Abstract
BACKGROUND
It is hypothesized that poor zinc nutritional status is associated with an increased risk of esophageal cancer (EC), but current evidence is contradictory. Since some factors may influence zinc absorption, its status may be better evaluated thorough biomarkers. The objectives of this study were to perform a systematic review on the association of zinc biomarkers with EC in observational studies and to evaluate the efficacy of zinc supplements in preventing EC in randomized trials.
METHODS
The MEDLINE database was searched in December 2013 for studies written in English with relevant keywords. Articles which met inclusion criteria were included in this study.
RESULTS
Eleven observational studies that measured zinc biomarkers and eight randomized trials which evaluated supplements containing zinc, met our inclusion criteria. The majority of studies suggested that higher zinc status was inversely associated with EC risk.
CONCLUSION
Most of the evidence for this hypothesis comes from case-control studies, which may introduce bias. Cohort studies are needed to establish whether poor zinc status is associated with increased risk for EC. Findings from trials are inconclusive as there is no data from single agent trials. However, the evidence is not still strong enough to conclude a protective role of zinc in EC.
Keywords: Zinc, Esophageal cancer, Minerals, Systematic review
INTRODUCTION
Zinc (Zn) is essential for the activity of more than 300 enzymes, immune function, and conformation of many transcription factors that control cell proliferation, apoptosis, and signaling.1 Zn is available from all food groups, but some important dietary sources of Zn include red meat, poultry, fish, other seafood, legumes, nuts, whole grains, and dairy products.2 However, the concentration of Zn in most foods is not inherent and the Zn content of foods depends on soil and water Zn concentrations or in the concentration in fodder. In addition, there are some physiologic factors such as age, genotype, and the quantity of Zn ingested, and the time over which Zn is ingested that may affect Zn absorption. Furthermore, the bioavailability of ingested Zn is dependent on the presence of phytate in foods, which inhibits Zn absorption.3,4 For these reasons, dietary intake methods are likely inaccurate for estimating Zn deficiency or Zn exposure and observational studies of Zn status may benefit from the use of biomarkers such as hair, nail, serum or plasma Zn concentrations.
Zn deficiency adversely affects the immune system, increases oxidative stress, and increases the generation of inflammatory cytokines.5 In animal models, a Zn deficient diet results in a precancerous condition in the upper digestive tract, including the esophagus 1 and enhances the effects of esophageal carcinogens (e.g., N-nitrosomethyl benzylamine) 6 by different mechanism including increased cell proliferation,7 cyclin D1 over expression 8 and p53 deficiency.9 Other mechanisms may include cyclooxygenase-2 (COX-2) over expression,10 activating S100A8 inflammation,1 P450-dependent metabolism of nitrosamines,11 and reduced alkyl guanine DNA methyltransferase activity.12 Moreover, in rodents, Zn supplementation may affect tumor progression13 by inducing apoptosis,14,15 and reversing over expression of the S100A8.16 In a rat model, a chronic Zn deficient diet induces a pro-tumorigenic micro RNA signature (miR-31 and miR-21) that fosters squamous cell carcinoma development.17 However, the effect of Zn on esophageal cancer (EC) risk in humans is uncertain.18-20
EC is the eighth most common cancer with respect to incidence and the sixth most common cancer with respect to mortality worldwide.21 EC is classified into two main types histologically: esophageal adenocarcinoma (EA) and esophageal squamous cell carcinoma (ESCC), each having different risk factors.19 Numerous observational studies have investigated the association between Zn biomarkers measured in nails, hair, plasma, or serum and EC risk. Furthermore, several randomized trials have tested whether Zn supplementation (in combinations with other nutrients) reduced the incidence of EC. However, the totality of evidence has not been systematically reviewed.
The objective of the present study was to review the results from observational studies about the association of Zn status (using all biomarkers of Zn) with EC and results of clinical trials about the efficacy of Zn supplements in preventing EC.
MATERIALS AND METHODS
Data sources, search strategy, and selection criteria
MEDLINE database was searched for observational studies and randomized trials investigating the relationship between Zn and EC. The following Medical Subject Headings (MeSH) terms were applied [“esophag*” AND (“cancer” OR “tumor” OR “carcinoma” OR “adenocarcinoma” OR “neoplasm”)]; and were combined with each of the terms “zinc”, “zn”, “zinc gluconate”, “zinc sulfate”, “zinc acetate”, “zinc oxide”, “methalothionein”, and “zinc isotope”. The potentially relevant articles were included if the full paper had been obtained. No time restrictions were added. Studies were restricted to human studies and publications in English. References of identified articles and reviews were also searched for additional relevant articles.
We aimed to identify all observational and randomized trials that assessed the association of Zn with EC, either alone or combined with other nutrients, for preventing EC. The endpoint was EC, which was defined as any combination of EA and ESCC. Studies reporting only EC without the type of pathology were also included. Articles with the following criteria were excluded:
1- Not original research (reviews, editorials, non-research letters);
2- Case reports or case series;
3- Ecologic studies;
4- Studies lacking a biomarker of Zn status.
In the case of several reports on one outcome from the same population, the last publication was enrolled 22-26 (Figure 1).
Fig. 1 .
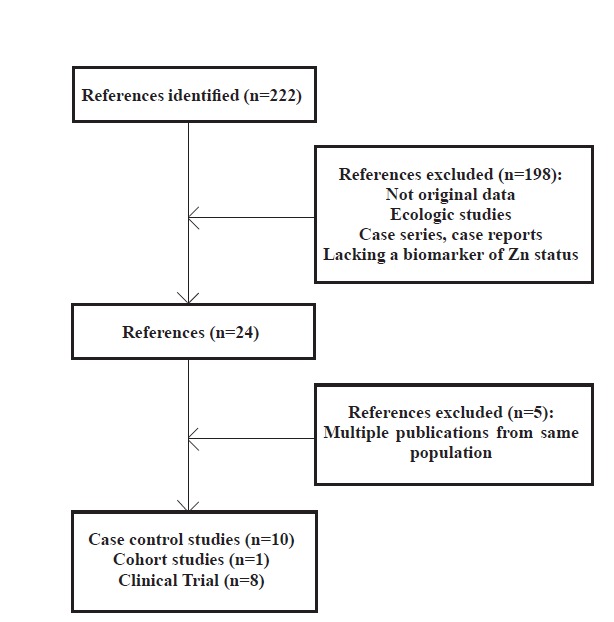
Flow diagram of study selection process
Data extraction and quality assessment
One investigator (MH) reviewed search results and extracted the study design, first author, year of publication, country, patient characteristics (sex and mean age), sample size, and the reported RR (OR) with 95% confidence intervals (CIs) for the highest versus lowest categories of Zn status from studies (table 1). The quality of observational studies was assessed according to the criteria used by Flores-Mateo et al.27 (appendix 1), and the quality of randomized trials was assessed according to the criteria of Jadad et al.28 (table 2).
Table 1 . Observational studies of Zn biomarkers and esophageal cancer1 .
| Author | Year | Design | country | Men among control % |
Mean age of control
y |
Type of control subjects | Source of case subjects | Outcome | No of case subjects/ non case subjects |
Zn assessment
(technique) |
Zn concentration |
Unadjusted OR
(95%CI)/ p |
Adjusted OR
(95%CI)/ p |
|
| Case subjects | Non case subjects | |||||||||||||
| O’Rorke 29 | 2012 | CCS | Ireland | 83.3 | 63.6 | General practitioner lists | Ireland case control study | EA incidence | 137/221 |
Toenail (INAA) |
70.7±21 µg/g |
70.1±18.5 µg/g |
0.87 (0.52-1.45)/ 0.55 |
0.86 (0.51-1.46)/ 0.56 |
| Ray30 | 2012 | CCS | South Africa | NR | NR | Volunteers from General population | Hospital | ESCC Prevalence | 30/30 | Hair (AAS) | 0.20±0.11 ppm |
0.39±0.10 ppm |
NR, p<0.0001 | NR |
| India | 0.54±0.21 ppm |
0.64±0.23 ppm |
NR, p=0.08 | NR | ||||||||||
| Sun31 | 2011 | CCO | China | 69 | 58 | Normal tissue from the same patient | Hospital | ESCC incidence | 36/36 | Tissue (AAS) |
16.51 ±1.28 µg/g |
20.44 ±1.55 µg/g |
NR, p<0.01 | NR |
| Dar 32 | 2008 | CCS | India | 65 | NR | NR | Institute of medical sciences |
ESCC prevalence |
55/55 | Plasma (AAS) | 86.8 µg/dl | 96.1 µg/dl | NR, p<0.0001 | NR |
| Nouri 33 | 2008 | CCS | Iran | 43 | NR | Hospital /family | Hospital |
ESCC incidence |
20/80 (60+20) | Nail (AAS) | 126.5 ±42 ppm |
Sari=173± 111 Tehran=251±213 Family=175±131 |
NR, p<0.001 | NR |
| Goyal 34 | 2006 | CCS | India | 69 | 44 | NR | NR |
ESCC incidence |
24/23 | Serum (AAS) | 75.20 ±5.57 µg/dl |
87.17 ±6.43 µg/dl |
NR, p<0.001 | NR |
| Dursun 35 | 2006 | CCS | Turkey | 50 | 50.2 | NR | NR | NR | 17/20 | RBC SOD | 1.87 ± 0.10 U/mg Hb |
1.67 ± 0.16 U/mg Hb |
NR, p < 0.001 | NR |
| Rogers 37 | 1993 | CCS | USA | 74 | NR | Cancer registry | General population |
EC incidence |
73/434 | Nail (NAA) | NR | NR | NR | 1.7 (0.7-4.1), NR |
| Prasad 38 | 1992 | CCS | India | 65 | 56.4 | Hospital | Hospital |
ESCC incidence |
35/35 | Plasma (AAS) | 10.2±0.22 µmol/l | 13.9±0.56 µmol/l | NR, p<0.001 | NR |
| Mellow 36 | 1983 | CCS | USA | 100 | 55 | Hospital personnel | Hospital |
ESCC incidence |
17/10 | Plasma (AAS) | 65.7 ± 3.3 µg/dl |
80.5±2.4 µg/dl |
NR, p<0.01 | NR |
| Abnet 39 | 2005 | Cohort | China | 47 |
55 (49-59) median |
Nested in cohort | Nested in cohort |
ESCC incidence |
60/72 | Tissue (X-ray fluorescence) |
44 (30-75) ng/cm 2 |
57 (47-108) ng/cm 2 |
NR |
HR=0.74 (0.56-0.97)/ 0..015 |
1AAS, Atomic Absorption Spectrometry; INAA, Instrumental Neutron Activation Analysis; CCS, Case-Control Study; CCO, Case Crossover Study; NR, Not Reported; RBC SOD, Red Blood Cell Super Oxide Dismutase; ppm, point per million; Hb, Hemoglobin
Appendix 1 . Quality criteria for observational studies on Zn and esophageal cancer .
| Case-control studies | Prospective cohort studies | ||||||||||
| reference number | 29 | 30 | 31 | 32 | 33 | 34 | 35 | 36 | 37 | 38 | 39 |
| All observational studies | |||||||||||
| Exposure was assessed at the individual level | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| Outcomes were based on objective tests or standard criteria in 90% of study participants | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ |
| The authors presented internal comparisons within study participants | √ | √ | √ | √ | √ | √ | √ | √ | |||
| The authors controlled for potential confounding risk factors in addition to age | √ | √ | √ | √ | √ | √ | √ | √ | |||
| Prospective cohort studies | |||||||||||
| Loss to follow-up was independent of exposure | √ | ||||||||||
| The intensity of search of disease was independent of exposure status | √ | ||||||||||
| Case-control studies | |||||||||||
| Data were collected in a similar manner for all participants | √ | √ | √ | √ | √ | √ | √ | √ | |||
|
The same exclusion criteria were applied to all participants |
√ | √ | √ | ||||||||
| The selection process for Non cases was described | √ | √ | √ | √ | √ | ||||||
| The study was based on incident cases of disease | √ | √ | √ | √ | √ | ||||||
Table 2 . Randomized trials of Zn and esophageal cancer .
| Author | Year | country | population | men | Mean age | Zn form (dose mg) | Other vitamins or minerals combined with Zinc | No of subjects | Factorial design | Placebo controlled/ Double blind |
Intervention
period |
Follow up
After trial |
Outcomes | Relative risk | Quality score 1 |
| Wang 40 | 2013 | China | Patients with dysplasia | 44 | 54 | Zn sulfate (45) | 14 vitamins & 12 minerals/ daily | 3318 | No | Yes | 6 y | 20 y | Total mortality/ Total cancer mortality/ EC mortality | No effect | 4 |
| 2Qiao41 | 2009 | China | Residents in Linxian | 45 | 52 at start | Zn oxide (22.5) | 5000IU retinol palmitate/ daily | 29584 | Yes | Yes | 5.25 y | 10 y | Total mortality/ Total cancer mortality/ EC mortality | Increased total and stroke mortality | 4 |
| Taylor 42 | 1995 | China | Patients with dysplasia | 44 | 54 | Zn sulfate (45) | 14 vitamins & 12 minerals/ daily | 396 | No | Yes | 30 mo | 0 | Reversion to non-dysplasia | 1.26 (1.06-1.46)/ p=0.005 | 4 |
| 72 mo | 0 | Reversion to non-dysplasia | 1.21 (1.02-1.40) / p=0.02 | ||||||||||||
| Zhang43 | 1995 | China | Residents in Linxian/ Patients with dysplasia | 45/ 44 | 52/ 54 | Zn oxide (22.5) / Zn sulfate (45) | 14 vitamins & 12 minerals/ daily | 400 /375 | Yes | Yes | 5.25 y/6 y | 0 | T cell response | No effect | 4 |
| Taylor 44 | 1994 | China | Rencun commune | 50 | 48 at start | Zn oxide (22.5) | 5000IU retinol palmitate/ daily | 391 | Yes | Yes | 5.25 y | 0 | Prevalence of esophageal cancer | OR=1.02 (0.36-2.91) | 4 |
| Prevalence of esophageal dysplasia or cancer |
OR= 1.12 (0.57-2.20) |
||||||||||||||
| Rao 45 | 1994 | China | Patients with dysplasia | 42 | 57 | Zn sulfate (45) | 14 vitamins & 12 minerals/ daily | 512 | No | Yes | 30 mo | 0 | Overall amount of proliferation | p>0.05 | 4 |
| Lower epithelial level | p>0.05 | ||||||||||||||
| Wahrendorf 46 | 1988 | China | Residents in Huixian | 50 | 35-64 | Zn (50 ) / weekly | 50000IU retinol, 200mg riboflavin/ weekly | 610 | No | Yes | 13.5 mo | 0 | Prevalence of precancerous lesions |
OR=0.78, p=0.05 |
3 |
| Munoz 47 | 1987 | China | Residents in Huixian | 50 | 35-64 | Zn (50 ) / weekly | 50000IU retinol, 200mg riboflavin/ weekly | 170 | No | Yes | 13.5 mo | 0 | Prevalence of micronuclei in esophageal cells |
OR=0.61, p=0.04 |
3 |
RESULTS
Observational studies
Ten case-control studies 29-38 and one prospective cohort study 39 were included in the study (figure 1). The studies were published between 1983 and 2013 (table 1). Most studies were performed on participants from Asia. The number of participants varied between 27 36 and 358 .29 The quality scores varied widely (appendix 1). Most articles which had evaluated the association between EC and Zn examined ESCC, with a single study of EA and one EC, where histology was not specified. The single case-control study of EA found no association,29 between Zn and the risk of EC while most studies on ESCC found an inverse association between Zn and the risk of EC (table 1).
Randomized trials
Eight trials 40-47 were included in this study, which were published between 1987 and 2013 (table 2). All trials used Zn combined with other vitamins or minerals. Zn doses were 22.5mg/d zinc oxide or 45mg/d zinc sulfate 33 or 50 mg zinc weekly. In two trials, the form of Zn was not specified. All trials were placebo-controlled and double-blinded. The length of intervention ranged from 13.5 months to 6 years, while some studies have included post-intervention follow-up of up to 20 years. All trials were performed in China and most of the reports came from the two Nutrition Intervention Trials (NIT) conducted in Linxian, China. In the NIT General Population Trial, nine nutrients including Zn were studied. Zn dose was 22.5 mg/d. At the end of this trial, an endoscopic survey was carried out.44 Other reports come from the NIT Dysplasia Trial. In the mentioned study, 3318 individuals who had been previously diagnosed with esophageal dysplasia by balloon cytology, received multivitamins and mineral supplements that included Zn, or placebo for 6 years. Three studies reported different outcomes from this trial.46,47
DISCUSSION
According to our knowledge, this systematic review is the first study evaluating the association between Zn biomarkers and EC. Nineteen studies were included in this review, and most of the observational studies reported an inverse association between Zn biomarkers and EC. This inverse association was observed in populations with different baseline Zn concentrations and in subjects from different countries. However, we found no single agent intervention study to summarize and the multi agent trials have produced conflicting results without clear evidence of benefit.
Most of the observational studies were case-control studies, which present more opportunities for bias than cohort studies. Thus the evidence for a protective effect of higher zinc status against EC is questionable. Observational study results are consistent with animal studies. In animal models, a Zn deficient diet causes a precancerous condition in esophagus 1 and enhances the effects of esophageal carcinogens (e.g., N -nitrosomethylbenzylamine) 6 by different mechanism.
Only one case-control study reported no association between a Zn biomarker (toenail concentration) and risk of EA. Two studies did not specify the histological type.35,37 All other studies which found a significant association were carried out using ESCC cases. The risk factors of these two types are different.19 This conclusion should be interpreted cautiously because only one EA was included in our study. This contradiction may be related to the geographic area, as well. This study was carried in Ireland while most ESCC studies were done with participants from different regions of Asia. A recent meta-analysis reported a significant association between Zn intake, estimated using FFQ, and the risk of digestive tract cancers in Asia, but not in European or American populations.48 The authors concluded that the different source of zinc intake may explain the different results in geographic region subgroups.
Future well designed studies examining the association between Zn biomarkers and EC are warranted. Careful consideration of choice of biomarkers will be important. All biomarkers of Zn, such as hair, nails, urine, or plasma may reflect Zn exposure to some degree.4,49,50 However, the interpretation of biomarkers is not simple because circulating Zn concentrations respond to conditions such as inflammation. Nails are susceptible to soil contamination. Contamination by coloring dyes and anti-dandruff shampoos may limit the suitability of hair. And all observational studies can be affected by confounding factors including socioeconomic status, smoking, or other EC risk factors which could cause the apparent inverse association observed between Zn biomarkers and EC.
In all reported trials, Zn was given combined with other vitamins or minerals. These interventions with supplements containing multiple nutrients do not allow evaluation of the effects of Zn alone. In addition, all reported trials were done in China. Baseline nutritional status of the populations may influence the results.
In the Linxian NIT trials, all studies were null, with the exception of one analysis which reported a positive effect of Zn on reversion to non-dysplasia after 30 and 72 month of starting trial. In the NIT General Population Trial, Zn was co-administered with retinol and there was no apparent effect on ESCC incidence or mortality. Two other trials in Huxian, China, assessed the effect of Zn in combination with retinol and riboflavin versus placebo; this combination was effective in reducing the prevalence of micronuclei in esophageal cells 47 and precancerous lesions.46
The discrepancy between observational studies and the intervention trials could be related to the dose of the intervention agent, the formula of the intervention agent, the age at which the intervention started, or the duration of the intervention. Observational studies may reflect long-term intake of nutrients, whereas trials, have relatively short intervention periods, while cancer has a long latency period. Moreover, different doses may lead to different results and subjects with high or low baseline status may react differently to the intervention.
Currently, observational studies of Zn biomarkers suggest that higher Zn status is associated with reduced risk of EC, but the evidence base is limited by the small number of studies and that many had weak study designs and small sample sizes. Well designed and larger cohort studies are needed before any conclusions can be drawn. Furthermore, current trial data does not suggest that supplements delivered in middle age are beneficial. The evidence base here is also limited by the lack of single agent Zn intervention trials and that most work has been conducted in a single population in China.
In conclusion, an inverse association between Zn concentrations and EC incidence was apparent in most of the reviewed observational studies, but the validity of such studies is uncertain. Randomized trials did not yield any evidence on the beneficence of Zn, but there are many limits to the current evidence base. Overall, the role of Zn in EC incidence is unclear and the benefits of Zn supplementation are not apparent. Yet the strong evidence from animal studies suggests that this hypothesis deserves further consideration.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
Please cite this paper as:
Hashemian M, Hekmatdoost A, Poustchi H, Mohammadi Nasrabadi F, Abnet CC, Malekzadeh R Systematic Review of Zinc Biomarkers and Esophageal Cancer Risk. Middle East J Dig Dis 2014;6:177-85.
References
- 1.Wan SG, Taccioli C, Jiang Y, Chen H, Smalley KJ, Huang K. et al. Zinc deficiency activates S100A8 inflammation in the absence of COX-2 and promotes murine oral-esophageal tumor progression. Int J Cancer. 2011;129:331–45. doi: 10.1002/ijc.25688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stathopoulou MG, Kanoni S, Papanikolaou G, Antonopoulou S, Nomikos T, Dedoussis G. Mineral intake. Prog Mol Biol Transl Sci. 2012;108:201–36. doi: 10.1016/B978-0-12-398397-8.00009-5. [DOI] [PubMed] [Google Scholar]
- 3.Hambidge KM, Miller LV, Westcott JE, Sheng X, Krebs NF. Zinc bioavailability and homeostasis. Am J Clin Nutr. 2010;91:1478S–83S. doi: 10.3945/ajcn.2010.28674I. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lowe NM, Dykes FC, Skinner AL, Patel S, Warthon-Medina M, Decsi T. et al. EURRECA-Estimating zinc requirements for deriving dietary reference values. Crit Rev Food Sci Nutr. 2013;53:1110–23. doi: 10.1080/10408398.2012.742863. [DOI] [PubMed] [Google Scholar]
- 5.Prasad AS, Beck FW, Snell DC, Kucuk O. Zinc in cancer prevention. Nutr Cancer. 2009;61:879–87. doi: 10.1080/01635580903285122. [DOI] [PubMed] [Google Scholar]
- 6.Fong LY, Sivak A, Newberne PM. Zinc deficiency and methylbenzylnitrosamine-induced esophageal cancer in rats. J Natl Cancer Inst. 1978;61:145–50. doi: 10.1093/jnci/61.1.145. [DOI] [PubMed] [Google Scholar]
- 7.Fong LY, Li JX, Farber JL, Magee PN. Cell proliferation and esophageal carcinogenesis in the zinc-deficient rat. Carcinogenesis. 1996;17:1841–8. doi: 10.1093/carcin/17.9.1841. [DOI] [PubMed] [Google Scholar]
- 8.Fong LY, Mancini R, Nakagawa H, Rustgi AK, Huebner K. Combined cyclin D1 overexpression and zinc deficiency disrupts cell cycle and accelerates mouse forestomach carcinogenesis. Cancer Res. 2003;63:4244–52. [PubMed] [Google Scholar]
- 9.Fong LY, Ishii H, Nguyen VT, Vecchione A, Farber JL, Croce CM. et al. P53 deficiency accelerates induction and progression of esophageal and forestomach tumors in zinc-deficient mice. Cancer Res. 2003;63:186–95. [PubMed] [Google Scholar]
- 10.Fong LY, Zhang L, Jiang Y, Farber JL. Dietary zinc modulation of COX-2 expression and lingual and esophageal carcinogenesis in rats. J Natl Cancer Inst. 2005;97:40–50. doi: 10.1093/jnci/dji006. [DOI] [PubMed] [Google Scholar]
- 11.Barch DH, Fox CC, Rosche WA, Rundhaugen LM, Wrighton SA. Inhibition of rat methylbenzylnitrosamine metabolism by dietary zinc and zinc in vitro. Gastroenterology. 1992;103:800–6. doi: 10.1016/0016-5085(92)90009-n. [DOI] [PubMed] [Google Scholar]
- 12.Fong LY, Cheung T, Ho YS. Effect of nutritional zinc-deficiency on O6-alkylguanine-DNA-methyl-transferase activities in rat tissues. Cancer Lett. 1988;42:217–23. doi: 10.1016/0304-3835(88)90308-4. [DOI] [PubMed] [Google Scholar]
- 13.Sun J, Liu J, Pan X, Quimby D, Zanesi N, Druck T. et al. Effect of zinc supplementation on N-nitrosomethylbenzylamine-induced forestomach tumor development and progression in tumor suppressor-deficient mouse strains. Carcinogenesis. 2011;32:351–8. doi: 10.1093/carcin/bgq251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu CG, Zhang L, Jiang Y, Chatterjee D, Croce CM, Huebner K. et al. Modulation of gene expression in precancerous rat esophagus by dietary zinc deficit and replenishment. Cancer Res. 2005;65:7790–9. doi: 10.1158/0008-5472.CAN-05-1345. [DOI] [PubMed] [Google Scholar]
- 15.Ishii H, Vecchione A, Furukawa Y, Croce CM, Huebner K, Fong LY. Differentially expressed genes execute zinc-induced apoptosis in precancerous esophageal epithelium of zinc-deficient rats. Oncogene. 2004;23:8040–8. doi: 10.1038/sj.onc.1207974. [DOI] [PubMed] [Google Scholar]
- 16.Taccioli C, Wan SG, Liu CG, Alder H, Volinia S, Farber JL. et al. Zinc replenishment reverses overexpression of the proinflammatory mediator S100A8 and esophageal preneoplasia in the rat. Gastroenterology. 2009;136:953–66. doi: 10.1053/j.gastro.2008.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alder H, Taccioli C, Chen H, Jiang Y, Smalley KJ, Fadda P. et al. Dysregulation of miR-31 and miR-21 induced by zinc deficiency promotes esophageal cancer. Carcinogenesis. 2012;33:1736–44. doi: 10.1093/carcin/bgs204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Islami F, Kamangar F, Nasrollahzadeh D, Moller H, Boffetta P, Malekzadeh R. Oesophageal cancer in Golestan Province, a high-incidence area in northern Iran - a review. Eur J Cancer. 2009;45:3156–65. doi: 10.1016/j.ejca.2009.09.018. [DOI] [PubMed] [Google Scholar]
- 19.Kamangar F, Chow WH, Abnet CC, Dawsey SM. Environmental causes of esophageal cancer. Gastroenterol Clin North Am. 2009;38:27–57, vii. doi: 10.1016/j.gtc.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamangar F, Malekzadeh R, Dawsey SM, Saidi F. Esophageal cancer in Northeastern Iran: a review. Arch Iran Med. 2007;10:70–82. [PubMed] [Google Scholar]
- 21.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 22.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey SM, Li B. The Linxian trials: mortality rates by vitamin-mineral intervention group. Am J Clin Nutr. 1995;62:1424S–6S. doi: 10.1093/ajcn/62.6.1424S. [DOI] [PubMed] [Google Scholar]
- 23.Blot WJ, Li JY, Taylor PR, Guo W, Dawsey S, Wang GQ. et al. Nutrition intervention trials in Linxian, China: supplementation with specific vitamin/mineral combinations, cancer incidence, and disease-specific mortality in the general population. J Natl Cancer Inst. 1993;85:1483–92. doi: 10.1093/jnci/85.18.1483. [DOI] [PubMed] [Google Scholar]
- 24.Mark SD, Liu SF, Li JY, Gail MH, Shen Q, Dawsey SM. et al. The effect of vitamin and mineral supplementation on esophageal cytology: results from the Linxian Dysplasia Trial. Int J Cancer. 1994;57:162–6. doi: 10.1002/ijc.2910570205. [DOI] [PubMed] [Google Scholar]
- 25.Dawsey SM, Wang GQ, Taylor PR, Li JY, Blot WJ, Li B. et al. Effects of vitamin/mineral supplementation on the prevalence of histological dysplasia and early cancer of the esophagus and stomach: results from the Dysplasia Trial in Linxian, China. Cancer Epidemiol Biomarkers Prev. 1994;3:167–72. [PubMed] [Google Scholar]
- 26.Wang GQ, Dawsey SM, Li JY, Taylor PR, Li B, Blot WJ. et al. Effects of vitamin/mineral supplementation on the prevalence of histological dysplasia and early cancer of the esophagus and stomach: results from the General Population Trial in Linxian, China. Cancer Epidemiol Biomarkers Prev. 1994;3:161–6. [PubMed] [Google Scholar]
- 27.Flores-Mateo G, Navas-Acien A, Pastor-Barriuso R, Guallar E. Selenium and coronary heart disease: a meta-analysis. Am J Clin Nutr. 2006;84:762–73. doi: 10.1093/ajcn/84.4.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jadad AR, Moore RA, Carroll D, Jenkinson C, Reynolds DJ, Gavaghan DJ. et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials. 1996;17:1–12. doi: 10.1016/0197-2456(95)00134-4. [DOI] [PubMed] [Google Scholar]
- 29.O’Rorke MA, Cantwell MM, Abnet CC, Brockman AJ, Murray LJ. Toenail trace element status and risk of Barrett’s oesophagus and oesophageal adenocarcinoma: results from the FINBAR study . Int J Cancer. 2012;131:1882–91. doi: 10.1002/ijc.27434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ray SS, Das D, Ghosh T, Ghosh AK. The levels of zinc and molybdenum in hair and food grain in areas of high and low incidence of esophageal cancer: a comparative study. Glob J Health Sci. 2012;4:168–75. doi: 10.5539/gjhs.v4n4p168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun Z-G, Song G-M, Zhang M, Wang Z. Clinical study on zinc, copper and manganese levels in patients with esophageal squamous cell cancer. Trace Elements and Electrolytes 2011. 2011;28:116–20. [Google Scholar]
- 32.Dar NA, Mir MM, Salam I, Malik MA, Gulzar GM, Yatoo GN. et al. Association between copper excess, zinc deficiency, and TP53 mutations in esophageal squamous cell carcinoma from Kashmir Valley, India--a high risk area. Nutr Cancer. 2008;60:585–91. doi: 10.1080/01635580802290231. [DOI] [PubMed] [Google Scholar]
- 33.Nouri M, Chalian H, Bahman A, Mollahajian H, Ahmadi-Faghih M, Fakheri H. et al. Nail molybdenum and zinc contents in populations with low and moderate incidence of esophageal cancer. Arch Iran Med. 2008;11:392–6. [PubMed] [Google Scholar]
- 34.Goyal MM, Kalwar AK, Vyas RK, Bhati A. A study of serum zinc, selenium and copper levels in carcinoma of esophagus patients. Indian J Clin Biochem. 2006;21:208–10. doi: 10.1007/BF02913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dursun H, Bilici M, Uyanik A, Okcu N, Akyuz M. Antioxidant enzyme activities and lipid peroxidation levels in erythrocytes of patients with oesophageal and gastric cancer. J Int Med Res. 2006;3:193–9. doi: 10.1177/147323000603400209. [DOI] [PubMed] [Google Scholar]
- 36.Mellow MH, Layne EA, Lipman TO, Kaushik M, Hostetler C, Smith JC, Jr Jr. Plasma zinc and vitamin A in human squamous carcinoma of the esophagus. Cancer. 1983;51:1615–20. doi: 10.1002/1097-0142(19830501)51:9<1615::aid-cncr2820510911>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 37.Rogers MA, Thomas DB, Davis S, Vaughan TL, Nevissi AE. A case-control study of element levels and cancer of the upper aerodigestive tract. Cancer Epidemiol Biomarkers Prev. 1993;2:305–12. [PubMed] [Google Scholar]
- 38.Prasad MP, Krishna TP, Pasricha S, Krishnaswamy K, Quereshi MA. Esophageal cancer and diet--a case-control study. Nutr Cancer. 1992;18:85–93. doi: 10.1080/01635589209514208. [DOI] [PubMed] [Google Scholar]
- 39.Abnet CC, Lai B, Qiao YL, Vogt S, Luo XM, Taylor PR. et al. Zinc concentration in esophageal biopsy specimens measured by x-ray fluorescence and esophageal cancer risk. J Natl Cancer Inst. 2005;97:301–6. doi: 10.1093/jnci/dji042. [DOI] [PubMed] [Google Scholar]
- 40.Wang JB, Abnet CC, Fan JH, Qiao YL, Taylor PR. The randomized Linxian Dysplasia Nutrition Intervention Trial after 26 years of follow-up: no effect of multivitamin supplementation on mortality. JAMA Intern Med. 2013;173:1259–61. doi: 10.1001/jamainternmed.2013.6066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qiao YL, Dawsey SM, Kamangar F, Fan JH, Abnet CC, Sun XD. et al. Total and cancer mortality after supplementation with vitamins and minerals: follow-up of the Linxian General Population Nutrition Intervention Trial. J Natl Cancer Inst. 2009;101:507–18. doi: 10.1093/jnci/djp037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Taylor PR, Wang GQ, Dawsey SM, Guo W, Mark SD, Li JY. et al. Effect of nutrition intervention on intermediate endpoints in esophageal and gastric carcinogenesis. Am J Clin Nutr. 1995:621420S–3S. doi: 10.1093/ajcn/62.6.1420S. [DOI] [PubMed] [Google Scholar]
- 43.Zhang YH, Kramer TR, Taylor PR, Li JY, Blot WJ, Brown CC. et al. Possible immunologic involvement of antioxidants in cancer prevention. Am J Clin Nutr. 1995;62:1477S–82S. doi: 10.1093/ajcn/62.6.1477S. [DOI] [PubMed] [Google Scholar]
- 44.Taylor PR, Li B, Dawsey SM, Li JY, Yang CS, Guo W. et al. Prevention of esophageal cancer: the nutrition intervention trials in Linxian, ChinaLinxian Nutrition Intervention Trials Study Group. Cancer Res. 1994;54:2029s–31s. [PubMed] [Google Scholar]
- 45.Rao M, Liu FS, Dawsey SM, Yang K, Lipkin M, Li JY. et al. Effects of vitamin/mineral supplementation on the proliferation of esophageal squamous epithelium in Linxian, China. Cancer Epidemiol Biomarkers Prev. 1994;3:277–9. [PubMed] [Google Scholar]
- 46.Wahrendorf J, Munoz N, Lu JB, Thurnham DI, Crespi M, Bosch FX. Blood, retinol and zinc riboflavin status in relation to precancerous lesions of the esophagus: findings from a vitamin intervention trial in the People's Republic of China. Cancer Res. 1988;48:2280–3. [PubMed] [Google Scholar]
- 47.Munoz N, Hayashi M, Bang LJ, Wahrendorf J, Crespi M, Bosch FX. Effect of riboflavin, retinol, and zinc on micronuclei of buccal mucosa and of esophagus: a randomized double-blind intervention study in China. J Natl Cancer Inst. 1987;79:687–91. [PubMed] [Google Scholar]
- 48.Li P, Xu J, Shi Y, Ye Y, Chen K, Yang J. et al. Association between zinc intake and risk of digestive tract cancers: A systematic review and meta-analysis. Clin Nutr. 2014;33:415–20. doi: 10.1016/j.clnu.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 49.Wolowiec P, Michalak I, Chojnacka K, Mikulewicz M. Hair analysis in health assessment. Clin Chim Acta . 2013;419:139–71. doi: 10.1016/j.cca.2013.02.001. [DOI] [PubMed] [Google Scholar]
- 50.Lowe NM, Medina MW, Stammers AL, Patel S, Souverein OW, Dullemeijer C. et al. The relationship between zinc intake and serum/plasma zinc concentration in adults: a systematic review and dose-response meta-analysis by the EURRECA Network. Br J Nutr. 2012;108:1962–71. doi: 10.1017/S0007114512004382. [DOI] [PubMed] [Google Scholar]


