Abstract
Brunner’s gland hamartoma is a rare benign tumour of the duodenum. It was first described by Cruveilhier in 1835. Presently around 200 cases have been reported in literature. No sex predilection is seen. Patients usually present in the fifth to sixth decades of life. They may be clinically silent or may present with variable symptoms and occasionally obstructive symptoms and chronic pancreatitis. Endoscopic presentation can be nodular, polypoid or diffuse glandular proliferation with thickening of duodenal wall and hence can be misdiagnosed as malignancy. We describe a case of duodenal tumor reported outside (on biopsy) as well differentiated adenocarcinoma which out as Brunner gland hamartoma upon complete resection. Brunner gland hamartoma may sometimes have a very unusual presentation. Extensive pre-operative evaluation is necessity to avoid radical surgical procedure.
Keywords: Brunner glands, Hamartoma, Duodenum, Malignancy
INTRODUCTION
Mucus secreting glands of the duodenum were first described by Brunner in 1688.1 Hyperplasia of these glands is rare with a reported incidence of 0.008%.2 The first case was described by Cruveilhier in 1835 and termed as hamartoma.3 Till date, less than 200 cases have been described with synonyms of Brunner gland adenoma, Brunner gland hamartoma (BGH) or Brunneroma.4 There is no sex predilection and patients present in the fifth to sixth decades of life.3 They are usually seen as incidental findings on imaging with duodenal bulb as the most common location.5 However, it may present with a variety of clinical presentations depending on location and tumor size such as abdominal pain, dyspepsia, nausea, vomiting, upper gastrointestinal (GI) bleeding and occasionally obstructive symptoms with associated chronic pancreatitis.
CASE REPORT
A 42-year-old man presented with history of pain epigastrium since one year and recurrent vomiting within half an hour of taking food since one month. His abdominal examination showed no abnormality. The patient underwent upper gastrointestinal endoscopy which showed a nodular stricture at D1/D2 junction. A biopsy of duodenum was performed by the same procedure which was diagnosed as well differentiated adenocarcinoma. The patient was further subjected to abdominal Computed Tomography (CT) which showed circumferential thickening of the second part of the duodenum (15.2 mm maximum thickness) abutting head of pancreas with loss of fat planes. Laboratory investigations showed normal blood counts, liver, and kidney function tests but elevated serum amylase and lipase levels. This patient underwent radical surgery (Whipple’s procedure) at our institute with clinical diagnosis of carcinoma periampullary region and the specimen was sent for histopathological examination. Gross examination of the specimen revealed circumferential thickening of the second part of the duodenum and bulky head of the pancreas. Cut section revealed a diffuse grey white area measuring 4x4 cm with few cystic and hemorrhagic areas. Six lymph nodes were isolated from the specimen and a single celiac node was examined which was received separately. Microscopy showed a tumor composed of lobules of Brunner’s glands with a dilatation of few glands. These lobules were separated by fine fibrous septa and constituted >50% of the thickness of the duodenal wall ( figures 1, 2 and 3). However, no malignancy was seen on extensive sampling. A diagnosis of Brunner’s gland hyperplasia was given. The patient had an uneventful recovery course with no complications.
Fig. 1 .
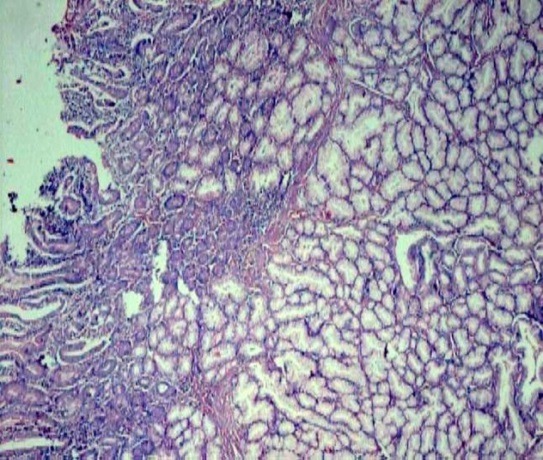
Hyperplasia of Brunner’s glands spanning >50% of wall thickness (H&E stain, 4x)
Fig. 2 .
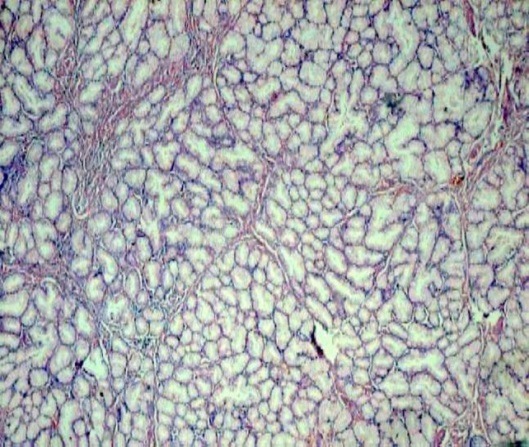
Lack of features of anaplasia in proliferating Brunner’s glands (H&E stain, 20x)
Fig. 3 .
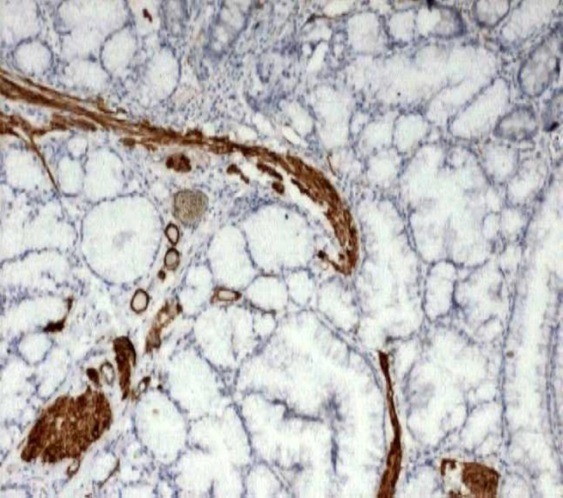
Smooth muscle actin stain highlighting the intervening smooth muscles (IHC, 10x)
DISCUSSION
Brunner glands are branched acinotubular glands with submucosal location, found exclusively between the pyloric ring and the papillae of vater. Their major role is the protection of duodenal epithelium from acidic gastric chyme by secretion of neutralizing alkaline fluid composed of viscous mucin. The pathogenesis of BGH has been linked to excessive local irritation from acidic gastric chyme, vagal stimuli, or unidentified antral hormones.6 Reduced lower esophageal sphincter tone and delayed gastric emptying have also been implicated. Hence, it is associated with conditions related to gastric hyperacidity and changes in gastric emptying such as esophagitis, hiatus hernia, and gastric-peptic ulcer. The association of this tumor with uremia and chronic pancreatitis has also been reported. In a study of patients undergoing pancreaticoduodenectomy, 76% of the patients had diffuse hyperplasia of Brunner’s gland, raising the question whether this is an adaptation to pancreatic insufficiency.7 An alternate suggestion for this hyperplasia is that these glands proliferate in reaction to inflammationas evidenced by presence of lymphocytes in the lesion .8 Support to this hypothesis is not rationalized as lymphocytes may be present in normal submucosa of the gastrointestinal tract. In a study on the role of Helicobacter pylori infection in the pathogenesis of BGH, 5 of 7 cases with BGH had concurrent Helicobacter pylori infection. A definite relationship has not yet been proven because of the high prevalence of Helicobacter pylori and rarity of this lesion in the general population.7
Initially, the hyperplasia of Brunner’s gland can be focal, localized, and solitary usually involving a lobule. However, if stimulus persists then the lesion enlarges involving multiple lobules leading to formation of diffuse and annular lesion infiltrating into the wall and circumference of the duodenum. Imaging studies are of little help in the diagnosis of this lesion. Endoscopically these can be nodular, polypoid or diffuse with thickening of the duodenal wall. Due to this presentation, these lesions can be misdiagnosed as gastrointestinal stromal tumor (GIST), lymphoma, carcinoid, Peutz Jeghers polyp, prolapsed pyloric mucosa, or aberrant pancreatic tissue.9 Hence, the best way to clarify the diagnosis of such lesions is biopsy. Although surface biopsy of this lesion can be misleading as in our case, a deeper biopsy will be more diagnostic. Microscopic diagnosis of hyperplasia requires presence of lobules of Brunner’s glands within the submucosa in at least 50% of the length of the biopsy showing no features of dysplasia. Some authors have further categorized these lesions. For lesion less than 1 cm, the term ’hyperplasia’ is used while the term ‘adenoma’ has been used for lesions >1cm and the term ‘hamartoma’ if it contains mesenchymal elements also.10 Conflicts regarding this terminology exist as some authors suggest that mere change in size indicate progression of hyperplasia and do not imply neoplastic behavior.11 Ductal malignancy arising from Brunner gland adenoma has been reported in one case 12 which is a very rare circumstance (0.5%). Brunner gland adenoma in the duodenal wall with a focus of atypical cells supported by MIB-1 and p53 positivity has also been reported.13
The treatment of BGH is done with surgical or endoscopic removal in symptomatic patients taking into account the size and gross appearance.14 Although endoscopic techniques of removal are more helpful, this procedure can be limited by difficult anatomical sites. Recurrence in such a lesion is not known.
Even though diagnosis of BGH can be made by various imaging studies, in our case these were non-conclusive. Even the small biopsy of duodenum was misleading. Hence, these possibilities should be kept in mind while dealing with duodenal masses.
ACKNOWLEDGEMENT
Department of pathology, PGIMS, Rohtak.
CONFLICT OF INTEREST
The authors declare no conflict of interest related to this work.
Please cite this paper as:
Sen R, Gupta V, Sharma N, Chawla N, Kumar S, Malik S. Brunner Gland Hamartoma Masquerading as Malignancy; A Rare Case Report. Middle East J Dig Dis 2014;6:237-40.
References
- 1.Abbas R, Al-Kawas FH. Review Brunner gland hamartoma. Gastroenterol Hepatol. 2008;4:473–8. [PMC free article] [PubMed] [Google Scholar]
- 2.Botsford TW, Crowe P, Croker DW. Tumours of the small intestineA review of experience with 115 cases including a report of a rare case of malignant hemangio-endothelioma. Am J Surg. 1962;103:358–65. doi: 10.1016/0002-9610(62)90226-x. [DOI] [PubMed] [Google Scholar]
- 3. Cruveilhier J. Anatomy of the human body. New York ,NY: Harper and Bros, 1844.
- 4.Yadav D, Hertan H, Pitchumoni CS. A giant Brunner’s gland adenoma presenting as gastrointestinal hemorrhage. J Clin Gastroenterol. 2001;32:448–50. doi: 10.1097/00004836-200105000-00020. [DOI] [PubMed] [Google Scholar]
- 5.Walden DT, Marcon NE. Endoscopic injection and polypectomy for bleeding Brunner’s gland hamartoma:case report and expanded literature review. Gastrointest Endosc. 1998;47:403–7. doi: 10.1016/s0016-5107(98)70228-7. [DOI] [PubMed] [Google Scholar]
- 6.Peison B, Benisch B. Brunner’s gland adenoma of the duodenal bulb. Am J Gastroenterol. 1982;77:276–8. [PubMed] [Google Scholar]
- 7.Stolte M, Schwabe H, Prestele H. Relationship between diseases of the pancreas and hyperplasia of Brunner glands. Virchows Arch A Pathol Anat Histol. 1981;394:75–87. doi: 10.1007/BF00431666. [DOI] [PubMed] [Google Scholar]
- 8.Gokhale U, Pillai RG. Large Brunner’s gland hamartoma:A case report. Oman Med J. 2009;24:41–3. doi: 10.5001/omj.2009.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yantis RK, Antonioli DA. Polyps of the small intestine.In:Odze RD, Goldblum JR, Crawford JM. Surgical pathology of GI tract, liver, biliary tract and pancreas. 1st ed. Pennysylvania: Saunders Press;2004.p.295-6.
- 10.Chong KC, Cheah WK, Lenzi JE, Goh PM. Benign duodenal tumours. Hepatogastroenterology. 2000;47:1298–1300. [PubMed] [Google Scholar]
- 11.Al-Hilli FA, Malik AK, George SM. The pathological expressions of Brunner’s gland hyperplasia into adenoma and hamartoma. Saudi Med J. 2003;24:1256–60. [PubMed] [Google Scholar]
- 12.Zanetti G, Casadei G. Brunner’s gland hamartoma with incipient ductal malignancy: report of a case. Tumori. 1981;67:75–8. doi: 10.1177/030089168106700114. [DOI] [PubMed] [Google Scholar]
- 13.Fujimaki E, Nakamura S, Sugai T, Takeda Y. Brunner’s gland adenoma with a focus of p53 positive atypical glands. J Gastroenterol. 2000;35:155–8. doi: 10.1007/s005350050029. [DOI] [PubMed] [Google Scholar]
- 14.Adeonigbagbe O, Lee C, Karowe M. A Brunner’s gland adenoma as a cause of anemia. J Clin Gastroenterol. 1999;29:193–6. doi: 10.1097/00004836-199909000-00020. [DOI] [PubMed] [Google Scholar]


