berger D. Prevention of parastomal hernias by prophylactic use of a specially designed intraperitoneal onlay mesh (Dynamesh IPST). Hernia. 2008;12(3):243–246 [DOI] [PubMed] [Google Scholar]
- 14. Moreno-Matias J, Serra-Aracil X, Darnell-Martin A. The prevalence of parastomal hernia after formation of an end colostomy. A new clinico-radiological classification. Colorectal Dis. 2009;11(2):173–177 [DOI] [PubMed] [Google Scholar]
- 15. Smietański M, Szczepkowski M, Alexandre JA. et al. European Hernia Society classification of parastomal hernias. Hernia. 2014;18(1):1–6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Muysoms FE, Miserez M, Berrevoet F, et al. Classification of primary and incisional abdominal wall hernias. Hernia. 2009;13(4):407–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Stechemesser B, Jacob DA, Schug-Paß C, Köckerling F. Herniamed: an internet-based registry for outcome research in hernia surgery. Hernia. 2012;16(3):269–276 [DOI] [PubMed] [Google Scholar]
- 18. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240(2):205–213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gill IS, Advincula AP, Aron M, et al. Consensus statement of the consortium for laparoendoscopic single-site surgery. Surg Endosc. 2010;24(4):762–768 [DOI] [PubMed] [Google Scholar]
- 20. Tran H. Safety and efficacy of laparoendoscopic single-site surgery for abdominal wall hernias. JSLS. 2012;16(2):242–249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Klinge U, Klosterhalfen B, Ottinger AP, Junge K, Schumpelick V. PVDF as a new polymer for the construction of surgical meshes. Biomaterials. 2002;23(16):3487–3493 [DOI] [PubMed] [Google Scholar]
- 22. Berger D, Bientzle M. Polyvinylidene fluoride: a suitable mesh material for laparoscopic incisional and parastomal hernia repair! A prospective, observational study with 344 patients. Hernia. 2009;13(2):167–172 [DOI] [PubMed] [Google Scholar]
- 23. Jänes A, Cengiz Y, Israelsson LA. Randomized clinical trial of the use of a prosthetic mesh to prevent parastomal hernia. Br J Surg. 2004;91(3):280–282 [DOI] [PubMed] [Google Scholar]
- 24. Jänes A, Cengiz Y, Israelsson LA. Experiences with a prophylactic mesh in 93 consecutive ostomies. World J Surg. 2010;34(7):1637–1640 [DOI] [PubMed] [Google Scholar]
- 25. Serra-Aracil X, Bombardo-Junca J, Moreno-Matias J, et al. Randomized, controlled, prospective trial of the use of a mesh to prevent parastomal hernia. Ann Surg. 2009;249(4):583–587 [DOI] [PubMed] [Google Scholar]
- 26. Slater NJ, Hansson BM, Buyne OR, Hendriks T, Bleichrodt RP. Repair of parastomal hernias with biologic grafts: a systematic review. J Gastrointest Surg. 201;15(7):1252–1258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Israelsson LA. Parastomal hernias. Surg Clin North Am. 2008;88(1):113–125 [DOI] [PubMed] [Google Scholar]