Abstract
Energy balance is regulated by circulating leptin concentrations and hypothalamic leptin receptor (ObRb) signaling via STAT3 but inhibited by SOCS3 & PTP1B. Leptin signaling enhances anorexigenic neuropeptides and receptor (POMC, MC3-R, MC4-R) activation while suppressing orexigenic neuropeptides (NPY, AgRP). We investigated in a sex-specific manner the early (PN2) and late (PN21) postnatal hypothalamic mechanisms in response to intrauterine (IUGR), postnatal (PNGR) and combined (IPGR) calorie and growth restriction. At PN2, while both male and female IUGR were hypoleptinemic, hypothalamic leptin signaling in females was activated as seen by enhanced STAT3. In addition, increased SOCS3 and PTP1B supported early initiation of leptin resistance in females that led to elevated AgRP but diminished MC3-R and MC4-R. In contrast, males demonstrated leptin sensitivity seen as a reduction in PTP1B and MC3-R and MC4-R with no effect on neuropeptide expression. At PN21 with adequate postnatal caloric intake, a sex-specific dichotomy in leptin concentrations was seen in IUGR with euleptinemia in males indicative of persisting leptin sensitivity and hyperleptinemia in females consistent with leptin resistance, both with normal hypothalamic ObRb signaling, neuropeptides and energy balance. In contrast, superimposition of PNGR upon IUGR (IPGR) led to diminished leptin concentrations with enhanced PTP1B and an imbalance in arcuate nuclear NPY/AgRP and POMC expression that favored exponential hyperphagia and diminished energy expenditure post-weaning. We conclude that IUGR results in sex-specific leptin resistance mainly observed in females while PNGR and IPGR abolish this sex-specificity setting the stage for acquiring obesity after weaning.
Keywords: Neuropeptide Y, Proopiomelanocortin, AgRP, metabolic programming, hyperphagia, obesity, energy expenditure
Introduction
Epidemiological investigations have linked postnatal exponential growth superimposed on low birth weight with adult onset chronic diseases that include obesity, type 2 diabetes mellitus, cardiovascular disease, stroke and cancers (Barker et al. 2005; Burdge et al. 2009; Evensen et al. 2009; Gluckman et al. 2009; Morrison et al. 2010; Zigman and Elmquist 2003). More recently, investigations have also demonstrated that slow growth in early childhood followed by exponential growth during late childhood could have a similar effect of acquiring adult onset diseases (Bhargava et al. 2004; Godfrey et al. 2010; Horton 2005). To determine the mechanisms responsible for this phenomenon, various investigators have engaged different animal models ranging from exposure to a low protein diet or total calorie restriction and bilateral uterine artery ligation (Bhasin et al. 2009; Lane et al. 2001; Thamotharan et al. 2005). While many changes have been described in various tissues, the key inciting factor responsible for the cascade of events that results in change during the entire life span has remained elusive. We have previously demonstrated that hypothalamic changes are encountered as early as in-utero, setting the stage for a series of postnatal events that perhaps culminate in the eventual adult phenotype (Rajakumar et al. 1998; Singh et al. 1997). In addition, we have observed that manipulation of the hypothalamus during the critical window of postnatal development, namely the suckling phase in rats, imposes long lasting sex-specific effects on the adult phenotype, a process known as developmental programming (Varma et al. 2004). Various other groups using differing animal models of intra-uterine growth restriction have demonstrated an imbalance in the hypothalamic orexigenic and anorexigenic neuropeptides separately in males and females (Breton et al. 2009; Chang et al. 2008; Cottrell et al. 2009; Delahaye et al. 2008; Luquet et al. 2005; Stevens et al.; Torrens et al. 2009), although the translation of these changes into phenotypic expression have been incomplete. Based on our previous observations and those of others, we hypothesized that prenatal and/or postnatal total calorie restriction associated with growth restriction will alter the hypothalamic leptin receptor signaling and neuropeptide balance in a sex-specific manner, thereby differentially regulating energy intake and expenditure, which collectively predetermine the ultimate phenotype. To test this hypothesis, we employed our previously characterized total calorie restriction rat model (Thamotharan et al. 2005) in which four experimental groups are generated by imposing postnatal cross fostering thereby creating controls, intra-uterine growth restriction (IUGR), postnatal growth restriction (PNGR) and the combined intra-uterine and postnatal growth restriction (IPGR) groups (table 1A). We observed early life adaptive changes which include circulating leptin and hypothalamic leptin receptor signaling perturbations with an imbalance of key hypothalamic neuropeptides that regulate the net energy balance and body weight with sex specificity. Mechanistically, differential DNA methylation of CpG islands in promoter regions of specific neuropeptides that were perturbed was not involved.
Table 1.
Experimental Groups: The four experimental groups are depicted at PN21 (male=M. female=F). Table 1.
| Experimental Group | Prenatal (e11-e21) | Postnatal (PN1–PN21) |
|---|---|---|
| CON (PN2andPN21) M+F | ad libitum (100%) | ad libitum (100%) |
| IUGR (PN2 and PN21) M+F | calroie restriction (50%) | ad libitum (100%) |
| IPGR (PN21) M+F | calroie restriction (50%) | calroie restriction (50%) |
| PNGR (PN21) M+F | ad libitum (100%) | calroie restriction (50%) |
Materials and Methods
Animals
Sprague-Dawley rats (Charles River Laboratories, Hollister, CA) were housed in individual cages in 12h light/dark cycles at 21–23°C, and allowed ad libitum access to standard rat chow (composition: carbohydrate 63.9%, fat 4% and protein 14.5%). The National Institutes of Health guidelines were followed as approved by the Animal Research Committee of the University of California, Los Angeles.
Maternal calorie restriction model
Pregnant rats received 50% of their daily food intake (~11g/day) beginning from day 11 through day 21 of gestation, which constitutes mid- to late gestation, compared with their control counterparts that received ad libitum access to rat chow (~20g/day) (Thamotharan et al. 2005). Both groups had ad libitum access to drinking water. At birth, the litter size was culled to six to ensure no inter-litter nutritional variability. Postnatally the cross-fostering of animals generated four experimental groups as previously described (Thamotharan et al. 2005). The newborn pups born to ad libitum feeding control mothers were reared by either mothers on semi-calorie restriction from PN1-PN21 (PNGR- ad libitum in prenatal and 50% calorie restriction in post natal periods) or by control mothers (CON-ad libitum in both pre- and post-natal periods). During the suckling phase, the intrauterine semi-calorie restricted progeny was fed either by control mothers with ad libitum access to foods (IUGR-50% calorie restriction in prenatal and ad libitum in post natal periods) representing intrauterine calorie restriction alone, or by semi-calorie restricted mothers (IPGR-50% calorie restriction in both pre- and post-natal periods) representing a combination of intrauterine and postnatal calorie restriction. After weaning from the mother, all animal groups had ad libitum access to food and water.
Calorie intake
a. Milk intake
Milk intake by rat pups from the four experimental groups was assessed as previously described (Fukushima et al. 2006). Briefly, pups were separated from mothers at 9 am, and maintained for 5 hours at 27±2°C on a temperature controlled heating pad with humidity of 30±2%. The pups were then returned to mothers’ cages for 1 hr, and subsequently milk intake was assessed in individual suckling pups by measuring body weight before and after this suckling phase at both PN7 and PN14.
b. Food and water intake
Immediately after weaning from mothers on day 22 of age, food intake was longitudinally assessed over a 24 hour period by weighing the rat chow that was placed in the food container before and remaining after the study period. Attention was paid to spilled food and evaporative losses. Similarly water intake was also longitudinally assessed over a 24 hour period by measuring the volume of water placed in the water container before and remaining after the study period. Evaporative losses were assessed by placing a water and food container attached to a cage with no animal, the differences assessed and subtracted from the final figures of food and water intake. All these measurements were performed first thing in the morning soon after the maximal ingestive phase.
Energy Expenditure and Respiratory Quotient Measurements
An indirect open circuit calorimeter (Oxymax, Columbus Instruments, Columbus, OH) was employed for these measurements. Briefly male and female rats were weighed and placed individually in sealed chambers of the calorimeter. After an optimal acclimation period, the calorimeter was calibrated and air flow rate adjusted according to animal weight. The sampling times between chambers were set to perform measurements every six minutes over one hour during the dark cycle. Energy expenditure and respiratory quotient values were generated using the Oxymax Lab Animal Monitoring system and computer software. Data was analyzed using the Oxymax Win v4.0 software package. A mean of 6–8 determined values per rat were averaged for the final value. Analysis of oxidation mixtures of carbohydrate and fat was derived using the table of Lusk (Peronnet and Massicotte 1991).
Plasma Assays
Plasma was separated and aliquots stored for measuring leptin. Leptin was quantified by the enzyme linked immuno-absorbent assay (ELISA) using rat standards and anti-rat leptin antibody (Linco Research Inc., St. Charles, Mo.; leptin sensitivity = 0.1 ng/ml).
Leptin administration
Intracerebroventricular (ICV) injections were performed with the use of conventional stereotaxic coordinates for the lateral ventricle, as previously described (Varma et al. 2004). The coordinates were set at 0.4 mm lateral to the midline, 0.4 mm posterior to the bregma, and 2.5 mm ventral to the skull surface. Individual pups in each litter were weighed daily between 8:00 AM and 10:00 AM. The litters of control pups (n=160 pups from 16 litters) alone were arbitrarily divided into two major groups. One received ICV murine leptin (1 μg.2.5 μl−1 dose−1; Linco Research, Inc., St. Charles, MI) daily between PN2-PN7 days of age (n=70 pups), and the second group received 2.5 μl of vehicle (n=90 pups) under mild restraint. The dose of leptin given during the torpor-like state of energy conservation in the morning translated into an age-dependent stepwise decrease with an initial dose of 0.16 μg· body wt. (BW)−1· dose−1 on day 2, 0.14 μg·g BW−1· dose−1 on day 3, 0.12 μg·g BW−1· dose−1 on day 4, 0.10 μg·g BW−1·dose−1 on day 5, 0.12 μg·g BW−1·dose−1 on day 6, and 0.08 μg·g BW−1·dose−1 on day 7. Leptin doses and route of administration were chosen on the basis of our preliminary experiments, which revealed that daily (PN2-PN7 days of age) ICV doses ranging from 0.05 to 1 μg·g BW−1·dose−1 led to a dose-dependent diminutive effect on the postnatal body weight gain pattern. The PN2-PN7 days of age were chosen for ICV leptin administration because this postnatal period was optimally responsive to leptin, as determined previously (Varma et al. 2004).
Immunoblotting for Hypothalamic signaling molecules
Hypothalamus separated microscopically from whole brain using established anatomical landmarks (Paxinos and Watson 1998) was homogenized either in PBS containing protease inhibitors (20 μg/ml pepstatin A, 20 μg/ml leupeptin, 30 μg/ml aprotinin and 2 mM PMSF), 1% Nonidet P-40 and 5 mM EDTA as previously described (Ogihara et al. 1997; Shin et al. 2004) or in cell lysis buffer (Cell Signaling Technology, Danvers, MA). Protein content was measured by the Bio-Rad Protein Assay (Bio-Rad, Hercules, CA). Homogenates (30 μg of protein) were subjected to sodium dodecyl sulfate (SDS) polyacrylamide gel electrophoresis and the separated proteins transferred to nitrocellulose membrane filters (Bio-Rad, Hercules, CA). The blotted membranes were sequentially incubated in 3% bovine serum albumin or 5% nonfat dry milk and the primary antibody consisting of the rabbit anti-STAT3, -SOCS3 (Cell Signaling Technology, Danvers, MA), -PTP1B IgG (BD Bioscience, San Jose, CA) and mouse anti-vinculin (internal loading control; Sigma, St. Louis, MO). The proteins were visualized in Typhoon 9410 Phosphorimager (GE Healthcare Biosciences, Piscataway, NJ) by blotting with the enhanced chemiluminescence (ECL) plus detection kit (GE Healthcare BioSciences Corp., Piscataway, NJ) following horseradish peroxidase-labeled anti-rabbit IgG for anti-STAT3 and -SOCS3 or anti-mouse IgG for anti-PTP1B and anti-vinculin (GE Healthcare Biosciences Corp., Piscataway, NJ). Each protein was quantified by using Image Quant 5.2 software (GE Healthcare Biosciences, Piscataway, NJ) and normalized to vinculin.
mRNA quantification by quantitative real-time polymerase chain reaction
Hypothalamic total RNA was extracted using RNAeasy lipid tissue kit (Qiagen, Valencia, CA). First strand cDNA was synthesized from 1 μg of DNase treated total RNA using Superscript II reverse transcriptase (Invitrogen Life Technologies, Carlsbad, CA), as previously described (Thamotharan et al. 2005).
Quantitative real time PCR was performed as previously described (Thamotharan et al. 2005). Primers and Taqman probes for detection of specific neuropeptide genes were designed using Primer Express Software (Applied Biosystems, Foster, CA) and are listed in table 3. These designed forward and reverse primers generate corresponding DNA fragments after amplification. Taqman probes were synthesized and labeled with fluorescent dye, 6-carboxyfluorescein (FAM) on the 5′-end and N,N,N′,N′-tetramethyl-6-carboxyrhodamine (TAMRA) on the 3′-end (Applied Biosystems, Foster CA). Taqman PCR was carried out using a StepOnePlus™ real-time PCR system or an ABI prism 7700 sequence detector (Applied Biosystems, Foster, CA). Real time PCR quantification was then performed using Taqman glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or eukaryotic 18S rRNA (Applied Biosystems, Foster, CA) as internal controls. PCR amplifications were performed in triplicates. The amplification cycles consisted of 12 min at 95°C (hot start), followed by 40 cycles at 95°C for 30 sec (denaturation), <56°C for ObRb and MC3-R; 58°C for NPY and MC4-R; 60°C for AgRP and POMC over 30 sec (annealing)>, and 72°C for 30 sec (extension), using reagents from Applied Biosystems (Foster, CA). Relative quantification of PCR products were based on value differences between the target and GAPDH or 18S rRNA control using the comparative CT method, as previously described (Thamotharan et al. 2005).
Table 3.
Target Genes for Real Time RT-PCR. Primers and probes of target genes are shown with expected sizes of amplification products. F; forward primer, R; reverse primer, P; probe
| Target Gene (Gene Bank Accession No.) | Sequence | PCR product size (bp) |
|---|---|---|
| NPY (M20373) | F: 5′ aatctcatcaccagacagagatatgg 3′ R: 5′ cattttctgtgctttctctcattaaga 3′ P: 5′ aagagatccagccctgagacactgatttca 3′ |
90 |
| Ob-Rb (D84550) | F: 5′ acttaatttccaaaagcctgaaaca 3′ R: 5′ ccagaagaagaggaccaaatatcac 3′ P: 5′ ttgagcatctttttaccaagcatgcagaatc3′ |
85 |
| AgRP (AF206017) | F: 5′ gcagaggtgctagatccacagaa 3′ R: 5′aggactcgtgcagccttacac 3′ P: 5′ cgagtctcgttctccgcgtcg 3′ |
72 |
| MCR-3 (X70667) | F: 5′ tatccgacgctgcctaacct 3′ R; 5′ ttgatgaaaacctgctcgca 3′ P: 5′ agcaaccggagtggcagtg 3′ |
94 |
| MCR-4 (U67863) | F: 5′ acccaccaccatggcatgta 3′ R: 5′cttccccagagactcgctggca 3′ P: 5′ cctccacctctggaaccgcagc 3′ |
92 |
| POMC (AH002232) | F: 5′ atagacgtgtggagctggtgc 3′ R: 5′ gcaagccagcaggttgct 3′ P: 5′ cagccagtgccaggacctcaccac 3′ |
80 |
In-situ hybridization histochemistry
cRNA probes
Rat NPY cDNA (+49 to +395, Gene bank accession number M2037), AgRP cDNA (+6 to +212, Gene bank accession number AF206017) and POMC cDNA (+61 to +540, Gene bank accession number AH002232) were generated by RT-PCR, using the first strand cDNA synthesized from 1 μg of DNase pre-treated rat hypothalamic RNA, as previously described (Thamotharan et al. 2005). Primers used for NPY were 5′-cggccgtctagaatgatgctaggtaacaa-3′ (forward) and 5′-gcgcgcgcatgcccacatggaagggtctt-3′ (reverse), for AgRP were 5′-gggcgccgagctccatttcccagagttctcaggtcta-3′ (forward) and 5′-cgcgcctctagagcggcagtagcacgtcttgaagaa3′ (reverse), and for POMC were 5′-cggccgtctagatccatagacgtgtggagctgg-3′ (forward) and 5′-gggcccgcatgccggctccaggacttgctccaa-3′ (reverse), PCR products were subcloned in pGEM-3Zf(+) vector with Xba I and Sph I sites for NPY and POMC, and with Sac I and Xba I sites for AgRP. Antisense RNA probes were prepared by in vitro transcription from the fragment of cDNA digested with Bam HI using Sp6 RNA polymerase, in the presence of 350 mM digoxigenin [DIG]-linked UTP in a 20 μl reaction mixture (Roche Applied Science, Penzberg, Germany), as previously described (Shin et al. 1998). A sense probe was similarly generated with T7 RNA polymerase from the Bam HI digested cDNA fragments.
Hypothalamic tissue preparation
Male and female animals were anesthetized with intraperitoneal injection of sodium pentobarbital (50mg/kg) and were perfused through the aorta with an isotonic sodium chloride solution to remove blood, followed by 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4 as previously described (Shin et al. 1998; Varma et al. 2004). Brains were removed from the cranium, washed with phosphate-buffered saline, pH 7.4, dehydrated through graded alcohols and embedded in paraplast wax. Serial sections (10 μM thickness) were cut, mounted on poly-L-lysine coated slides, and stored at room temperature until use.
In situ hybridization
In situ hybridization was performed as previously described (Shin et al. 1998). Briefly, after de-waxing and rehydration, tissue sections were digested with 5 μg/ml proteinase K at room temperature for 20 min, then fixed in 0.4% paraformaldehyde at 4°C for 10 min and incubated overnight at 55°C in a hybridization solution containing digoxigenin-labeled riboprobes (for NPY, AgRP or POMC cDNA), 50% formamide, 10 mM Tris-HCl, pH 7.5, 300 mM NaCl, 1 mM EDTA, 0.25% SDS, 1 X Denhardt’s solution, 200 μg/ml yeast tRNA, and 10% dextran sulfate. Following hybridization, tissue sections were washed in 2 X SSC, 50% formamide at 58°C for 30 min, incubated with 1 μg/ml RNase A solution at 37°C for 30 min, and washed once in 2 X SSC and twice in 0.2 X SSC at 50°C for 20 min each. Following hybridization, sections with DIG-labeled NPY, AgRP or POMC riboprobes were incubated with polyclonal sheep anti-DIG Fab antibody conjugated with alkaline phosphatase (Roche Applied Science, Penzberg, Germany). Detection of the label was accomplished with NBT (nitroblue tetrazolium chloride) and BCIP (5-bromo-4-chloro-3-indolylphosphate) (Roche Applied Science, Penzberg, Germany). Color development was carried out at room temperature for 2h and then the sections were washed in TE buffer (10 mM Tris-HCl, 1 mM EDTA, pH 7.5). Slides were mounted with a mixture containing 24% polyvinylalcohol, 12% glycerol and 59 mM Tris-HCl (pH 7.5) (Shin et al. 1998). Controls consisted of the corresponding sense RNA probes employed in serial sections and were noted to completely abolish the final immunoreactivity.
Quantification
Color images captured by a Nikon E-600 microscope (Nikon, Melville, NY) equipped with a cooled charge coupled device (CCD) camera (Cool SNAP HQ Monochrome, Roper Scientific, Tucson, AZ) were analyzed by the Metamorph image analysis system (Molecular Devices, CA) using integrated optical density (OD) as previously described (Kimura et al. 2004; Niswender et al. 2003). Briefly, color images attained by NBT/BCIP staining were observed in bright field. Images were acquired at 12-bit gray level resolution and displayed in pseudocolor (monochrome) for analysis, with NPY, AgRP and POMC represented by dark brown or black color (NBT/BCIP). The Metamorph image analysis computer software program was used to measure integrated optical density for expression or count numbers of NPY, AgRP and POMC neurons. Sampling was done bilaterally in the arcuate nuclei with a 10 and 20 X objective. Results were standardized to measured optical density using threshold images of color development by corresponding sense RNA probes in a microscopic field of the arcuate nuclei. Arcuate nuclear regions by first highlighting the labeled cells were measured using a Metamorph analysis program in the control animal group for NPY, AgRP and POMC image channel, and then integrated grains were counted corresponding to the same regions from bright-field images in the calorie restriction group using an image transfer and expressed as grains per arcuate region.
Hypothalamic NPY, AgRP and POMC gene promoter DNA methylation
To explore the presence of differential DNA methylation of key hypothalamic neuropeptides, genomic DNA was extracted and CpG islands present within the promoter regions subjected to bisulfite modification. DNA methylation was quantified by pyrosequencing. Pyrosequencing for allele quantification (PSQ H96A, Biotage, Sweden) is a real-time sequencing-based DNA analysis software that quantifies multiple and individual consecutive CpG sites. Briefly 1000 ng of sample DNA was bisulfite treated using the Zymo DNA Methylation Kit (Zymo research, Orange, CA). Bisulfite treated DNA was eluted in10 μl volume and 1 μl of it was used for each PCR. PCR was performed using 10 X PCR buffer, 3.0 mM MgCl2, 200 μM of each dNTP, 0.2 μM each of forward and reverse primers, 1.25 U of Hot Start DNA polymerase (Qiagen Inc.), and ~10 ng of bisulfite converted DNA per 50 μl reaction. PCR cycling conditions were: 94°C over 15 min; 45 cycles of 94°C for 30 s; 53°C over 30 s; 72°C for 30 s; 72°C over 5 min; and the amplification products were maintained at 4°C.
PCR was performed with one of the PCR primers biotinylated to convert the PCR product to single-stranded DNA templates. The PCR products (each 10 μl) were sequenced by the Pyrosequencing PSQ96 HS System (Biotage AB) following the manufacturer’s instructions (Biotage, Kungsgatan, Sweden). The methylation status of each locus was analyzed individually as T/C SNP using QCpG software (Biotage, Kungsgatan, Sweden).
Data Analysis
Data is presented as Mean±SE. Pair-wise comparisons were performed when only two groups were compared using the unpaired Student’s t-test. The null hypothesis of equal means was rejected at a p<0.05 level of significance. When all four experimental groups were compared, following establishment of normality, ANOVA was employed and F values determined. Once significance was established, inter-group differences were validated by the Fisher’s paired least significance difference test and significance assigned when p values were less than 0.05.
Results
Body weights
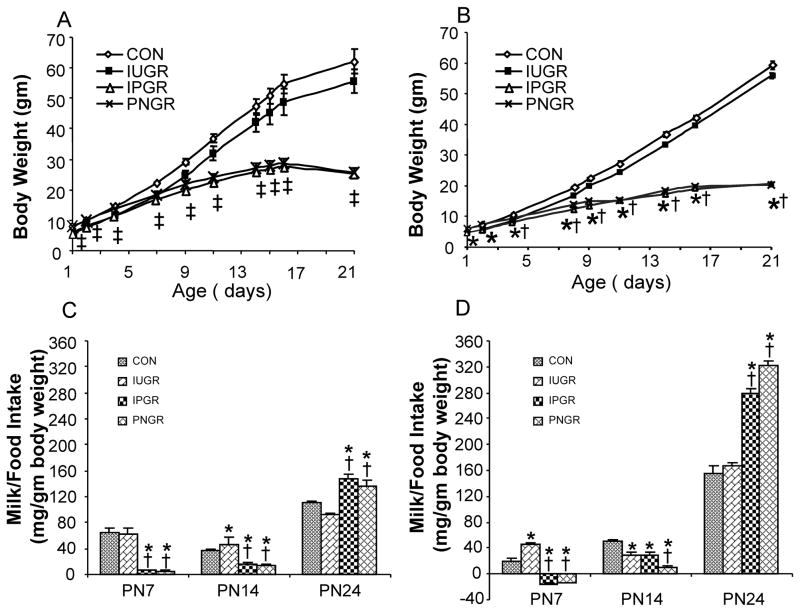
The experimental groups are shown in table 1 demonstrating the PN1-2 control and IUGR groups (Thamotharan et al. 2005). These two groups were further split into four groups namely the CON pups were reared as CON or PNGR and the IUGR pups were reared either as IUGR or IPGR. Body weights during the suckling phase are depicted in figure 1A (male) and figure 1B (female) in a sex-specific manner. While both male and female pups in the IUGR group exposed to ad libitum milk intake from a CON mother demonstrated “catch-up” growth to that of the CON counterpart as early as PN7-8, the males and females, thereafter exhibit body weights lower than the age-matched and sex-matched CON, although not statistically different. In contrast, the PNGR and IPGR exhibit persistent growth restriction becoming evident by PN8-9 in the suckling phase in males and females.
Figure 1.
Growth curves of male (A) and female (B) pups from the four experimental groups: CON, IUGR, IPGR and PNGR from birth to PN 21 are shown in the upper panels. N= 6 for male and n=18 for female per each group. In males, p< 0.02 compared with CON (*) or IUGR (†). In female, p<0.003 compared with CON (*) or IUGR (†). ‡ represents the combination of (*) and (†). Calorie intake: i.e. milk intake is shown at PN7 and PN14 (N=6 each) and food intake in mg/gm body weight per day is shown at PN24 (N=12 to 18 each) in males (C) and females (D) in the lower panels. In male, p<0.04 compared with CON (*) or IUGR (†). In female, p< 0.001 compared with CON (*) or IUGR (†).
Suckling phase – Energy balance
Milk intake
This growth pattern reflects milk intake which peaks at PN7 and PN14 in male (Fig. 1C) and at PN7 alone in female (Fig. 1D) IUGR groups. In contrast, the IPGR and PNGR groups demonstrate uniformly diminished milk intake that reaches a nadir at PN7 partially recovering but still remaining low at PN14 in both males (Fig. 1C) and females (Fig. 1D). Thus, early calorie intake, particularly during the postnatal phase of development, has a critical impact on the growth pattern and ultimate body weight.
Post-weaned Energy balance
Food intake
Post-weaning, food intake (mg/gm body weight/day) in males was higher in PNGR and IPGR at PN24 versus CON and IUGR (Fig. 1C), being no different at PN60-PN120 of age (data not shown). In females, food intake (mg/gm body weight/day) was higher in PNGR and IPGR at PN24 versus CON (Fig. 1D). Water intake (ml/gm body weight/day) at PN24 in males and females mimicked the pattern of food intake in PNGR and IPGR versus that of CON and IUGR (data not shown).
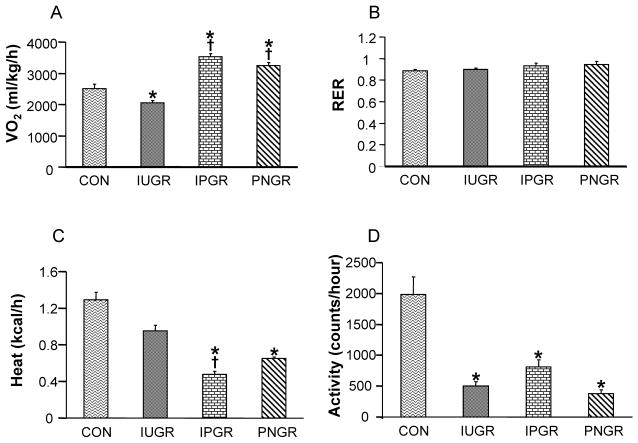
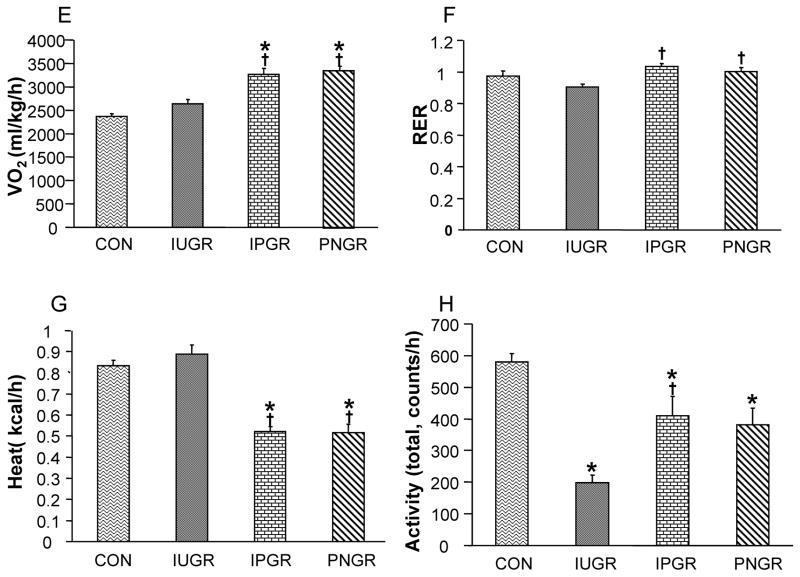
Energy Expenditure
O2 consumption (VO2), respiratory exchange rate (RER), heat production and locomotive activity of the four experimental groups in PN21 male (Fig. 2A–D) and PN21 female rats (Fig. 2E–H) are depicted. Both IPGR and PNGR demonstrated increased VO2 compared to CON in males (Fig. 2A) and females (Fig. 2E). RER was similar among the four groups in males (Fig. 2B), while IPGR and PNGR expressed an increase in females compared to the sex-matched IUGR. Heat production in IPGR and PNGR was decreased, compared to CON in males (Fig. 2C) and females (Fig. 2G). Total locomotive activity was decreased in IUGR, IPGR and PNGR in males (Fig. 2D) and females (Fig. 2H). The inter-group differences noted at PN21 were no longer evident at PN60 in both males and females (data not shown).
Figure 2.
O2 consumption (VO2) (A), respiratory exchange rate (RER) (B), heat production (C) and locomotor activity (D) are shown in the four experimental groups at 21 days. Male (A–D) and female (E–H), N = 6 in each group. p<0.02 compared with CON (*), p<0.05 compared with IUGR (†).
Leptin concentrations
Circulating leptin concentrations at PN2 of age in males and females were significantly lower in IUGR versus the sex-matched and age-matched CON group (table 2a). While the leptin concentration in male IUGR at PN21 was similar to that of CON, in the female IUGR, circulating leptin concentration was almost twice that of CON females (table 2b). Leptin concentrations were undetectable in the IPGR and PNGR groups in both males and females (table 2b).
Table 2.
Circulating plasma leptin concentrations. (a) PN2; IUGR male pups, *p=0.05 (N=6, each for CON and IUGR), IUGR female pups, *p<0.04 (N=6, each for CON and IUGR), (b) PN21; Both IPGR and PNGR expressed negative values. Female IUGR, *p<0.002 versus CON.
| (a) | |||
|---|---|---|---|
| PN2 Male | Leptin (ng/ml) | PN2 Female | Leptin (ng/ml) |
| Control | 1.30 ± 0.55 | Control | 2.70 ± 0.44 |
| IUGR | 0.16 ± 0.06* | IUGR | 0.96 ± 0.54* |
| (b) | |||
|---|---|---|---|
| PN21 Male | Leptin (ng/ml) | PN21 Female | Leptin (ng/ml) |
| Control | 5.32 ±0.69 | Control | 5.28 ± 0.34 |
| IUGR | 5.10 ± 0.51 | IUGR | 9.63 ±1.25* |
| IPGR | undetectable | IPGR | undetectable |
| PNGR | undetectable | PNGR | undetectable |
Hypothalamic neuropeptides
We next examined the leptin-regulated hypothalamic neuropeptides that are responsible for central control of energy balance. Since, IUGR males and females at PN2 expressed low circulating leptin concentrations (table 2a) and the female IUGR developed high concentrations, while male and female IPGR and PNGR groups at PN21 developed undetectable concentrations of circulating leptin, it was important to determine the effects of these perturbed leptin concentrations on the hypothalamus at PN2 and PN21 in an age- and sex-specific manner. We investigated the effect on the hypothalamic leptin receptor (ObRb) and both sets of hypothalamic neuropeptides, namely the orexigenic NPY and AgRP peptides that enhance energy intake while suppressing energy expenditure thereby resulting in obesity, and the anorexigenic peptide POMC, a prohormone precursor of α-MSH and corticotropin, both of which mediate diminution in energy intake while promoting energy expenditure, thereby resulting in loss of body weight in adults (Ahima and Osei 2004). Melanocortin receptor isoforms (MC3-R & MC4-R) in turn mediate the action of α-MSH.
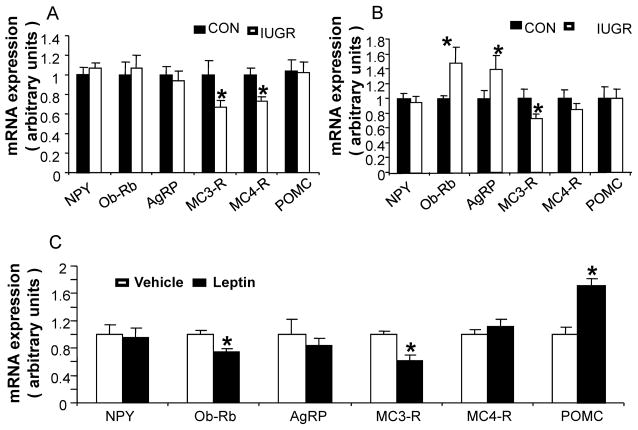
Table 3 shows target genes that were quantified by quantitative real time PCR using TaqMan probes with sequence-specific oligonucleotides. MC3-R and MC4-R were decreased versus CON (Fig. 3A) at PN2 of age in males, At PN2 of age in females, while no change in NPY, POMC and MC4-R mRNA concentrations were noted, an increase in ObRb and AgRP with a reduction in MC3-R mRNA was observed (Fig. 3B). Since there was an inter-group difference in circulating leptin concentrations, we next undertook exogenous leptin administration in CON female pups during the suckling phase. Intra-cerebroventricular administration of exogenous leptin from PN2 to PN7 revealed a reduction in hypothalamic ObRb and MC3-R, an increase in POMC with no change in NPY, AgRP and MC4-R mRNA in PN8 CON females when compared to the age-matched respective vehicle (VEH) treated group (Fig. 3C). Next, we examined the four experimental groups of CON, IUGR, PNGR and IPGR at PN21 of age. At PN21, the IPGR and PNGR males demonstrated a significant increase in NPY, Ob-Rb and AgRP expression, but a significant decrease in POMC expression (Fig. 3D). Similarly the PN21 old females also showed a significant increase in NPY, Ob-Rb, AgRP and MC4-R expression, but a significant reduction in POMC expression in IPGR and PNGR groups (Fig. 3E).
Figure 3.
Real-time quantitative RT-PCR analysis of hypothalamic NPY, Ob-Rb, AgRP, MC3-R, MC4-R and POMC expression. Control and IUGR pups at postnatal 2 day (A–B), and in saline and leptin treated female pups (C). A: Male, n=10 in both groups. *p<0.02 IUGR versus CON. B: Female, n=10 in both groups. *p<0.05, IUGR versus CON. C: Postnatal intracerebroventricular (ICV) leptin (LEP) and the respective vehicle (VEH) treatment. n = 15 pups for the LEP group, and n = 15 pups for the VEH group. *p<0.02, LEP versus VEH. D and E, Four experimental groups at postnatal 21 day; N=6 in each group. p<0.04 versus CON (*) or IUGR(†).
In situ hybridization
To specifically quantify the arcuate nuclear expression of NPY, AgRP and POMC genes because ARC is a key site for metabolic regulation in the hypothalamus (Coppari et al. 2005; Enriori et al. 2007), we undertook in-situ hybridization experiments for CON and IPGR in the ARC region of hypothalamic sections using DIG-labeled isoform specific RNA probes. At PN21 in IPGR males and females, an increase in arcuate nuclear NPY mRNA (Fig. 4A–D) and AgRP mRNA (Fig. 4E–H) was observed, while the expression of POMC mRNA was decreased in the arcuate nucleus of IPGR males and females (Fig. 4I–L). Quantification of NPY mRNA and AgRP mRNA by the Metamorph image analysis system using integrated optical density revealed a three- or ten-fold increase in male IPGR and a two- or three-fold increase in female IPGR (Fig. 4M) for each neuropeptide when compared to respective sex-matched CON. These changes could only partially be explained by the increase in NPY positive neuronal number, i.e. only a 1.7 fold increase in male IPGR and a 1.2 fold increase in female IPGR versus the respective CON (Fig. 4N). Especially in male, the expression of AgRP mRNA revealed a large increase with a concomitant increase reflected in the number of AgRP positive cells. In the case of POMC, quantification of POMC revealed a three-fold decrease in the male and female IPGR (Fig. 4M) with a concomitant 1.8 to 2-fold reduction in POMC positive cells (Fig. 4N). Thus the decrease in POMC mRNA is also perhaps related to a decrease in POMC expression per cell, albeit to a lesser extent than NPY mRNA.
Figure 4.
In situ hybridization histochemistry demonstrating arcuate nuclear NPY, AgRP and POMC gene expression in rat hypothalamus at postnatal day 21. A–D for NPY, E–H for AgRP and I–L for POMC. Bars: 100 μm. M, Integrated optical density of intense signals obtained with DIG-labeled antisense cRNA probes in both male and female. * p<0.002 for intergroup comparisons. N, NPY, AgRP and POMC positive-neuronal cells in both male and female. N=3 per group, 6 to 9 sections per each group. * p<0.002 for intergroup comparisons.
NPY, AgRP and POMC gene promoter DNA methylation in hypothalamus
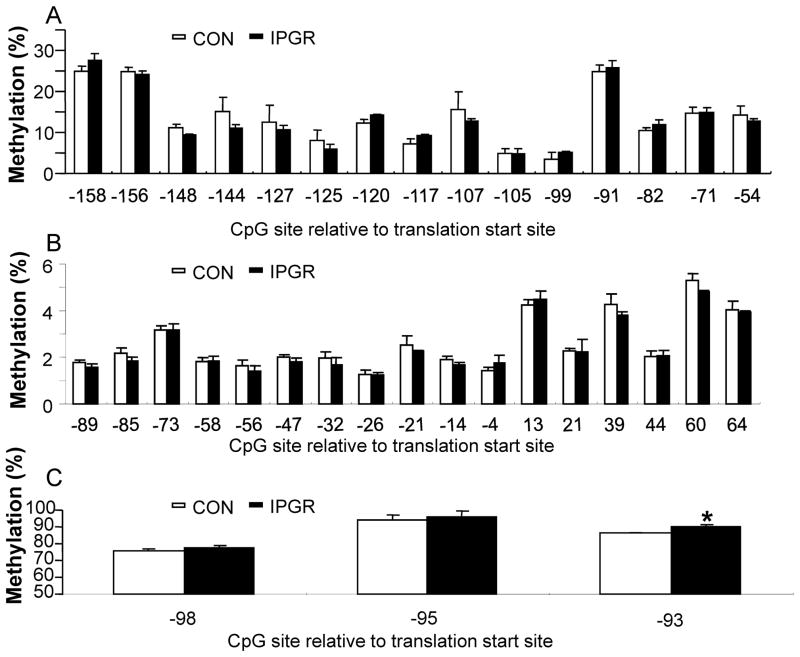
To investigate the mechanism by which circulating leptin concentrations regulate key hypothalamic neuropeptide gene expression particularly at PN21, we examined DNA methylation of the 5′-flanking region responsible for transcriptional regulation. Generally the level of DNA methylation of CpG islands found is close proximity to either the promoter or enhancer elements inversely correlates with transcription of the downstream gene expression. Bisulfite modification followed by DNA pyrosequencing was performed using genomic DNA extracted from PN21 male CON and IPGR hypothalami. Using gene specific primers, C to T conversion of CpG islands was assessed in POMC, NPY and AgRP genes which spanned 3–5 kb length of 5′-flanking regions (Fig. 5), CpG islands within the NPY and POMC gene promoters demonstrated no differential methylation between CON and IPGR (Fig. 5A and B). In contrast, the three CpG sites (in the absence of CpG islands) in the AgRP promoter region were differentially methylated between CON and IPGR, although the range of methylation varied from 75% to 96%.
Figure 5.
DNA methylation analysis of hypothalamic NPY, AgRP and POMC promoter regions in CON and IPGR. Genomic DNA from postnatal 21 day males was processed by pyrosequencing. A. POMC, B. NPY, C. AgRP. *p<0.04 for intergroup comparisons.
Hypothalamic leptin post-receptor or ObRb signaling molecules
We next examined the effect of perturbed circulating leptin concentrations on hypothalamic post-leptin receptor key signaling molecules in order to determine age- and sex-specific differences in leptin sensitivity versus resistance. Activation of STAT3 signifies leptin sensitivity while activation of SOCS3 inhibits leptin signaling(Sahu 2004). PTP1B on the other hand inhibits PI-3-kinase activation in response to both insulin and leptin receptor activation (Cheng et al. 2002; Cook and Unger 2002). Immunoblotting using antibodies against STAT3, SOCS3 and PTP1B related to leptin receptor signaling was performed using hypothalamic homogenates. At PN2 in males, while no change in hypothalamic STAT3 and SOCS3 protein concentrations was observed, a reduction in PTP1B was noted (Fig. 6A). In contrast, in age-matched females, an increase in hypothalamic STAT3, SOCS3 and PTP1B concentrations was observed (Fig. 6B). At PN21 of age, in both males (Fig. 6C) and females (Fig. 6D) while the changes in hypothalamic STAT3 that augments leptin signaling and SOCS3 that inhibits leptin signaling were no longer noted, an increase in PTP1B that inhibits leptin and insulin signaling was observed in IPGR and PNGR groups versus age-matched and sex-matched CON and IUGR.
Figure 6.
Western blot analysis of leptin post-receptor signaling molecules, PTP1B, STAT3 and SOCS3 proteins. A & B, postnatal day 2. C & D, postnatal day 21. upper panel: representative Western blots, lower panel: densitometric quantification of corresponding protein concentrations. A & C, male; B & D, female. N=7 in each group of A & B panels, *p<0.03 for intergroup comparisons. N=6 for each of the four groups in B & D panels. * p< 0.05 compared with CON (*), # p<0.05 compared with IUGR..
Discussion
The hypothalamus is a major site that integrates peripheral signals and central mechanisms underlying calorie intake, body weight regulation and energy homeostasis (Sahu 2004). In our present investigation we have systematically examined the sex specific effects of intra-uterine and postnatal calorie restriction on leptin-mediated hypothalamic mechanisms during the suckling phase of development. These mechanisms may predispose towards the subsequent post-weaned phenotype of hyperphagia and diminished energy expenditure.
Intra-uterine effects
Intra-uterine calorie restriction resulted in reduced circulating leptin concentrations both in males and females. However, examination of the hypothalamus revealed a sex-specific differential effect with an increase in ObRb and AgRP expression observed only in females. This change was associated with an increase in STAT3 and SOCS3, two post-leptin receptor signaling molecules, STAT3 reflects leptin sensitivity (Bates et al. 2005; Becskei et al. 2010; Ferezou-Viala et al. 2007; Hosoi et al. 2002) and SOCS3 reflects leptin resistance (Howard et al. 2004; Mori et al. 2004). In addition, the increase in PTP1B, a molecule that functions against both insulin and leptin signaling (Banno et al. 2010; Bence et al. 2006; Cheng et al. 2002; Haj et al. 2003; Tao et al. 2001; Zabolotny et al. 2002), is noted only in females. In contrast, in males while no change in either ObRb or AgRP is noted, there is also no change in STAT3 or SOCS3 with a decrease in PTP1B supporting leptin and insulin sensitivity. In both sexes while there was a decline in the melanocortin receptor (-3 and -4) expression, no changes in either NPY or POMC mRNAs were observed. Our present observations support the fact that low circulating leptin concentrations are able to enhance hypothalamic leptin sensitivity seen as an increase in ObRb, STAT3 but only AgRP in females. Since no effect is seen on NPY and POMC despite stimulation of leptin signaling this early in life in both sexes, immaturity of the leptin-POMC/NPY circuitry may underlie this lack of response (Bouret et al. 2004; Cone and Simerly 2011). Alternatively, since PN2 pups are consuming a high fat diet (milk), perhaps AgRP (responsible for regulating fat intake) (Tracy et al. 2008) particularly in the females rather than NPY and POMC (responsible for regulating carbohydrate intake) (Kinzig et al. 2007) are responsive to the low circulating leptin concentrations encountered. In contrast to these findings, the elevation in SOCS3 and PTP1B again only in females suggests the early emergence of leptin resistance that follows the chronic intra-uterine stimulation of enhanced hypothalamic leptin sensitivity perhaps present throughout late gestation fetal stage. In contrast, the hypothalamic mechanism of enhanced leptin (and insulin) sensitivity encountered in males is different and mediated through lowered PTP1B concentrations. These observations support sex-specific changes in the hypothalamus in response to intra-uterine calorie restriction associated with a similar decrease in circulating leptin concentrations. Previous investigations have demonstrated that the leptin sensitivity of the hypothalamus is enhanced in females while the effect of insulin is apparent in both males and females (Hamed et al. 2009; Panarotto et al. 2000; Ramel et al. 2010). We have previously demonstrated that intra-uterine calorie restriction had a minimal effect on PN2 circulating insulin concentrations (Thamotharan et al. 2005). This previous observation coupled with our present measurement of reduced leptin concentrations may formulate yet another explanation for our present sex-specific differences noted in the PN2 offspring exposed to intrauterine calorie restriction.
Postnatal effects
Ad libitum exposure to calorie intake postnatally superimposed on IUGR normalized the phenotype during the suckling phase with regain in body weight reflecting that of controls. In the presence of adequate milk intake, no changes in post-weaned food intake, physical activity (only a decline in females), oxygen consumption or energy expenditure were evident. This is despite a sex-dependent divergence in circulating leptin concentrations, the males being normal and the females being hyperleptinemic. Instead, we ensured that the control female suckling offspring was not leptin resistant by administering exogenous leptin and observing a reduction in hypothalamic ObRb and MC3-R with induction of POMC expression, consistent with intact leptin sensitivity. However, the increased endogenous leptin concentrations in female IUGR at this postnatal stage with no similar changes in hypothalamic neuropeptides (see below) as noted in response to exogenous leptin administration, may signify a disconnect between circulating high leptin concentrations and the effect on hypothalamic neuropeptides. This observation in females may be the beginning of leptin resistance which in turn may predict the development of obesity in the IUGR at a subsequent adult stage as has been previously described (Garg et al. 2006; Thamotharan et al. 2005).
Our phenotypic investigations targeting the PNGR and IPGR groups that continued to be hypoleptinemic and growth restricted, demonstrated diminished milk intake, energy expenditure and physical activity with an increase in oxygen consumption of both sexes. While no change in RER was seen in males, a distinct increase in RER was evident in females. This was related to enhanced reliance on carbohydrates to fuel oxidation in females when compared to males (data not shown). In both sexes by PN60, these parameters were similar (data not shown). Despite this difference in RER at PN21, there was no difference in the PN24 post-weaned food intake of the PNGR and IPGR in the two sexes, being increased when compared to controls and IUGR. Because of this increase in post-weaned food intake of the two groups, namely PNGR and IPGR, with no such change in the IUGR, we next focused on the hypothalamus in all groups with localization studies specifically in the IPGR.
At PN21 after differential exposure to postnatal calorie intake, both sexes demonstrated similar changes in post-leptin signaling molecules, namely an increase in PTP1B in only the IPGR and PNGR with no change in the IUGR versus control. This was associated with no major changes in the IUGR, but an imbalance in the neuropeptides that influence the energy balance in the PNGR and IPGR. These changes consisted of an increase in ObRb, NPY and AgRP with a decrease in POMC (associated with an increase in MCRs), consistent with the reduced circulating leptin concentrations. In-situ hybridization experiments confirmed that these changes were related to changes in cell numbers and expression levels. These key hypothalamic changes at PN21 preempted the increased food intake in both sexes post-weaning. Thus postnatal calorie restriction induced hypothalamic changes that were independent of the intra-uterine calorie restriction induced perturbations lacked sex-specificity. The only sex-specific changes that were evident consisted of the arcuate nuclear exaggerated increase in the male versus the female IUGR AgRP expression over their respective controls. In addition the greater decrease in the female POMC expression versus that of the male IUGR was seen versus the respective control. However, these hypothalamic neuropeptide perturbations preempted the increased post-weaned food intake in both sexes, when food was available ad libitum. This increased food intake regulated by the perturbed hypothalamic mechanisms may relate to self-preservation in the face of nutritional adversity. The enhanced ability to consume food when freely available may be responsible for the subsequent catch up growth experienced by both the PNGR and IPGR as previously described (Garg et al. 2006; Thamotharan et al. 2005). During this phase of catch-up growth, if the offspring is exposed to a high calorie diet, the opportunity exists for developing obesity later in life, particularly visceral adiposity (Morrison et al. 2010).
Previous investigations have detailed changes in the developing hypothalamus however sex-specificity has not been detailed. For example, in the bilateral uterine artery ligation rat model, we observed an increase in hypothalamic NPY and CRF peptides in the fetus, which persisted until PN21 (Rajakumar et al. 1998), however no sex-specific differences were examined. Previous investigations administering leptin during the postnatal period demonstrated an impact on hypothalamic neuropeptides with phenotypic changes mainly in females (Varma et al. 2004). Other groups employing a low protein diet during gestation and lactation demonstrated increased paraventricular nuclear NPY peptide concentrations in the PN20 male offspring (Plagemann et al. 2000). The low protein dietary intervention led to a diminution of circulating insulin concentrations with no change in leptin concentrations (Plagemann et al. 2000). Another recent study also concentrated mainly on the male offspring at PN21 of a total calorie restricted mother during gestation and lactation, where hypothalamic ObRb and STAT3 were assessed. In response to exogenous leptin administration during the suckling phase (PN3-PN13) a reversal of the adult hyperphagic obese phenotype due to perinatal undernutrition and post-weaning exposure to a high fat diet were observed, thereby preventing the subsequent development of hyperleptinemia and hyperinsulinemia in females (Vickers et al. 2005). These same female animals at 24 weeks of age in the absence of postnatal leptin intervention revealed increased hypothalamic ObRb, NPY and POMC mRNAs but decreased AgRP mRNA in the undernourished (IUGR) post weaned high fat obese group alone (Ikenasio-Thorpe et al. 2007). In contrast, introduction of postnatal leptin led to a bidirectional effect in PN170 female offspring independent of the post-weaned diet (regular chow or high fat), with increased expression of hepatic 11β-HSD2, glucocorticoid receptor, PPARα and PEPCK in the IUGR with postnatal ad libitum feeds versus a decrease or no change with postnatal calorie restriction (IPGR) (Gluckman et al. 2007). These changes were dependent on gene promoter CpG differential DNA methylation (Gluckman et al. 2007).
In the case of NPY and POMC genes, incongruous correlation of POMC mRNA expression and DNA methylation of its gene promoter (Coupe et al. 2010) were noted. In response to a low protein diet, hypothalamic POMC mRNA expression was low in the face of decreased methylation of the POMC gene promoter (Coupe et al. 2010). Similar to these results, in our present study, we did not demonstrate any effect of gene promoter DNA methylation on the expression of all three neuropeptides, i.e. NPY, POMC or AgRP. Despite an increase in AgRP expression in IPGR versus CON, it is difficult to relate this increase in mRNA expression with the enhanced methylation of the promoter region because the displayed methylation levels were rather high ranging from 75% to 96%. These high levels of DNA methylation are similar to the levels reported previously with neuropeptides (Coupe et al. 2010). Thus, the mechanisms responsible for postnatal calorie restriction induced changes in hypothalamic neuropeptides may not be reliant on DNA methylation.
While previous studies explored the developing hypothalamus in various calorie restricted offspring, sex-specificity and the impact of prenatal versus postnatal calorie restriction on hypothalamic neuropeptides and leptin post-receptor signaling molecules along with phenotypic characterization has been lacking. Our present study is the first that explored these aspects completely providing sex-specific differences as early as PN2 and differences related to pre- versus postnatal calorie restriction. Our investigations demonstrate calorie restriction induced hypothalamic neuropeptide changes that preempt feeding behavior post-weaning, perhaps providing an explanation for the phenomenon of subsequent “catch-up” growth. Previous investigations in adult animals have demonstrated a dichotomy in the hypothalamic response to exogenous leptin in males and females using tissue or gene specific knockout mice (Bruning et al. 2000; Lewitt and Brismar 2002; Shi et al. 2010). In addition, when the insulin receptor was specifically mutated in brain, both females and males demonstrated centrally acquired-obesity and glucose intolerance with higher plasma leptin concentrations in the female alone (Bruning et al. 2000). These adult studies support our present observation of higher circulating leptin concentration in the PN21 female IUGR which incidentally had higher plasma insulin concentrations as well (unpublished data). Despite a difference between the PN21 female and male circulating leptin concentrations, the absence of sex-specific hypothalamic changes supports adaptive responses in play. These adaptive responses may be essential for normalizing the IUGR hypothalamus when exposed to adequate milk (calorie) intake postnatally. Future studies are necessary to uncover intermediary processes that lead to such postnatal adaptation despite differences in circulating leptin concentrations. These responses in turn may underlie the development of hypothalamic leptin resistance mainly in female IUGR while maintaining leptin sensitivity in male IUGR at PN21. However, the superimposition of postnatal calorie restriction on IUGR (IPGR) resulted in lowering circulating leptin concentrations which in turn reversed this propensity for leptin resistance seen in female IUGR towards a state of leptin sensitivity seen in males. Thus, postnatal dietary manipulation can alter the female-specific hypothalamic leptin resistant state which forms the early signature of IUGR.
In summary, we have described the effect of intrauterine calorie restriction on the early PN2 hypothalamus which demonstrates sex-specificity, in that the female demonstrates early emergence of hypothalamic leptin resistance. These sex-specific changes are further exaggerated with ad libitum exposure to adequate milk intake during the postnatal period providing the basis for subsequent obesity. In contrast, postnatal calorie restriction by itself or superimposed on intrauterine calorie restriction results in lowering circulating leptin concentrations associated with compensatory hypothalamic neuropeptide changes without major sex-specificity. These changes in hypothalamic neuropeptide gene expression are not associated with changes in DNA methylation of their promoter regions. However these hypothalamic changes set the stage for relative hyperphagia and energy conservation during the post-weaned stage, targeting the phenomenon of “catch-up” growth that may be vital for survival.
Acknowledgments
This work was supported by grants from NIH HD 25024 and 41230. We acknowledge the experimental assistance of Mami Matsuyoshi, Akta Patel, Maelaine Rodero, Hoang Dinh Tran and Shanthie Thamotharan.
Abbreviations
- STAT3
signal transducer and activator of transcription 3
- SOCS3
suppressor of cytokine signaling 3
- PTP1B
protein tyrosine phosphatase 1B
- AgRP
agouti-related peptide
- MC3-R
melanocortin-3 receptor
- MC4-R
melanocortin-4 receptor
- POMC
pro-opiomelanocortin
- NPY
neuropeptide Y
- ObRb
leptin receptor long form
- α -MSH
α -melanocyte stimulating hormone
- ARC
arcuate nucleus
References
- Ahima RS, Osei SY. Leptin signaling. Physiol Behav. 2004;81(2):223–241. doi: 10.1016/j.physbeh.2004.02.014. [DOI] [PubMed] [Google Scholar]
- Banno R, Zimmer D, De Jonghe BC, Atienza M, Rak K, Yang W, Bence KK. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest. 2010;120(3):720–734. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353(17):1802–1809. doi: 10.1056/NEJMoa044160. [DOI] [PubMed] [Google Scholar]
- Bates SH, Kulkarni RN, Seifert M, Myers MG., Jr Roles for leptin receptor/STAT3-dependent and -independent signals in the regulation of glucose homeostasis. Cell Metab. 2005;1(3):169–178. doi: 10.1016/j.cmet.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Becskei C, Lutz TA, Riediger T. Reduced fasting-induced activation of hypothalamic arcuate neurons is associated with hyperleptinemia and increased leptin sensitivity in obese mice. Am J Physiol Regul Integr Comp Physiol. 2010;299(2):R632–641. doi: 10.1152/ajpregu.00674.2009. [DOI] [PubMed] [Google Scholar]
- Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12(8):917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- Bhargava SK, Sachdev HS, Fall CH, Osmond C, Lakshmy R, Barker DJ, Biswas SK, Ramji S, Prabhakaran D, Reddy KS. Relation of serial changes in childhood body-mass index to impaired glucose tolerance in young adulthood. N Engl J Med. 2004;350(9):865–875. doi: 10.1056/NEJMoa035698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhasin KK, van Nas A, Martin LJ, Davis RC, Devaskar SU, Lusis AJ. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes. 2009;58(3):559–566. doi: 10.2337/db07-1530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB. Trophic action of leptin on hypothalamic neurons that regulate feeding. Science. 2004;304(5667):108–110. doi: 10.1126/science.1095004. [DOI] [PubMed] [Google Scholar]
- Breton C, Lukaszewski MA, Risold PY, Enache M, Guillemot J, Riviere G, Delahaye F, Lesage J, Dutriez-Casteloot I, Laborie C, Vieau D. Maternal prenatal undernutrition alters the response of POMC neurons to energy status variation in adult male rat offspring. Am J Physiol Endocrinol Metab. 2009;296(3):E462–472. doi: 10.1152/ajpendo.90740.2008. [DOI] [PubMed] [Google Scholar]
- Bruning JC, Gautam D, Burks DJ, Gillette J, Schubert M, Orban PC, Klein R, Krone W, Muller-Wieland D, Kahn CR. Role of brain insulin receptor in control of body weight and reproduction. Science. 2000;289(5487):2122–2125. doi: 10.1126/science.289.5487.2122. [DOI] [PubMed] [Google Scholar]
- Burdge GC, Lillycrop KA, Jackson AA. Nutrition in early life, and risk of cancer and metabolic disease: alternative endings in an epigenetic tale? Br J Nutr. 2009;101(5):619–630. doi: 10.1017/S0007114508145883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Gaysinskaya V, Karatayev O, Leibowitz SF. Maternal high-fat diet and fetal programming: increased proliferation of hypothalamic peptide-producing neurons that increase risk for overeating and obesity. J Neurosci. 2008;28(46):12107–12119. doi: 10.1523/JNEUROSCI.2642-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2(4):497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- Cone RD, Simerly RB. Leptin grows up and gets a neural network. Neuron. 2011;71(1):4–6. doi: 10.1016/j.neuron.2011.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook WS, Unger RH. Protein tyrosine phosphatase 1B: a potential leptin resistance factor of obesity. Dev Cell. 2002;2(4):385–387. doi: 10.1016/s1534-5807(02)00158-2. [DOI] [PubMed] [Google Scholar]
- Coppari R, Ichinose M, Lee CE, Pullen AE, Kenny CD, McGovern RA, Tang V, Liu SM, Ludwig T, Chua SC, Jr, Lowell BB, Elmquist JK. The hypothalamic arcuate nucleus: a key site for mediating leptin’s effects on glucose homeostasis and locomotor activity. Cell Metab. 2005;1(1):63–72. doi: 10.1016/j.cmet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Cottrell EC, Cripps RL, Duncan JS, Barrett P, Mercer JG, Herwig A, Ozanne SE. Developmental changes in hypothalamic leptin receptor: relationship with the postnatal leptin surge and energy balance neuropeptides in the postnatal rat. Am J Physiol Regul Integr Comp Physiol. 2009;296(3):R631–639. doi: 10.1152/ajpregu.90690.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coupe B, Amarger V, Grit I, Benani A, Parnet P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology. 2010;151(2):702–713. doi: 10.1210/en.2009-0893. [DOI] [PubMed] [Google Scholar]
- Delahaye F, Breton C, Risold PY, Enache M, Dutriez-Casteloot I, Laborie C, Lesage J, Vieau D. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology. 2008;149(2):470–475. doi: 10.1210/en.2007-1263. [DOI] [PubMed] [Google Scholar]
- Enriori PJ, Evans AE, Sinnayah P, Jobst EE, Tonelli-Lemos L, Billes SK, Glavas MM, Grayson BE, Perello M, Nillni EA, Grove KL, Cowley MA. Diet-induced obesity causes severe but reversible leptin resistance in arcuate melanocortin neurons. Cell Metab. 2007;5(3):181–194. doi: 10.1016/j.cmet.2007.02.004. [DOI] [PubMed] [Google Scholar]
- Evensen KA, Steinshamn S, Tjonna AE, Stolen T, Hoydal MA, Wisloff U, Brubakk AM, Vik T. Effects of preterm birth and fetal growth retardation on cardiovascular risk factors in young adulthood. Early Hum Dev. 2009;85(4):239–245. doi: 10.1016/j.earlhumdev.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Ferezou-Viala J, Roy AF, Serougne C, Gripois D, Parquet M, Bailleux V, Gertler A, Delplanque B, Djiane J, Riottot M, Taouis M. Long-term consequences of maternal high-fat feeding on hypothalamic leptin sensitivity and diet-induced obesity in the offspring. Am J Physiol Regul Integr Comp Physiol. 2007;293(3):R1056–1062. doi: 10.1152/ajpregu.00117.2007. [DOI] [PubMed] [Google Scholar]
- Fukushima N, Yokouchi K, Kawagishi K, Moriizumi T. Effect of maternal deprivation on milk intake in normal and bilaterally facial nerve-injured developing rats. Neurosci Res. 2006;54(2):154–157. doi: 10.1016/j.neures.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Garg M, Thamotharan M, Rogers L, Bassilian S, Lee WN, Devaskar SU. Glucose metabolic adaptations in the intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab. 2006;290(6):E1218–1226. doi: 10.1152/ajpendo.00474.2005. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Buklijas T, Low FM, Beedle AS. Epigenetic mechanisms that underpin metabolic and cardiovascular diseases. Nat Rev Endocrinol. 2009;5(7):401–408. doi: 10.1038/nrendo.2009.102. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Lillycrop KA, Vickers MH, Pleasants AB, Phillips ES, Beedle AS, Burdge GC, Hanson MA. Metabolic plasticity during mammalian development is directionally dependent on early nutritional status. Proc Natl Acad Sci U S A. 2007;104(31):12796–12800. doi: 10.1073/pnas.0705667104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey KM, Gluckman PD, Hanson MA. Developmental origins of metabolic disease: life course and intergenerational perspectives. Trends Endocrinol Metab. 2010;21(4):199–205. doi: 10.1016/j.tem.2009.12.008. [DOI] [PubMed] [Google Scholar]
- Haj FG, Markova B, Klaman LD, Bohmer FD, Neel BG. Regulation of receptor tyrosine kinase signaling by protein tyrosine phosphatase-1B. J Biol Chem. 2003;278(2):739–744. doi: 10.1074/jbc.M210194200. [DOI] [PubMed] [Google Scholar]
- Hamed SA, Fida NM, Hamed EA. States of serum leptin and insulin in children with epilepsy: risk predictors of weight gain. Eur J Paediatr Neurol. 2009;13(3):261–268. doi: 10.1016/j.ejpn.2008.05.005. [DOI] [PubMed] [Google Scholar]
- Horton TH. Fetal origins of developmental plasticity: animal models of induced life history variation. Am J Hum Biol. 2005;17(1):34–43. doi: 10.1002/ajhb.20092. [DOI] [PubMed] [Google Scholar]
- Hosoi T, Okuma Y, Nomura Y. Leptin regulates interleukin-1beta expression in the brain via the STAT3-independent mechanisms. Brain Res. 2002;949(1–2):139–146. doi: 10.1016/s0006-8993(02)02974-8. [DOI] [PubMed] [Google Scholar]
- Howard JK, Cave BJ, Oksanen LJ, Tzameli I, Bjorbaek C, Flier JS. Enhanced leptin sensitivity and attenuation of diet-induced obesity in mice with haploinsufficiency of Socs3. Nat Med. 2004;10(7):734–738. doi: 10.1038/nm1072. [DOI] [PubMed] [Google Scholar]
- Ikenasio-Thorpe BA, Breier BH, Vickers MH, Fraser M. Prenatal influences on susceptibility to diet-induced obesity are mediated by altered neuroendocrine gene expression. J Endocrinol. 2007;193(1):31–37. doi: 10.1677/joe.1.07017. [DOI] [PubMed] [Google Scholar]
- Kimura T, Kosaka J, Nomura T, Yamada T, Miki Y, Takagi K, Kogami T, Sasaki J. Quantification of in situ hybridization signals in rat testes. J Histochem Cytochem. 2004;52(6):813–820. doi: 10.1369/jhc.4A6249.2004. [DOI] [PubMed] [Google Scholar]
- Kinzig KP, Hargrave SL, Hyun J, Moran TH. Energy balance and hypothalamic effects of a high-protein/low-carbohydrate diet. Physiol Behav. 2007;92(3):454–460. doi: 10.1016/j.physbeh.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lane RH, Kelley DE, Gruetzmacher EM, Devaskar SU. Uteroplacental insufficiency alters hepatic fatty acid-metabolizing enzymes in juvenile and adult rats. Am J Physiol Regul Integr Comp Physiol. 2001;280(1):R183–190. doi: 10.1152/ajpregu.2001.280.1.R183. [DOI] [PubMed] [Google Scholar]
- Lewitt MS, Brismar K. Gender difference in the leptin response to feeding in peroxisome-proliferator-activated receptor-alpha knockout mice. Int J Obes Relat Metab Disord. 2002;26(10):1296–1300. doi: 10.1038/sj.ijo.0802135. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD. NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science. 2005;310(5748):683–685. doi: 10.1126/science.1115524. [DOI] [PubMed] [Google Scholar]
- Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10(7):739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- Morrison JL, Duffield JA, Muhlhausler BS, Gentili S, McMillen IC. Fetal growth restriction, catch-up growth and the early origins of insulin resistance and visceral obesity. Pediatr Nephrol. 2010;25(4):669–677. doi: 10.1007/s00467-009-1407-3. [DOI] [PubMed] [Google Scholar]
- Niswender KD, Gallis B, Blevins JE, Corson MA, Schwartz MW, Baskin DG. Immunocytochemical detection of phosphatidylinositol 3-kinase activation by insulin and leptin. J Histochem Cytochem. 2003;51(3):275–283. doi: 10.1177/002215540305100302. [DOI] [PubMed] [Google Scholar]
- Ogihara T, Shin BC, Anai M, Katagiri H, Inukai K, Funaki M, Fukushima Y, Ishihara H, Takata K, Kikuchi M, Yazaki Y, Oka Y, Asano T. Insulin receptor substrate (IRS)-2 is dephosphorylated more rapidly than IRS-1 via its association with phosphatidylinositol 3-kinase in skeletal muscle cells. J Biol Chem. 1997;272(19):12868–12873. doi: 10.1074/jbc.272.19.12868. [DOI] [PubMed] [Google Scholar]
- Panarotto D, Ardilouze JL, Tessier D, Maheux P. The degree of hyperinsulinemia and impaired glucose tolerance predicts plasma leptin concentrations in women only: a new exploratory paradigm. Metabolism. 2000;49(8):1055–1062. doi: 10.1053/meta.2000.7745. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Academic Press; 1998. [DOI] [PubMed] [Google Scholar]
- Peronnet F, Massicotte D. Table of nonprotein respiratory quotient: an update. Can J Sport Sci. 1991;16(1):23–29. [PubMed] [Google Scholar]
- Plagemann A, Waas T, Harder T, Rittel F, Ziska T, Rohde W. Hypothalamic neuropeptide Y levels in weaning offspring of low-protein malnourished mother rats. Neuropeptides. 2000;34(1):1–6. doi: 10.1054/npep.1999.0778. [DOI] [PubMed] [Google Scholar]
- Rajakumar PA, He J, Simmons RA, Devaskar SU. Effect of uteroplacental insufficiency upon brain neuropeptide Y and corticotropin-releasing factor gene expression and concentrations. Pediatr Res. 1998;44(2):168–174. doi: 10.1203/00006450-199808000-00005. [DOI] [PubMed] [Google Scholar]
- Ramel A, Arnarson A, Parra D, Kiely M, Bandarra NM, Martinez JA, Thorsdottir I. Gender difference in the prediction of weight loss by leptin among overweight adults. Ann Nutr Metab. 2010;56(3):190–197. doi: 10.1159/000281833. [DOI] [PubMed] [Google Scholar]
- Sahu A. Minireview: A hypothalamic role in energy balance with special emphasis on leptin. Endocrinology. 2004;145(6):2613–2620. doi: 10.1210/en.2004-0032. [DOI] [PubMed] [Google Scholar]
- Shi H, Sorrell JE, Clegg DJ, Woods SC, Seeley RJ. The roles of leptin receptors on POMC neurons in the regulation of sex-specific energy homeostasis. Physiol Behav. 2010;100(2):165–172. doi: 10.1016/j.physbeh.2010.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin BC, McKnight RA, Devaskar SU. Glucose transporter GLUT8 translocation in neurons is not insulin responsive. J Neurosci Res. 2004;75(6):835–844. doi: 10.1002/jnr.20054. [DOI] [PubMed] [Google Scholar]
- Shin BC, Suzuki M, Inukai K, Anai M, Asano T, Takata K. Multiple isoforms of the regulatory subunit for phosphatidylinositol 3-kinase (PI3-kinase) are expressed in neurons in the rat brain. Biochem Biophys Res Commun. 1998;246(2):313–319. doi: 10.1006/bbrc.1998.8606. [DOI] [PubMed] [Google Scholar]
- Singh BS, Westfall TC, Devaskar SU. Maternal diabetes-induced hyperglycemia and acute intracerebral hyperinsulinism suppress fetal brain neuropeptide Y concentrations. Endocrinology. 1997;138(3):963–969. doi: 10.1210/endo.138.3.5001. [DOI] [PubMed] [Google Scholar]
- Stevens A, Begum G, Cook A, Connor K, Rumball C, Oliver M, Challis J, Bloomfield F, White A. Epigenetic changes in the hypothalamic proopiomelanocortin and glucocorticoid receptor genes in the ovine fetus after periconceptional undernutrition. Endocrinology. 2010;151(8):3652–3664. doi: 10.1210/en.2010-0094. [DOI] [PubMed] [Google Scholar]
- Tao J, Malbon CC, Wang HY. Galpha(i2) enhances insulin signaling via suppression of protein-tyrosine phosphatase 1B. J Biol Chem. 2001;276(43):39705–39712. doi: 10.1074/jbc.M105216200. [DOI] [PubMed] [Google Scholar]
- Thamotharan M, Shin BC, Suddirikku DT, Thamotharan S, Garg M, Devaskar SU. GLUT4 expression and subcellular localization in the intrauterine growth-restricted adult rat female offspring. Am J Physiol Endocrinol Metab. 2005;288(5):E935–947. doi: 10.1152/ajpendo.00342.2004. [DOI] [PubMed] [Google Scholar]
- Torrens C, Hanson MA, Gluckman PD, Vickers MH. Maternal undernutrition leads to endothelial dysfunction in adult male rat offspring independent of postnatal diet. Br J Nutr. 2009;101(1):27–33. doi: 10.1017/S0007114508988760. [DOI] [PubMed] [Google Scholar]
- Tracy AL, Clegg DJ, Johnson JD, Davidson TL, Benoit SC. The melanocortin antagonist AgRP (83–132) increases appetitive responding for a fat, but not a carbohydrate, reinforcer. Pharmacol Biochem Behav. 2008;89(3):263–271. doi: 10.1016/j.pbb.2007.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varma A, He J, Shin BC, Weissfeld LA, Devaskar SU. Postnatal intracerebroventricular exposure to leptin causes an altered adult female phenotype. Am J Physiol Endocrinol Metab. 2004;287(6):E1132–1141. doi: 10.1152/ajpendo.00228.2004. [DOI] [PubMed] [Google Scholar]
- Vickers MH, Gluckman PD, Coveny AH, Hofman PL, Cutfield WS, Gertler A, Breier BH, Harris M. Neonatal leptin treatment reverses developmental programming. Endocrinology. 2005;146(10):4211–4216. doi: 10.1210/en.2005-0581. [DOI] [PubMed] [Google Scholar]
- Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2(4):489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- Zigman JM, Elmquist JK. Minireview: From anorexia to obesity--the yin and yang of body weight control. Endocrinology. 2003;144(9):3749–3756. doi: 10.1210/en.2003-0241. [DOI] [PubMed] [Google Scholar]