Abstract
Objective
The molecular mechanisms leading to the development of abdominal aortic aneurysms (AAAs) remain poorly understood. The aim of this study was to determine the expression of Sonic Hedgehog (SHh), transforming growth factor β (TGF-β), and Notch signaling components in human aneurysmal and nonaneurysmal aorta in vivo.
Methods
Paired tissue samples were obtained from aneurysmal and nonaneurysmal (control) segments of the aortic wall of eight patients with suitable anatomy undergoing open repair of infrarenal AAAs. Protein and messenger RNA (mRNA) expression levels were determined by Western blot and quantitative real-time polymerase chain reaction analysis.
Results
Aneurysm development resulted in a significant reduction in vascular smooth muscle (vSMC) differentiation genes α-actin and SMC22α at both mRNA and protein levels. In parallel experiments, an 80.0% ± 15% reduction in SHh protein expression was observed in aneurysmal tissue compared with control. SHh and Ptc-1 mRNA levels were also significantly decreased, by 82.0% ± 10% and 75.0% ± 5%, respectively, in aneurysmal tissue compared with nonaneurysmal control tissue. Similarly, there was a 50.0% ± 9% and 60.0% ± 4% reduction in Notch receptor 1 intracellular domain and Hrt-2 protein expression, respectively, in addition to significant reductions in Notch 1, Notch ligand Delta like 4, and Hrt-2 mRNA expression in aneurysmal tissue compared with nonaneurysmal tissue. There was no change in Hrt-1 expression observed in aneurysmal tissue compared with control. In parallel experiments, we found a 2.2 ± 0.2-fold and a 5.6 ± 2.2-fold increase in TGF-β mRNA and protein expression, respectively, in aneurysmal tissue compared with nonaneurysmal tissue. In vitro, Hedgehog signaling inhibition with cyclopamine in human aortic SMCs resulted in decreased Hedgehog/Notch signaling component and vSMC differentiation gene expression. Moreover, cyclopamine significantly increased TGF-β1 mRNA expression by 2.6 ± 0.9-fold.
Conclusions
These results suggest that SHh/Notch and TGF-β signaling are differentially regulated in aneurysmal tissue compared with nonaneurysmal tissue. Changes in these signaling pathways and the resulting changes in vSMC content may play a causative role in the development of AAAs.
Abdominal aortic aneurysms (AAAs) are acquired dilations of the infrarenal artery of up to 1.5 times the normal size, mainly occurring in older adults. At present, nearly 10% of Americans older than 65 years have some degree of aortic enlargement, with approximately 15,000 such individuals dying each year of resulting complications of aortic aneurysms, representing the 13th leading cause of death in the United States.1 The mechanisms involved in the formation of these aortic aneurysms are poorly understood. What is known is that local inflammation in the aortic wall leads to cytokine production, with macrophages and vascular smooth muscle cells (vSMCs) releasing proteases including matrix metalloproteinases. These events lead to the destruction of the extracellular matrix proteins collagen and elastin, which is followed in late aneurysmal development by vSMC apoptosis.2 It is this loss of vSMCs and the resulting degradation of collagen and elastin that is now recognized as a potentially important factor in aneurysm development and progression. The growth of vSMCs, that is, the balance between proliferation and apoptosis (programmed cell death), plays an integral role in vessel homeostasis and during pathologic remodeling of the vessel wall.3 AAA formation represents a dramatic example of vessel wall remodeling; recent studies suggest that increased vSMC death makes an important contribution to this disease by eradicating the very cells required for connective tissue repair. Therefore, a greater knowledge of the potential signaling pathways regulating vSMC survival in aneurysmal development will prove fundamental to understanding the etiology of AAAs.
Hedgehog (Hh) signaling, which plays an integral role in the development of embryonic lineages, has been shown to promote vSMC growth and survival in adult tissue.4-6 Hedgehogs are a class of 19-kDa morphogens that interact with heparin on the cell surface through an N-terminal basic domain and are tethered to the surface through cholesterol and fatty acyl modification.7 Sonic hedgehog (SHh) is the most widely expressed hedgehog during development, in which lack of SHh is embryonically lethal.7 Signaling occurs through interaction of SHh with the Patched receptors Ptc-1 and Ptc-2, which then activate the transcription factors Gli-1, Gli-2, and Gli-3. The downstream targets of the Gli gene products include both Ptc and Gli themselves; thus, Ptc and Gli are both components and targets of the Hh signaling pathway. In addition, Notch signaling has been implicated as a critical determinant of vSMC survival and vascular structure through modulation of signaling pathways that regulate growth.8-11 We and others have demonstrated that Hh can regulate the expression of Notch target genes in a variety of cell types, supporting an interaction between these two pathways.12-14 Moreover, in a recent study, we successfully prevented injury-induced intimal and medial vSMC growth by localized knockdown of Ptc-1 in the carotid artery, an effect mimicked by localized knockdown of Notch 1 receptors in the same vessels.4,15 In this context, we examined whether decreased Hh signaling promotes vSMC loss, which is fundamental to AAA development by Notch repression of vSMC differentiation. In doing so, we analyzed the expression levels of specific Hh and Notch signaling components in human AAA tissue samples (ie, paired aneurysmal and nonaneurysmal segments of the aortic wall from patients undergoing open repair of infrarenal AAAs) in addition to smooth muscle cell (SMC) differentiation marker expression, α-actin and SM22α. A greater understanding of Hh/Notch signaling in aneurysmal development may help provide novel therapeutic and drugable targets to combat this dangerous condition in which once survival of vSMCs is compromised and arterial wall integrity is diminished, patient outcome is very poor. However, it is clear that further studies will be required.
METHODS
Tissue harvest
Tissue samples were obtained from eight patients with suitable anatomy undergoing open repair of infrarenal AAAs. The aneurysmal sample was from the midportion of the aneurysmal sac; the nonaneurysmal sample was taken from the proximal normal aortic neck. The “normal” aorta was taken from what was deemed to be nonaneurysmal aortic tissue from the infrarenal or juxtarenal aorta by the attending surgeon during the course of the operation. This process was not standardized. All aneurysmal and nonaneurysmal specimens analyzed were paired from the same patient and stored in Allprotect tissue reagent (Qiagen, Valencia, Calif) at −80°C for future protein and RNA analysis.
Inclusion and exclusion criteria
All subjects enrolled in this study were male and female subjects older than 18 years and undergoing treatment at Strong Memorial Hospital for cardiovascular disease. These subjects agreed to participate in the study, and informed consent was obtained. Patients were excluded from the study for the following reasons: pediatric patients (<18 years of age), patients who carried a preoperative diagnosis suggestive of primary or metastatic malignant disease, patients who carried a preoperative diagnosis of a primary cardiovascular infection, and patients with known infectious diseases (such as acquired immunodeficiency syndrome, hepatitis, and tuberculosis). The University of Rochester Institutional Review Board approved this study.
Western blot analysis
For protein expression analysis, tissue samples were pulverized with a hand-held homogenizer, and 12 to 15 μg of sample protein was resolved on sodium dodecyl sulfate-polyacrylamide gel electrophoresis (12% resolving, 5% stacking) before transfer onto nitrocellulose membrane (Amersham Biosciences, Piscataway, NJ). Membranes were stained with Ponceau S and probed for glyceraldehyde-3-phosphate dehydrogenase to ensure equal protein loading and transfer and rinsed in wash buffer (phosphate-buffered saline containing 0.05% Tween-20) before being probed as described previously.16 All antibodies were purchased from Abcam (Cambridge, Mass) and were used according to the manufacturer’s instructions.
Quantitative real-time reverse transcription-polymerase chain reaction (RT-PCR)
Aortic wall biopsy specimens or cultured SMCs in RNAlater solution (Ambion, Austin, Tex) were used to isolate total RNA (0.5-1 μg) with Qiagen RNeasy kit (Valencia, Calif). Total RNA was reverse-transcribed with iScript cDNA Synthesis kit from Bio-Rad (Carlsbad, Calif). The gene-specific oligonucleotide sequences were as previously described.16 Real-time RT-PCR was performed with the Stratagene Mx3005 machine and the SYBR Green JumpStart PCR kit (Sigma, St. Louis, Mo) as described by the manufacturer.
Cell culture
Human aortic smooth muscle cells (HASMCs) were obtained from Lonza (Walkersville, Md) and cultured in optimized Smooth Muscle Cell Medium (Clonetics, Lonza), supplemented with human epidermal growth factor, insulin, human fibroblast growth factor, and 10% fetal calf serum. Cells were characterized by staining positive for SMC α-actin, calponin, myosin, and smoothelin.
Data analysis
All results are expressed as mean ± standard error of the mean. Experimental points were performed in triplicate, with a minimum of three independent experiments (VSMC), or a minimum of eight patient samples for nonaneurysmal control and aneurysmal. A Wilcoxon signed rank test was used for comparison of two groups compared with normalized control. A value of P ≤ .05 was considered significant.
RESULTS
Hh expression and SMC marker expression are decreased in aneurysmal tissue samples
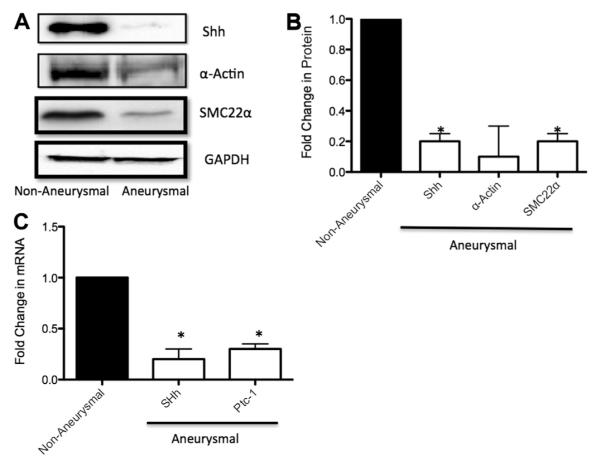
The effect of aneurysmal development on vSMC content as determined by SMC differentiation marker gene expression was undertaken in parallel with Hh component expression by Western blot and quantitative RT-PCR analysis. Aneurysmal tissue paired with nonaneurysmal tissue from a pool of eight patients was analyzed for vSMC markers α-actin and SMC22α concomitantly with the expression of Hh signaling components SHh and Ptc-1 (Fig 1). Aneurysm development resulted in a significant 80% and 90% reduction in SMC22 and α-actin protein expression, respectively (Fig 1, A and B). In parallel experiments, an 80% reduction in SHh protein expression was observed in aneurysmal tissue compared with control tissue (Fig 1, A and B). SHh and Ptc-1 messenger RNA (mRNA) levels were also significantly decreased, by 82.0% ± 10% and 75.0% ± 5%, respectively, in aneurysmal tissue compared with nonaneurysmal control tissue (Fig 1, C).
Fig 1.
Hedgehog (Hh)/vascular smooth muscle cell (vSMC) differentiation gene expression in nonaneurysmal and aneurysmal tissue from patients undergoing open repair of infrarenal abdominal aortic aneurysm (AAA). A and B, Representative Western blot (A) and cumulative protein data (B) for Sonic Hedgehog (SHh), α-actin, and SMC22α expression levels in nonaneurysmal and aneurysmal tissue samples of patients. C, Quantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis of SHh and Ptc-1 messenger RNA (mRNA) levels in nonaneurysmal and aneurysmal tissue samples of patients. Data are normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and represent the mean ± standard error of the mean; n = 8.
Altered Notch and TGF-β expression in aneurysmal tissue
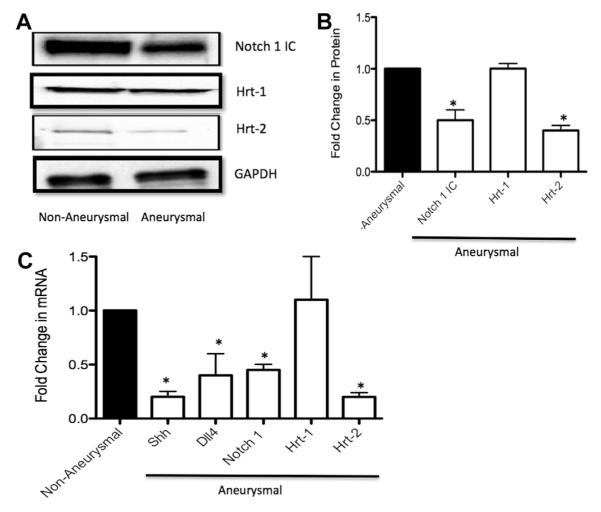
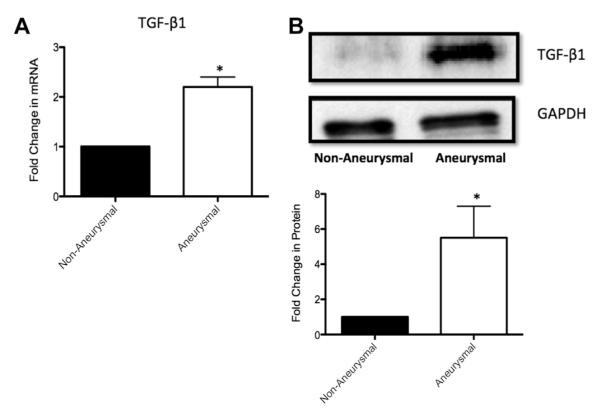
Paired nonaneurysmal and aneurysmal tissue from eight patients was pooled and also analyzed for Notch signaling components: Notch receptor 1 intracellular domain (Notch 1 IC), Notch ligand Delta like 4 (Dll4), and Notch target genes Hrt-1 and Hrt-2 (Fig 2). Expression levels of TGF-β1 were examined in parallel. There was a 50% ± 9% and 60% ± 4% reduction in Notch 1 IC and Hrt-2 protein expression, respectively, in aneurysmal tissue compared with nonaneurysmal tissue, concomitant with no significant change in Hrt-1 protein expression between samples (Fig 2, A and B). Similarly, there were significant reductions in Notch 1, Dll4, and Hrt-2 mRNA expression in aneurysmal tissue compared with nonaneurysmal tissue, with no significant change in Hrt-1 mRNA expression (Fig 2, A). In parallel experiments, we found a 2.2-fold and a 5.6-fold increase in TGF-β mRNA and protein expression, respectively, in aneurysmal tissue compared with nonaneurysmal tissue (Fig 3, A and B).
Fig 2.
Hedgehog (Hh)/Notch signaling component expression in nonaneurysmal and aneurysmal tissue from patients undergoing open repair of infrarenal abdominal aortic aneurysm (AAA). A and B, Representative Western blot (A) and cumulative protein data (B) for Notch receptor 1 intracellular domain (Notch 1 IC) and Hrt-1 and Hrt-2 expression levels in nonaneurysmal and aneurysmal tissue samples of patients. C, Quantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis of Sonic Hedgehog (SHh), Notch ligand Delta like 4 (Dll4), Notch 1, and Hrt-1 and Hrt-2 messenger RNA (mRNA) levels in nonaneurysmal and aneurysmal tissue samples of patients. Data are normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and represent the mean ± standard error of the mean; n = 8.
Fig 3.
Transforming growth factor β1 (TGF-β1) expression in nonaneurysmal and aneurysmal tissue segments from patients undergoing open repair of infrarenal abdominal aortic aneurysm (AAA). A, Quantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis of TGF-β1 messenger RNA (mRNA) levels in nonaneurysmal and aneurysmal tissue samples of patients. B, Representative Western blot and cumulative protein data for TGF-β expression levels in nonaneurysmal and aneurysmal tissue samples of patients. Data are normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and represent the mean ± standard error of the mean; n = 8.
Hh signaling modulates vSMC marker expression through a Notch-dependent pathway
We have previously shown that Hh modulates vSMC growth in vitro through a Notch-dependent pathway.13 Moreover, selective small interfering RNA knockdown of Hh signaling in vivo resulted in attenuation of medial and intimal hyperplasia, concomitant with reduced Notch signaling components, an effect mimicked by a similar targeted inhibition of the Notch 1 receptor in vivo.4,15 Here we examined whether vSMC differentiation gene expression, which is significantly decreased in aneurysmal tissue in parallel with decreased Hh/Notch expression, could be similarly inhibited in vitro by blocking of Hh signaling with cyclopamine. In HASMCs, we observed a significant decrease in Hh signaling components Ptc-1 and Gli-2 after cyclopamine (40 μmol/L) treatment for 24 hours (Fig 4). Moreover, cyclopamine treatment also significantly inhibited Notch signaling, as evidenced by decreased Notch 1 and Hrt-2 mRNA expression, while also inhibiting α-actin expression (Fig 4). In contrast, inhibition of Hh signaling with cyclopamine resulted in a 2.6-fold increase in TGF-β1 mRNA in HASMCs (Fig 4). These in vitro data demonstrate that Hh signaling differentially modulates both vSMC differentiation gene expression and TGF-β1, effects that may be relevant in aneurysm formation in vivo.
Fig 4.
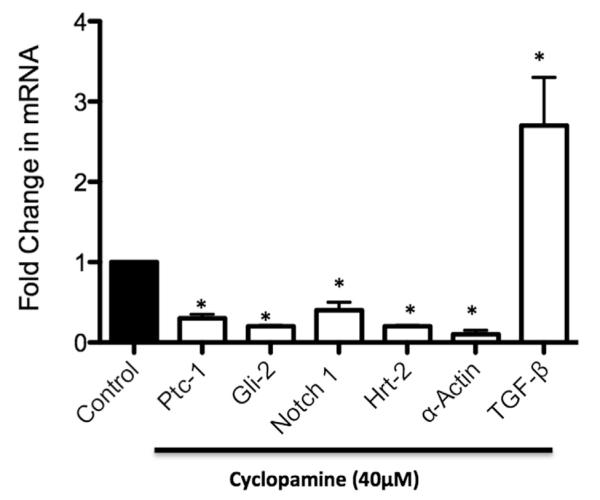
The effect of Hedgehog (Hh) signaling inhibition on Notch/transforming growth factor β (TGF-β)/α-actin messenger RNA (mRNA) expression in human aortic smooth muscle cells (HASMCs). Quantitative reverse transcription-polymerase chain reaction (RT-PCR) analysis of mRNA levels of Ptc-1, Gli-2, Notch 1, Hrt-2, α-actin, and TGF-β1 in HASMCs treated with cyclopamine (40 μM) for 24 hours. Data are normalized to glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and represent the mean ± standard error of the mean; n = 4.
DISCUSSION
Research strategies, both scientific and clinical, on AAA development and treatment remain poorly elucidated, despite AAAs being responsible for substantial mortality in men older than 65 years and despite an ever-expanding aging population in the United States today. The contribution of vSMCs in AAA development and especially their survival, which is fundamental to maintaining arterial wall integrity, is of great interest as it likely plays a critical role in this pathologic process. The cell signaling mechanisms involved in AAA development are also poorly understood. Changes in vSMC phenotype are coordinated by the regulation of vSMC differentiation marker gene expression in addition to the production of matrix metalloproteinases in response to injury.2,17,18 Our research to date has specifically investigated signaling pathways regulating cell phenotype and has focused on identifying the key signaling cascades causing changes in vSMC phenotype that are the hallmarks of atherosclerosis.14,19,20 In a somewhat similar fashion, AAA may also be a vascular disease wherein aberrant vSMC phenotype modulation is fundamental to its development. Surprisingly, however, little is known about what signaling mechanisms dictate changes in vSMC growth in the context of AAAs. For the past decade, we and others have characterized the role of Hh/Notch signaling in promoting the vSMC growth evident in vascular injury-induced stenosis of arterial vessels.4,13,21,22 In that setting, injury-induced changes in hemodynamic forces drive a Hh/Notch signaling cascade, which promotes the growth of medial and intimal vSMCs. Could similar external cues, such as hemodynamic forces, or chemical entities, such as tobacco, a known influential factor of this disorder, similarly regulate this Hh/Notch signaling cascade and by doing so arbitrate vSMC phenotype in a manner that could negatively affect vSMC survival in AAA development? Here, we show for the first time that together with decreased vSMCs present in aneurysm tissue, there is marked downregulation of Hh and Notch signaling components that may result in increased TGF-β1 compared with paired nonaneurysmal tissue.
Using paired biopsy samples of aneurysmal and nonaneurysmal tissue from patients undergoing open repair of infrarenal AAAs, we established that vSMC differentiation-genes SMC22α and α-actin are significantly decreased in aneurysmal samples, indicative of a reduced SMC component. In addition, this decreased vSMC content and marker gene expression was accompanied by a marked decrease in Hh and Notch signaling component expression at a protein and mRNA level. To investigate whether reduced vSMC content was potentially a Hh/Notch-dependent event, we inhibited Hh signaling in vitro in HASMCs by use of a well-established Hh inhibitor, cyclopamine. Inhibition of Hh signaling in this manner in vitro caused a significant decrease in Hh and Notch signaling component expression in parallel with decreased vSMC differentiation gene expression. We have previously shown that inhibiting the Hh signaling pathway both in vitro and in vivo inhibits vSMC growth through a Notch-dependent pathway.4,13 Moreover, we recently reported that inhibition of Hh signaling prevented intimal and medial vSMC growth in the injured carotid artery concomitant with downregulation of Notch signaling.4 Specifically, inhibition of Ptc-1 by perivascular delivery of small interfering RNAs decreased ligation injury-induced vSMC growth and promoted vSMC apoptosis by increasing the ratio of Bax:Bcl-XL and enhancing caspase-3 activity.4 This resulted in decreased arterial wall vSMC content in a manner somewhat analogous to the compromised vSMC population we see in AAA. Our data now suggest that the Hh/Notch signaling axis may also play a role in the decreased vSMC growth and survival that we see during aneurysm development.
In this study, we also examined the expression of TGF-β1, a protein that controls proliferation and cell differentiation, in the biopsy samples of patients. TGF-β1 has been shown to promote thoracic aortic aneurysm development, and although similar to AAA, there is disparity, however, in the origin of the vSMCs present in these aneurysm types.23 Interestingly, vSMCs that originate from different embryonic origins have been shown to respond differently to TGF-β1.24 TGF-β1 has been reported to promote thoracic aortic aneurysm development while playing a protective role in AAA development.23 This protective role of TGF-β1 in AAA development may be further explained in this study as we found TGF-β1 expression to be significantly elevated in aneurysmal tissue compared with control nonaneurysmal tissue. Furthermore, increased interest in the role of TGF-β1 signaling in the pathogenesis of AAA has emerged in recent years because of genetic studies demonstrating an association between gene mutations in components of TGF-β signaling and AAA.25 In addition, in a recent study by Dennler et al, the Hh target gene Gli-2 was shown to be a target of TGF-β signaling, thus providing an alternative way to stimulate Gli-dependent transcription in the absence of a Hh ligand.26 Moreover, a recent study by Biros et al suggests that impaired TGF-β signaling is accompanied by a downregulation of the Notch signaling pathway that contributes to the pathogenesis of AAAs.27 Taken together with our study, there is therefore much evidence supporting crosstalk between these pathways. Our data showing Hh inhibition mediating TGF-β1 expression suggest a novel Hh/Notch/TGF-β signaling cascade in dictating vSMC survival in AAA development.
Our previous studies indicate that altered biomechanical forces regulate vSMC growth through a Hh/Notch-dependent pathway both in vitro and in the ligation injury-induced remodeled vessel in vivo.5,21,28 As AAA development is a vascular disease also indicative of altered biomechanical forces and remodeling, it is tempting to speculate that it is these altered biomechanical forces that are regulating vSMC survival through a similar Hh/Notch-dependent pathway. A number of clinical studies investigating biomechanical profiles for AAAs have begun.29 Large population-based studies for the validation of patient-specific biomechanical profiles with accompanying rupture risk assessment and outcome are being carried out with the introduction of AAA screening programs.28,29 AAAs are predominantly asymptomatic until rupture occurs, resulting in a mortality rate of nearly 85%. AAA wall thickness is altered as a result of remodeling and compromised vSMC content, which influences the mechanical properties and response to stress. This in turn affects wall stress distribution. Therefore, these biomechanical profiling studies in AAA development and rupture present a valuable aid in patient-specific risk assessment and treatment decision.29 As our previous work has clearly indicated a link between altered biomechanical regulation of vSMC growth and Hh/Notch signaling expression, Hh and Notch signaling components may themselves be suitable biomarkers in determining rupture risk assessment and in eventual treatment strategies.
CONCLUSIONS
Ongoing studies in our laboratory will further elucidate the role of the Hh/Notch signaling pathways in AAA development by knockin/knockout studies of specific Hh signaling components in the elastase perfusion mouse model of AAA.1 These studies will focus on specific components in the Hh/Notch signaling pathways and their effect on overall AAA development. In doing so, these targets will provide novel therapeutic goals that could lead to drugable targets to combat a condition that is a leading cause of death in the United States for men older than 65 years. With an ever-expending baby boomer population and subsequent spiraling Medicare costs, drugable targets for AAA, which is predominant in the older patient, certainly offer a preferred alternative to the only present available treatment, that being surgery.
Clinical Relevance.
Abdominal aortic aneurysms represent a major problem for many older patients, and as the population of the United States ages, their prevalence is set to increase. Vascular smooth muscle cell (vSMC) death is pivotal to abdominal aortic aneurysm development and ultimately leads to the deterioration of the arterial wall and subsequent rupture. This study addresses the role of the Hedgehog signaling pathway in mediating this vSMC survival. This study will help provide novel therapeutic targets to combat a condition in which once survival of vSMCs is compromised and arterial wall integrity is diminished, 90% do not survive.
Footnotes
AUTHOR CONTRIBUTIONS
Conception and design: DM, DG, JC
Analysis and interpretation: DM, ER, PK
Data collection: DM
Writing the article: AD, DM
Critical revision of the article: ER, PC, DM
Final approval of the article: DM
Statistical analysis: DM
Obtained funding: DM
Overall responsibility: DM
Presented as a podium presentation at the Society for Vascular Surgery Vascular Research Initiatives Conference, Chicago, Ill, April 18-20, 2012.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Cao RY, Amand T, Ford MD, Piomelli U, Funk CD. The murine angiotensin II-induced abdominal aortic aneurysm model: rupture risk and inflammatory progression patterns. Front Pharmacol. 2010;1:9. doi: 10.3389/fphar.2010.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ailawadi G, Moehle CW, Pei H, Walton SP, Yang Z, Kron IL, et al. Smooth muscle phenotypic modulation is an early event in aortic aneurysms. J Thorac Cardiovasc Surg. 2009;138:1392–9. doi: 10.1016/j.jtcvs.2009.07.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Owens GK, Kumar MS, Wamhoff BR. Molecular regulation of vascular smooth muscle cell differentiation in development and disease. Physiol Rev. 2004;84:767–801. doi: 10.1152/physrev.00041.2003. [DOI] [PubMed] [Google Scholar]
- 4.Redmond EM, Hamm K, Cullen JP, Hatch E, Cahill PA, Morrow D. Inhibition of patched-1 prevents injury-induced neointimal hyperplasia. Arterioscler Thromb Vasc Biol. 2013;33:1960–4. doi: 10.1161/ATVBAHA.113.301843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Morrow D, Sweeney C, Birney YA, Guha S, Collins N, Cummins PM, et al. Biomechanical regulation of hedgehog signaling in vascular smooth muscle cells in vitro and in vivo. Am J Physiol Cell Physiol. 2007;292:C488–96. doi: 10.1152/ajpcell.00337.2005. [DOI] [PubMed] [Google Scholar]
- 6.Morrow D, Sweeney C, Birney YA, Cummins PM, Guha S, Murphy R, et al. Sonic hedgehog regulates vascular smooth muscle cell fate in vitro through VEGF activation of notch signaling. Circulation. 2005;112:U70. [Google Scholar]
- 7.Hooper JE, Scott MP. Communicating with Hedgehogs. Nat Rev Mol Cell Biol. 2005;6:306–17. doi: 10.1038/nrm1622. [DOI] [PubMed] [Google Scholar]
- 8.Lai EC. Keeping a good pathway down: transcriptional repression of Notch pathway target genes by CSL proteins. EMBO Rep. 2002;3:840–5. doi: 10.1093/embo-reports/kvf170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iso T, Hamamori Y, Kedes L. Notch signaling in vascular development. Arterioscler Thromb Vasc Biol. 2003;23:543–53. doi: 10.1161/01.ATV.0000060892.81529.8F. [DOI] [PubMed] [Google Scholar]
- 10.Harper JA, Yuan JS, Tan JB, Visan I, Guidos CJ. Notch signaling in development and disease. Clin Genet. 2003;64:461–72. doi: 10.1046/j.1399-0004.2003.00194.x. [DOI] [PubMed] [Google Scholar]
- 11.Sweeney C, Morrow D, Birney YA, Coyle S, Hennessy C, Scheller A, et al. Notch 1 and 3 receptor signaling modulates vascular smooth muscle cell growth, apoptosis, and migration via a CBF-1/RBP-Jκ dependent pathway. FASEB J. 2004;18:1421–3. doi: 10.1096/fj.04-1700fje. [DOI] [PubMed] [Google Scholar]
- 12.Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, et al. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–11. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- 13.Morrow D, Cullen JP, Liu W, Guha S, Sweeney C, Birney YA, et al. Sonic Hedgehog induces Notch target gene expression in vascular smooth muscle cells via VEGF-A. Arterioscler Thromb Vasc Biol. 2009;29:1112–8. doi: 10.1161/ATVBAHA.109.186890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morrow D, Guha S, Sweeney C, Birney Y, Walshe T, O’Brien C, et al. Notch and vascular smooth muscle cell phenotype. Circ Res. 2008;103:1370–82. doi: 10.1161/CIRCRESAHA.108.187534. [DOI] [PubMed] [Google Scholar]
- 15.Redmond EM, Liu W, Hamm K, Hatch E, Cahill PA, Morrow D. Perivascular delivery of Notch 1 siRNA inhibits injury-induced arterial remodeling. PLoS One. 2014;9:e84122. doi: 10.1371/journal.pone.0084122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Morrow D, Cullen JP, Cahill PA, Redmond EM. Cyclic strain regulates the Notch/CBF-1 signaling pathway in endothelial cells: role in angiogenic activity. Arterioscler Thromb Vasc Biol. 2007;27:1289–96. doi: 10.1161/ATVBAHA.107.142778. [DOI] [PubMed] [Google Scholar]
- 17.Longo GM, Xiong W, Greiner TC, Zhao Y, Fiotti N, Baxter BT. Matrix metalloproteinases 2 and 9 work in concert to produce aortic aneurysms. J Clin Invest. 2002;110:625–32. doi: 10.1172/JCI15334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mack CP, Owens GK. Regulation of smooth muscle alpha-actin expression in vivo is dependent on CArG elements within the 5′ and first intron promoter regions. Circ Res. 1999;84:852–61. doi: 10.1161/01.res.84.7.852. [DOI] [PubMed] [Google Scholar]
- 19.Morrow D, Guha ST, Birney Y, Sweeney C, Cummins P, Walls D, et al. Hedgehog regulation of Notch signaling in vascular smooth muscle cells in vitro. Arterioscler Thromb Vasc Biol. 2005;25:E86. [Google Scholar]
- 20.Morrow D, Scheller A, Birney YA, Sweeney C, Guha S, Cummins PM, et al. Notch-mediated CBF-1/RBP-Jκ–dependent regulation of human vascular smooth muscle cell phenotype in vitro. Am J Physiol Cell Physiol. 2005;289:C1188–96. doi: 10.1152/ajpcell.00198.2005. [DOI] [PubMed] [Google Scholar]
- 21.Li Y, Takeshita K, Liu PY, Satoh M, Oyama N, Mukai Y, et al. Smooth muscle Notch1 mediates neointimal formation after vascular injury. Circulation. 2009;119:2686–92. doi: 10.1161/CIRCULATIONAHA.108.790485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morrow D, Cullen JP, Liu W, Cahill PA, Redmond EM. Alcohol inhibits smooth muscle cell proliferation via regulation of the Notch signaling pathway. Arterioscler Thromb Vasc Biol. 2010;30:2597–603. doi: 10.1161/ATVBAHA.110.215681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Krishna S, Walker PJ, Norman P, Golledge J. Transforming growth factor-β and abdominal aortic aneurysms. Cardiovasc Pathol. 2013;22:126–32. doi: 10.1016/j.carpath.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 24.Topouzis S, Majesky MW. Smooth muscle lineage diversity in the chick embryo. Two types of aortic smooth muscle cell differ in growth and receptor-mediated transcriptional responses to transforming growth factor-β. Dev Biol. 1996;178:430–45. doi: 10.1006/dbio.1996.0229. [DOI] [PubMed] [Google Scholar]
- 25.Lin F, Yang X. TGF-β signaling in aortic aneurysm: another round of controversy. J Genet Genomics. 2010;37:583–91. doi: 10.1016/S1673-8527(09)60078-3. [DOI] [PubMed] [Google Scholar]
- 26.Dennler S, Andre J, Alexaki I, Li A, Magnaldo T, ten Dijke P, et al. Induction of sonic hedgehog mediators by transforming growth factor-β: Smad3-dependent activation of Gli2 and Gli1 expression in vitro and in vivo. Cancer Res. 2007;67:6981–6. doi: 10.1158/0008-5472.CAN-07-0491. [DOI] [PubMed] [Google Scholar]
- 27.Biros E, Walker PJ, Nataatmadja M, West M, Golledge J. Down-regulation of transforming growth factor, beta receptor 2 and Notch signaling pathway in human abdominal aortic aneurysm. Atherosclerosis. 2012;221:383–6. doi: 10.1016/j.atherosclerosis.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 28.Kontopodis N, Georgakarakos E, Metaxa E, Pagonidis K, Papaharilaou Y, Ioannou CV. Estimation of wall properties and wall strength of aortic aneurysms using modern imaging techniques. One more step towards a patient-specific assessment of aneurysm rupture risk. Med Hypotheses. 2013;81:212–5. doi: 10.1016/j.mehy.2013.04.048. [DOI] [PubMed] [Google Scholar]
- 29.Malkawi AH, Hinchliffe RJ, Xu Y, Holt PJ, Loftus IM, Thompson MM. Patient-specific biomechanical profiling in abdominal aortic aneurysm development and rupture. J Vasc Surg. 2010;52:480–8. doi: 10.1016/j.jvs.2010.01.029. [DOI] [PubMed] [Google Scholar]