Abstract
BACKGROUND
Furazolidone has been used as an alternative for clarithromycin or metronidazole in Helicobacterpylori (H.pylori) eradication regimens. In Iran, 14-day Furazolidone-containing quadruple regimens have shown promising eradication rates, but short-course, low dose therapies are always attractive. Therefore, we designed a study to compare the efficacy of two 10-day triple regimens containing moderate and high dose furazolidone for H.pylori eradication.
METHODS
Two hundred and ten patients with peptic ulcer disease who were naïve to H.pylori treatment were included. They were randomized into 2 groups: 105 patients received omeprazole 20mg, amoxicillin 1000mg, and furazolidone 200mg(OAF-400), all twice a day for ten days.And the remaining 105 patients received omeprazole 20mg twice a day, amoxicillin 1000mg twice a day and furazolidone 200mg three times a day for ten days(OAF-600). Urease breath test was performed 8 weeks after the treatment to confirm H. pylori eradication.
RESULTS
The intention-to-treat eradication rate was 76.19% in group OAF-400 and 80.95% in group OAF-600 (pp=0.38). Per protocol eradication rates were 81.63% and 89.47%, respectively (p= 0.11).Severe adverse effects were reported by 8.6% of the patients in group OAF-400 and 5.7% of the patient in group OAF-600 (p=0.1). However, the total side effects (including mild, moderate, and severe ones) were significantly more prevalent in the OAF-600 group (p=0.001).
CONCLUSION
None of our triple furazolidone-based regimens (moderate- and high-dose) could achieve the standard eradication rate, and therefore, cannot be considered as a suitable option for first-line treatment.
Keywords: Helicobacter pylori, Eradication, Furazolidone
INTRODUCTION
Helicobacter pylori(H. pylori) is the most common bacterial infection, affecting almost half of the world’s population.1 It is a known cause for peptic ulcer disease, as well as gastric adenocarcinoma, and lymphoma.1 Although numerous studies have been performed to determine the ideal regimen for eradicating the bacteria, results vary among different geographic regions and sometimes they had been disappointing. The reasons for failure to treatment are numerous, but the most important factors include primary resistance to antibiotics and differences in bacterial strains.2,3
Clarithromycin is an antibiotic that has been successfully used as an alternative to metronidazole in standard triple therapy for H. pylori eradication. But massive use of the drug has led to decrease in the eradication rates, sometimes even to lower than 70%.4 On the other hand, clarithromycin is an expensive drug and not simply available in developing countries.
Furazolidone has also been used as an alternative to clarithromycin or metronidazole in H. pylori eradication regimens. The drug is readily available with low costs. In Iran, 14-day furazolidone-containing quadruple regimens have shown promising eradication rates,5-8 but short-course, low dose therapies have always been attractive. Therefore, we designed a study to compare the efficacy of two 10-day triple regimens containing moderate and highdose furazolidone for H. pylori eradication.
MATERIALS AND METHODS
Two hundred and ten patients with endoscopically confirmed peptic ulcer disease and positive H. pylori infection, documented by antral biopsy and rapid urease test, entered the study. All the patients were naïve to H. pylori treatment. Upper endoscopy and biopsy sampling were performed using Fujinon EG-250WR5 videogastroscope(Fuji Photo Optical Ltd, Japan).
The exclusion criteria were: age less than 18 years, significant underlying disease including liver, cardiac, pulmonary, and renal diseases, neoplasia, coagulopathy, history of gastric surgery, pregnancy, breast-feeding, glucose-6-phosphate dehydrogenase deficiency, and history of allergic reactions to any of the medications used in this protocol.
Demographic information, smoking habits, history of previous upper gastrointestinal bleeding (GIB), and some endoscopic findings including ulcer type, ulcer diameter, and number of ulcers were recorded in questionnaires.
The patients were randomly assigned to one of these 2 groups using a computer generated randomization system: 105 patients received omeprazole 20mg, amoxicillin 1000mg, and furazolidone 200mg(OAF-400), all twice a day for ten days. And the remaining 105 patients received omeprazole 20mg twice a day, amoxicillin 1000mg twice a day and furazolidone 200mg three times a day for ten days(OAF-600).
The patients were provided by written instructions and written informed consents were obtained from all of them. They were also informed about avoiding some special foods due to mono-amine oxidase inhibition by furazolidone.
The patients were asked to record the side effects of the medications on a daily basis and were also advised to call the doctor in case of severe side effects. After the treatment course, they were visited and were asked about their compliance to treatment and side effects of therapy. The severity of side effects were classified as: No side effect, mild (not interfering with daily activities), moderate (partially interfering with daily activities), and severe (abandoning daily activities).
The compliance to treatment was considered to be excellent if the patient had used more than 80% of the medications, moderate if the patient used 60-80% of the medications, and poor in case of taking less than 60% of the prescribed drugs. Eight weeks after the treatment course, H. pylori eradication was assessed by C¹⁴-urea breath test (UBT) (Heliprobe Breath Card and Analyser, Kibion AB, Uppsala, Sweden).
Statistical Analysis
According to the results of previous studies performed on Furazolidone-containing regimens, we assumed to have about 8% difference between the two regimens in their ability to eradicate H. pylori.9-11 We included 105 patients in each group.
To calculate the intention to treat eradication rates, everyone who entered the study was considered and to calculate per-protocol eradication rates, only those who completed the entire protocol with more than 80% compliance to treatment were considered in the analysis. Data were analyzed using SPSS software for windows (version 17) and Chi-square and t tests were used as appropriated. P values less than 0.05 were considered statistically significant. The statistician who analyzed the final results was blinded to patients’ allocation.
RESULTS
Two hundred and ten patients were included in the study. Demographic and baseline characteristics of the patients were not statistically different between the two groups (table 1). One hundred and ninety nine patients completed the study. Four patients did not perform UBT (two patients in each group). Also, four patients in OAF-400 and three patients in OAF-600 groups discontinued treatment due to adverse effects of therapy.
Table 1 . Demographic, clinical characteristics and endoscopic findings of the patients .
| Variable | OAF-400 * | OAF-600 * | p -value | |
|
Male/ Female Age(mean±SD)(years) Smokers N (%) |
53/52 42.4 ± 11.9 17 (16.1) |
57/48 42.2 ± 14.1 19 (18.1) |
0.35 0.54 0.4 |
|
| Number of ulcers |
Single Multiple No ulcer (erosion) |
45 (42.8%) 24 (22.8%) 36 (34.3%) |
61 (58.1%) 15 (14.2%) 29 (27.6%) |
0.42 |
|
Type of ulcer |
Duodenal ulcer Gastric ulcer Duodenal+Gastric ulcer Duodenal erosion Gastric erosion Duodenal+Gastric erosion |
53 (50.5%) 14 (13.3%) 2 (1.9%) 15 (14.3%) 21 (20%) 0 (0%) |
65 (61.9%) 10 (9.5%) 1 (0.9%) 5 (4.8%) 21 (20%) 3 (2.9%) |
0.07 |
* OAF: Omeprazole, Amoxicillin, Furazolidone
Compliance to treatment was excellent in 95.2% of the patients in OAF-400 and 90.5% in OAF-600 groups. One of the patients in OAF-400 and seven patients in OAF-600 groups had used 60-80% of the prescribed drugs. Furthermore, four and three patients had used less than 60% of the medications in the two groups, respectively. But totally, these rates were not significantly different between the two groups (p= 0.9).
According to intention to treat (ITT) analysis, eradication rates were 76.19% (80/105) (95% CI=68-84.2) in OAF-400group and 80.95% (85/105) (95% CI=73.4-88.4) in OAF-600group (p=0.38). Per-protocol (pp) eradication rates were 81.63% (80/98) (95% CI=74-89.2) and89.47% (85/95) (95% CI=83.3-95.5), respectively (p= 0.11, figure 1).
Fig. 1 .
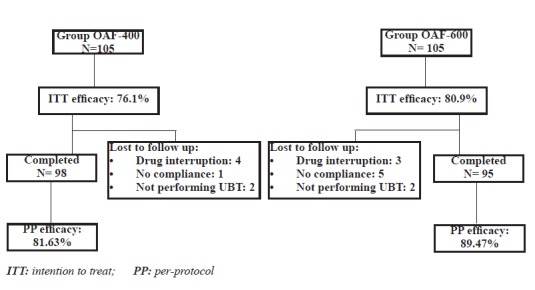
Method of follow up & treatment efficacy
It should be noted that three of the patients who had used 60-80% of medications (2 in OAF-400 and 1 in OAF-600) had negative UBT results, but they were not included in PP analysis. Also one of the patients who had used less than 60% of medications in OAF-600 group had negative UBT result. He, too, was not considered in PP analysis.
Severe side effects occurred in 9 (8.6%) of the patients in OAF-400 and 6 (5.7%) in OAF-600 groups, but the rates were not significantly different between the two groups (p=0.1). However, the total side effects (including mild, moderate, and severe side effects) were significantly more prevalent in the OAF-600 group (p=0.001). The most common side effects were nausea, malaise, and dizziness, being significantly more common in OAF-600 group (table 2). Also, most adverse effects occurred during the second half of treatment.
Table 2 . Adverse effects reported by the patients during treatment .
| Adverse effect | OAF-400 | OAF-600 | p -value | |
| Malaise | 0 (0%) | 7 (6.7%) | 0.007 | |
| Nausea & Vomiting | 2 (1.9%) | 19 (18.1%) | 0.04 | |
| Bitter taste | 0 (0%) | 2 (1.9%) | 0.15 | |
| Dizziness | 4 (3.8%) | 12 (11.4%) | 0.03 | |
| Dyspepsia | 0 (0%) | 3 (2.9%) | 0.08 | |
| Rash | 0 (0%) | 1 (0.9%) | 0.31 | |
| Arthralgia | 1 (0.9%) | 2 (1.9%) | 0.56 | |
| Orthostatic hypotension | 0 (0%) | 8 (7.6%) | 0.004 | |
| Urticaria | 1 (0.9%) | 1 (0.9%) | 0.1 | |
| Pruritus | 1 (0.9%) | 0 (0%) | 0.31 | |
| Headache | 1 (0.9%) | 6 (5.7%) | 0.055 | |
| Abdominal pain | 1 (0.9%) | 1 (0.9%) | 1 | |
| Chest pain | 0 (0%) | 1 (0.9%) | 0.31 | |
| Diarrhea | 1 (0.9%) | 2 (1.9%) | 0.56 | |
| Bloating | 2 (1.9%) | 0 (0%) | 0.15 | |
| Fever | 1 (0.9%) | 8 (6.7%) | 0.01 | |
| Anorexia | 1 (0.9%) | 2 (1.9%) | 0.56 | |
| Severity of side effects N (%) | Mild | 4(3.8%) | 34 (31.4%) | 0.001 |
| Moderate | 4(3.8%) | 14 (13.3%) | ||
| Severe | 9 (8.6%) | 6 (5.7%) | ||
| Drug interruption due to side effects | 4 (3.8%) | 3 (2.8%) |
DISCUSSION
H. pylori eradication rates have been mostly lower in developing countries compared with the developed ones.12 It seems that high resistance to antibiotics, especially to metronidazole (40-70%) has played the most important role for the difference.13-19 Therefore, metronidazole-containing regimens are not usually recommended for the developing countries.
Clarithromycin is an antibiotic that is widely used as an alternative to metronidazole in H. pylori eradication regimens. But common use of the drug has led to decrease in eradication rates, even to unacceptable level in some studies.20-24 De Boer and colleagues have reported the evolution of clarithromycin resistance from 1% to 13% in the previous years.25 Furthermore, Graham and co-workers believe that resistance to clarithromycin is the most important factor in the current failure rates.26 On the other hand, clarithromycin is an expensive drug and is not simply available in developing countries.
Furazolidone has also been used as an alternative toclarithromycin or metronidazole in H. pylori eradication regimens.27-30 Furazolidone does not have cross-resistance with metronidazole and therefore, is recommended to be used in areas with high metronidazole resistance.31 The drug is readily available with low costs.
The first studies evaluating the effects of furazolidone-containing regimens were reported in the early 1990’s.32 In Iran the first reports were in 200028 and the resistance rate to furazolidone has increased during the previous 10 years, but the change in resistance rate was not significant (from 0% in 2003 to 4.5% in 2010).33
In Iran, 14-day furazolidone-containing quadruple regimens have shown promising eradication rates.6,9,11,34 However, the side effects of the drug have limited its use. The most common adverse effects include sudden hypotension, fever, headache, abdominal pain, and skin rash. It seems that they mostly occur due to inhibition of mono-amine oxidase.2 Although these reactions lead to cessation of therapy by some patients, they mostly occur in the second week of treatment.11 Therefore, some researchers modified their treatment strategies, administering furazolidone just during the first or the second week of treatment or using short term protocols.
In 2011, Fakheri and colleagues evaluated two modified quadruple therapies: the first group received omeprazole, amoxicillin, and metronidazole for 2 weeks and the second group received omeprazole, amoxicillin, and bismuth for 2 weeks. Both groups also received furazolidone just during the first week of treatment. The per-protocol eradication rates were 86.36% and 90.27%, respectively. Adverse effects of treatment were significantly lower in the second group (12.8% vs. 2.5%).35
Fakheri and colleagues also performed another study in which low-dose furazolidone (100mg twice a day) was administered in triple and quadruple regimens. But per-protocol eradication rates were not acceptable (54% and 72%, respectively).33
Consequently, two other bismuth-based quadruple therapies were conducted in 10- and 14-day regimens in which metronidazole was administered over the first half of therapy and was then replaced by furazolidone over the second half. Both protocols had ideal results with fewer side effects.34,36
Khatibian and colleagues performed a study comparing 14-day omeprazole-amoxicillin-bismuth-furazolidone with a 14-day quadruple regimen in which metronidazole was given during the first week and then was replaced by furazolidone during the second week. Per-protocol eradication rates were 95.2% and 95.3%, respectively.34
In another study, Ghadir and colleagues compared the efficacy of omeprazole-amoxicillin-furazolidone triple therapy with omeprazole-amoxicillin-bismuth-furazolidone quadruple therapy. PP eradication rates were 61.1% vs. 85.3%, respectively (p< 0.05).10
Daryani and colleagues also assessed the efficacy of a 14-day furazolidone-containing quadruple therapy. The eradication rates were 71.4% and 87.7% in men and women, respectively.37
Totally, furazolidone has been used in nine quadruple therapies in Iran, four of which showed optimal efficacy. However, severe side effects were reported in 14-day regimens.5,6,10,20,28,33,34,36-38 Also, five studies have investigated the effects of 4-, 7- and 14-day furazolidone-containing triple therapies in Iran.6,9-11,39 But only a 7-day protocol, in which a probiotic had been added to the regimen, could achieve >90% PP eradication rate.9
In the present study, we evaluated the effects of moderate- and high-dose furazolidone-containing regimens on H. pylori eradication. Our first hypothesis was to assess whether moderate-dose furazolidone-containing treatment could be as effective as the high-dose regimen. The ITT eradication rates were 76.19% vs. 80.95% and PP eradication rates were 81.36% vs. 89.47%, respectively. The differences in the eradication rates were not statistically significant. However, if we consider H. pylori infection as an infectious disease, the ideal regimen is the one that can eradicate H. pylori infection in more than 95% of cases. Graham classified the efficacy of treatment according to per protocol success as A: excellent (>95% eradication rate), B: good (90-95%), C: fair (85-89%), D: poor (81-84%) and F: unacceptable (≤80%).40 Accordingly, although the regimen containing high-dose furazolidone could achieve higher eradication rate, it is classified in group C, and therefore, cannot be recommended as a suitable first-line option.
The only previous study evaluating the effects of high-dose furazolidone (600mg/day) was performed by Frota and colleagues. According to their study, a triple regimen containing omeprazole, tetracycline, and high-dose furazolidone could achieve 91.8% per-protocol eradication rate. But the duration of treatment was 14 days that was longer than our protocol.41
Another important issue in introducing an ideal option for H. pylori eradication is the occurrence of side effects. A standard H. pylori eradication regimen should have less than 5% severe adverse effects. But in our study, although the OAF-600 group had near-optimal eradication rate, it had more than 5% adverse effects.
Previous studies have shown that most side effects of furazolidone-containing regimens occur in the second week of treatment.5,42 This is in concordance with our study. These data support the use of shorter duration of furazolidone in treatment protocols.
Our second hypothesis was that moderate-dose regimen would lead to higher compliance to treatment than the high-dose therapy. As it was assumed, the number of patients who had less than 80% compliance to treatment was higher in OAF-600 than the OAF-400 group (8 patients vs. 5 patients), but the difference was not statistically significant.
Finally, it should be mentioned that the main limitation of our study was the unavailability of H. pylori culture. However the latest study in the same geographic region has reported 73.4% resistance to metronidazole, 30% to clarithromycin, 6.8% to amoxicillin, and 9% to tetracycline.43
In conclusion, none of our triple furazolidone-based regimens (moderate- and high-dose) could achieve the standard eradication rate, and therefore, cannot be considered as a suitable option for first-line treatment. Due to the ineffectiveness of furazolidone-containing triple regimens, we recommend that in future studies, the efficacy of furazolidone in quadruple regimens be investigated.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
Please cite this paper as:
Hosseini V, Mokhtare M, Gholami M, Taghvaei T, Maleki I, Valizadeh M, Bari Z, Fakheri H. A Comparison between Moderate- and High-dose Furazolidone in Triple Regimens for Helicobacterpylori Eradication in Iran. Middle East J Dig Dis 2014;6:195-202.
References
- 1.Go MF. Review article: natural history and epidemiology of Helicobacter pylori infection. Aliment Pharmacol Ther. 2002;16Suppl 1:3–15. doi: 10.1046/j.1365-2036.2002.0160s1003.x. [DOI] [PubMed] [Google Scholar]
- 2.Graham DY, Fischbach L. Helicobacter pylori treatment in the era of increasing antibiotic resistance. Gut. 2010;59:1143–53. doi: 10.1136/gut.2009.192757. [DOI] [PubMed] [Google Scholar]
- 3.van Doorn LJ, Schneeberger PM, Nouhan N, Plaisier AP, Quint WG, de Boer WA. Importance of Helicobacter pylori cagA and vacA status for the efficacy of antibiotic treatment. Gut. 2000;46:321–6. doi: 10.1136/gut.46.3.321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jafri NS, Hornung CA, Howden CW. Meta-analysis: sequential therapy appears superior to standard therapy for Helicobacter pylori infection in patients naive to treatment. Ann Intern Med. 2008;148:923–31. doi: 10.7326/0003-4819-148-12-200806170-00226. [DOI] [PubMed] [Google Scholar]
- 5.Daghaghzadeh H, Emami MH, Karimi S, Raeisi M. One-week versus two-week furazolidone-based quadruple therapy as the first-line treatment for Helicobacter pylori infection in Iran. J Gastroenterol Hepatol. 2007;22:1399–403. doi: 10.1111/j.1440-1746.2007.05029.x. [DOI] [PubMed] [Google Scholar]
- 6.Fakheri H, Merat S, Hosseini V, Malekzadeh R. Low-dose furazolidone in triple and quadruple regimens for Helicobacter pylori eradication. Aliment Pharmacol Ther. 2004;19:89–93. doi: 10.1046/j.1365-2036.2003.01822.x. [DOI] [PubMed] [Google Scholar]
- 7.Fakheri H, Bari Z, Sardarian H. A modified bismuth-containing quadruple therapy including a short course of furazolidone for Helicobacter pylori eradication after sequential therapy failure. Helicobacter. 2012;17:264–8. doi: 10.1111/j.1523-5378.2012.00946.x. [DOI] [PubMed] [Google Scholar]
- 8.Fakheri H, Taghvaei T, Hosseini V, Bari Z. A comparison between sequential therapy and a modified bismuth-based quadruple therapy for Helicobacter pylori eradication in Iran: a randomized clinical trial. Helicobacter. 2012;17:43–8. doi: 10.1111/j.1523-5378.2011.00896.x. [DOI] [PubMed] [Google Scholar]
- 9.Ahmad K, Fatemeh F, Mehri N, Maryam S. Probiotics for the treatment of pediatric helicobacter pylori infection: a randomized double blind clinical trial. Iran J Pediatr. 2013;23:79–84. [PMC free article] [PubMed] [Google Scholar]
- 10.Ghadir MR, Shafaghi A, Iranikhah A, Pakdin A, Joukar F, Mansour-Ghanaei F. Furazolidone, amoxicillin and omeprazole with or without bismuth for eradication of Helicobacter pylori in peptic ulcer disease. Turk J Gastroenterol. 2011;22:1–5. [PubMed] [Google Scholar]
- 11.Roghani HS, Massarrat S, Shirekhoda M, Butorab Z. Effect of different doses of furazolidone with amoxicillin and omeprazole on eradication of Helicobacter pylori. J Gastroenterol Hepatol. 2003;18:778–82. doi: 10.1046/j.1440-1746.2003.03058.x. [DOI] [PubMed] [Google Scholar]
- 12.Malfertheiner P, Megraud F, O'Morain C, Bazzoli F, El-Omar E, Graham D. et al. Current concepts in the management of Helicobacter pylori infection: the Maastricht III Consensus Report. Gut. 2007;56:772–81. doi: 10.1136/gut.2006.101634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abu-Mahfouz MZ, Prasad VM, Santogade P, Cutler AF. Helicobacter pylori recurrence after successful eradication: 5-year follow-up in the United States. Am J Gastroenterol. 1997;92:2025–8. [PubMed] [Google Scholar]
- 14.Dani R, Queiroz DM, Dias MG, Franco JM, Maqalhaes LC, Mendes GS. et al. Omeprazole, clarithromycin and furazolidone for the eradication of Helicobacter pylori in patients with duodenal ulcer. Aliment Pharmacol Ther. 1999;13:1647–52. doi: 10.1046/j.1365-2036.1999.00653.x. [DOI] [PubMed] [Google Scholar]
- 15.Drouin E. Helicobacter pylori: novel therapies. Can J Gastroenterol. 1999;13:581–3. doi: 10.1155/1999/485237. [DOI] [PubMed] [Google Scholar]
- 16.Kim JJ, Reddy R, Lee M, Kim JG, El-Zaatari FA, Osato MS. et al. Analysis of metronidazole, clarithromycin and tetracycline resistance of Helicobacter pylori isolates from Korea. J Antimicrob Chemother. 2001;47:459–61. doi: 10.1093/jac/47.4.459. [DOI] [PubMed] [Google Scholar]
- 17.Megraud F. Resistance of Helicobacter pylori to antibiotics. Aliment Pharmacol Ther. 1997;11 Suppl 1:43–53. doi: 10.1046/j.1365-2036.11.s1.11.x. [DOI] [PubMed] [Google Scholar]
- 18.Osato MS, Reddy R, Graham DY. Metronidazole and clarithromycin resistance amongst Helicobacter pylori isolates from a large metropolitan hospital in the United States. Int J Antimicrob Agents. 1999;12:341–7. doi: 10.1016/s0924-8579(99)00079-5. [DOI] [PubMed] [Google Scholar]
- 19.Segura AM, Gutierrez O, Otero W, Angel A, Genta RM, Graham DY. Furazolidone, amoxycillin, bismuth triple therapy for Helicobacter pylori infection. Aliment Pharmacol Ther. 1997;11:529–32. doi: 10.1046/j.1365-2036.1997.00172.x. [DOI] [PubMed] [Google Scholar]
- 20.Taghavi SA, Jafari A, Eshraghian A. Efficacy of a new therapeutic regimen versus two routinely prescribed treatments for eradication of Helicobacter pylori: a randomized, double-blind study of doxycycline, co-amoxiclav, and omeprazole in Iranian patients. Dig Dis Sci. 2009;54:599–603. doi: 10.1007/s10620-008-0374-z. [DOI] [PubMed] [Google Scholar]
- 21.Uygun A, Kadayifci A, Yesilova Z, Savas MC, Ates Y, Karslioglu Y. et al. Recent success of pantoprazole -or lansoprazole- based clarithromycin plus amoxicillin treatment in the eradication of Helicobacter pylori. Turk J Gastroenterol. 2004;15:219–24. [PubMed] [Google Scholar]
- 22.Yasar B, Abut E, Kayadibi H, Toros B, Sezikli M, Akkan Z. et al. Efficacy of probiotics in Helicobacter pylori eradication therapy. Turk J Gastroenterol. 2010;21:212–7. doi: 10.4318/tjg.2010.0090. [DOI] [PubMed] [Google Scholar]
- 23.Alkim H, Iscan M, Oz F. Effectiveness of ranitidine bismuth citrate and proton pump inhibitor based triple therapies of Helicobacter pylori in Turkey. Libyan J Med. 2011;6 doi: 10.3402/ljm.v6i0.8412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Songur Y, Senol A, Balkarli A, Basturk A, Cerci S. Triple or quadruple tetracycline-based therapies versus standard triple treatment for Helicobacter pylori treatment. Am J Med Sci. 2009;338:50–3. doi: 10.1097/MAJ.0b013e31819c7320. [DOI] [PubMed] [Google Scholar]
- 25.de Boer W, Driessen W, Jansz A, Tytgat G. Effect of acid suppression on efficacy of treatment for Helicobacter pylori infection. Lancet. 1995;345:817–20. doi: 10.1016/s0140-6736(95)92962-2. [DOI] [PubMed] [Google Scholar]
- 26.Graham DY, Qureshi WA. Antibiotic-resistant Hpylori infection and its treatment. Curr Pharm Des. 2000;6:1537–44. doi: 10.2174/1381612003399077. [DOI] [PubMed] [Google Scholar]
- 27.Sotoudehmanesh R, Malekzadeh R, Vahedi H, Dariani NE, Asgari AA, Massarrat S. Second-line Helicobacter pylori eradication with a furazolidone-based regimen in patients who have failed a metronidazole-based regimen. Digestion. 2001;64:222–5. doi: 10.1159/000048865. [DOI] [PubMed] [Google Scholar]
- 28.Malekzadeh R, Ansari R, Vahedi H, Siavoshi F, Alizadeh BZ, Eshraghian MR. et al. Furazolidone versus metronidazole in quadruple therapy for eradication of Helicobacter pylori in duodenal ulcer disease. Aliment Pharmacol Ther. 2000;14:299–303. doi: 10.1046/j.1365-2036.2000.00709.x. [DOI] [PubMed] [Google Scholar]
- 29.Graham DY, Osato MS, Hoffman J, Opekun AR, Anderson SY, El-Zimaity HM. Furazolidone combination therapies for Helicobacter pylori infection in the United States. Aliment Pharmacol Ther. 2000;14:211–5. doi: 10.1046/j.1365-2036.2000.00640.x. [DOI] [PubMed] [Google Scholar]
- 30.Liu WZ, Xiao SD, Shi Y, Wu SM, Zhang DZ, Xu WW. et al. Furazolidone-containing short-term triple therapies are effective in the treatment of Helicobacter pylori infection. Aliment Pharmacol Ther. 1999;13:317–22. doi: 10.1046/j.1365-2036.1999.00492.x. [DOI] [PubMed] [Google Scholar]
- 31.Fennerty MB, Lieberman DA, Vakil N, Magaret N, Faigel DO, Helfand M. Effectiveness of Helicobacter pylori therapies in a clinical practice setting. Arch Intern Med. 1999;159:1562–6. doi: 10.1001/archinte.159.14.1562. [DOI] [PubMed] [Google Scholar]
- 32.Coelho LG, Passos MC, Chausson Y, Castro Lde P. Five-day bismuth-free triple therapy for the eradication of Helicobacter pylori and reduction of duodenal ulcer relapse. Am J Gastroenterol. 1991;86:971–5. [PubMed] [Google Scholar]
- 33.Fakheri H, Malekzadeh R, Merat S, Khatibian M, Fazel A, Alizadeh BZ. et al. Clarithromycin vsfurazolidone in quadruple therapy regimens for the treatment of Helicobacter pylori in a population with a high metronidazole resistance rate. Aliment Pharmacol Ther. 2001;15:411–6. doi: 10.1046/j.1365-2036.2001.00931.x. [DOI] [PubMed] [Google Scholar]
- 34.Khatibian M, Ajvadi Y, Nasseri-Moghaddam S, Ebrahimi-Daryani N, Vahedi H, Zendehdel N. et al. Furazolidone-based, metronidazole-based, or a combination regimen for eradication of Helicobacter pylori in peptic ulcer disease. Arch Intern Med. 2007;10:161–7. [PubMed] [Google Scholar]
- 35.Fakheri H, Fakhri M, Shahmohammadi S. Efficacy of short-duration Furazolidone in two different quadruple regimens for Hpylori eradication. Pak J Med Sci. 2011;27:887–91. [Google Scholar]
- 36.Riahizadeh S, Malekzadeh R, Agah S, Zendehdel N, Sotoudehmanesh R, Ebrahimi-Daryani N. et al. Sequential metronidazole-furazolidone or clarithromycin-furazolidone compared to clarithromycin-based quadruple regimens for the eradication of Helicobacter pylori in peptic ulcer disease: a double-blind randomized controlled trial. Helicobacter. 2010;15:497–504. doi: 10.1111/j.1523-5378.2010.00798.x. [DOI] [PubMed] [Google Scholar]
- 37.Ebrahimi-Dariani N, Mirmomen S, Mansour-Ghanaei F, Noormohammadpoor P, Sotodehmanesh R, Haghpanah B. et al. The efficacy of furazolidone-based quadruple therapy for eradication of Hpylori infection in Iranian patients resistant to metronidazole-based quadruple therapy. Med Sci Monit. 2003;9:105–8. [PubMed] [Google Scholar]
- 38.Agah S, Shazad B, Abbaszadeh B. Comparison of azithromycin and metronidazole in a quadruple-therapy regimen for Helicobacter pylori eradication in dyspepsia. Saudi J Gastroenterol. 2009;15:225–8. doi: 10.4103/1319-3767.56091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mirzaee V, Rezahosseini O. Randomized control trial: Comparison of Triple Therapy plus Probiotic Yogurt vsStandard Triple Therapy on Helicobacter Pylori Eradication. Iran Red Crescent Med J. 2012;14:657–66. [PMC free article] [PubMed] [Google Scholar]
- 40.Graham DY, Lu H, Yamaoka Y. A report card to grade Helicobacter pylori therapy. Helicobacter. 2007;12:275–8. doi: 10.1111/j.1523-5378.2007.00518.x. [DOI] [PubMed] [Google Scholar]
- 41.Frota LC, da Cunha Mdo P, Luz CR, de Araujo-Filho AH, Frota LA, Braga LL. Helicobacter pylori eradication using tetracycline and furazolidone versus amoxicillin and azithromycin in lansoprazole based triple therapy: an open randomized clinical trial. Arq Gastroenterol. 2005;42:111–5. doi: 10.1590/s0004-28032005000200009. [DOI] [PubMed] [Google Scholar]
- 42.Megraud F, Lamouliatte H. Review article: the treatment of refractory Helicobacter pylori infection. Aliment Pharmacol Ther. 2003;17:1333–43. doi: 10.1046/j.1365-2036.2003.01592.x. [DOI] [PubMed] [Google Scholar]
- 43.Talebi Bezmin Abadi A, Mobarez AM, Taghvaei T, Wolfram L. Antibiotic resistance of Helicobacter pylori in Mazandaran, North of Iran. Helicobacter. 2010;15:505–9. doi: 10.1111/j.1523-5378.2010.00795.x. [DOI] [PubMed] [Google Scholar]


