Abstract
The transverse carpal ligament (TCL) is a significant constituent of the wrist structure and forms the volar boundary of the carpal tunnel. It serves biomechanical and physiological functions, acting as a pulley for the flexor tendons, anchoring the thenar and hypothenar muscles, stabilizing the bony structure, and providing wrist proprioception. This article mainly describes and reviews our recent studies regarding the biomechanical role of the TCL in the compliant characteristics of the carpal tunnel. First, force applied to the TCL from within the carpal tunnel increased arch height and area due to arch width narrowing from the migration of the bony insertion sites of the TCL. The experimental findings were accounted for by a geometric model that elucidated the relationships among arch width, height, and area. Second, carpal arch deformation showed that the carpal tunnel was more flexible at the proximal level than at the distal level and was more compliant in the inward direction than in the outward direction. The hamate-capitate joint had larger angular rotations than the capitate-trapezoid and trapezoid-trapezium joints for their contributions to changes of the carpal arch width. Lastly, pressure application inside the intact and released carpal tunnels led to increased carpal tunnel cross-sectional areas, which were mainly attributable to the expansion of the carpal arch formed by the TCL. Transection of the TCL led to an increase of carpal arch compliance that was nine times greater than that of the intact carpal tunnel. The carpal tunnel, while regarded as a stabile structure, demonstrates compliant properties that help to accommodate biomechanical and physiological variants such as changes in carpal tunnel pressure.
Keywords: ligament, carpal, pressure, wrist, surgery
Carpal Tunnel Structure
The carpal tunnel is a fibro-osseous structure formed by the carpal bones interconnected by numerous ligaments. The eight irregularly shaped carpal bones are intricately arranged with the intercarpal ligaments to establish the medial, lateral, and dorsal boundaries of the tunnel. The proximal row of the carpal bones consists of the pisiform, triquetrum, lunate, and scaphoid, while the distal row comprises the hamate, capitate, trapezoid, and trapezium. The volar aspect of the carpal tunnel is spanned by the transverse carpal ligament (TCL). This band of fibrous tissue inserts radially into the scaphoid tuberosity and trapezial ridge, and ulnarly into the pisiform and the hook of the hamate. The thenar and hypothenar muscles are an integral part of the carpal tunnel structure, because the TCL serves as attachment sites for these hand muscles,1 2 and contraction of these muscles causes biomechanical interactions with the carpal tunnel via the TCL.3 Within the carpal tunnel, the space is occupied by the median nerve, nine tendons of the extrinsic flexors of the thumb and fingers, and other connective tissues (e.g., bursae, synovium). The sophisticated anatomical configuration of the carpal tunnel results in complex biomechanical properties of the tunnel structure and also renders it vulnerable to pathomechanics.
Biomechanics of the Carpal Tunnel
Carpal tunnel mechanics and pathomechanics are clinically relevant in the management of many hand and wrist disorders, including carpal tunnel syndrome. Compression of the median nerve in the carpal tunnel is attributable to many deleterious mechanical factors such as forceful use of the hand and fingers,4 5 awkward postures of the wrist,6 7 volume increases in the carpal tunnel contents,8 9 10 11 12 muscle intrusion,11 13 elevated pressure within the tunnel,6 14 15 and hypertrophy or stiffening of the TCL.16 17 Attempts have been made to elucidate the mechanisms of these mechanical factors and their relationships and to utilize the knowledge of carpal tunnel biomechanics to help decompress the median nerve.
In an attempt to increase the carpal tunnel cross-sectional area, Li et al applied palmarly directed force onto the TCL from within the tunnel.18 The force application caused the TCL to form a larger arch, with increases in arch height and in tunnel cross-sectional area. Interestingly, the increased carpal arch area was due to arch width narrowing from the migration of the bony insertion sites of the TCL, and was not caused by elongation of the TCL. Subsequently, a geometric model of the carpal tunnel was developed to reveal the relationships among arch width, arch height, arch area, and TCL length. The model showed that increases in the carpal arch area were especially sensitive to narrowing of the carpal arch width,18 explaining the experimental finding that carpal arch area increases are effectively attainable by decreases of the arch width although the TCL length remains unchanged.
To understand the deformability of the carpal tunnel, Xiu et al studied the force-deformation relationship of the carpal arch width while inward and outward forces were applied to the TCL insertion sites.19 It was found that carpal tunnel compliance was location- and direction-dependent; the carpal tunnel was more flexible at the proximal level than at the distal level and was more compliant under inward force application than under outward force application. The difference in compliance in the inward and outward directions is likely due to the direction-dependent stabilizing role of the TCL as well as the other intercarpal ligaments.20 21 22 The role of the intercarpal ligaments in stabilizing the bony arch was also shown by Garcia-Elias et al, who applied compressive force to the carpal tunnel in the dorsal-palmar direction to quantify the rigidity of the tunnel with and without an intact TCL.23 In contrast to the compliance in the radio-ulnar direction,19 the carpal tunnel stiffness in the dorsal-palmar direction23 was not affected by transecting the TCL because of the other intercarpal ligaments in the tunnel structure. An investigation into the relative mobility of the intercarpal joints within the distal carpal row by Gabra et al showed that the hamate-capitate joint had larger angular rotations than the capitate-trapezoid and trapezoid-trapezium joints20; this is consistent with the concept that the scaphotrapeziotrapezoid joint (STT) complex is relatively rigid, tightly held together by intercarpal ligaments. Furthermore, manipulating the carpal arch width caused complex, three-dimensional motion patterns of the carpal bones, which results from peculiar bony arrangement, elaborate ligamentous constraints, and oblique orientation of the carpal arch width.
Pressure Induced Morphological Changes of the Carpal Tunnel
The structural change of the carpal tunnel in response to pressure is another clinically meaningful phenomenon of carpal tunnel mechanics. There is a well-established association between carpal tunnel pressure and carpal tunnel syndrome. The pressure within the carpal tunnel is relatively low in a normal physiological state: less than 15 mm Hg when the wrist is at its anatomical neutral position.6 14 15 However, the carpal tunnel pressure in patients with carpal tunnel syndrome is known to be consistently elevated, and it is not uncommon to exceed even 200 mm Hg.6 15 Such an elevation of pressure may be attributable to the relatively inflexible carpal tunnel structure compounded by space-occupying factors such as fluid retention, edema, muscle intrusion, or tissue hypertrophy. Yet, it has been shown that carpal tunnel syndrome is associated with greater palmar bowing of the TCL.24 25 26 27 For example, Uchiyama et al showed a linear correlation between carpal tunnel area and TCL palmar bowing for the patients with carpal tunnel syndrome, but not for controls.24 Mesgarzadeh et al used a bowing ratio (i.e., TCL palmar deviation divided by carpal arch width) to quantify the amount of bowing and found that carpal tunnel syndrome patients had a bowing ratio of 18.1% in comparison to a 5.8% ratio for controls.27 Together, the pressure elevation and carpal arch change associated with carpal tunnel syndrome suggests that the tunnel exhibit some degree of compliance.
To understand the compliant nature of the carpal tunnel, Li et al performed a cadaveric experiment to examine the change in carpal tunnel area and shape in response to increasing pressure within the tunnel.28 Controlled pressure (0–200 mm Hg) was applied to the carpal tunnel through a custom balloon device, and the resulting cross sections of the carpal tunnel were recorded using magnetic resonance imaging (MRI) (Fig. 1). The technique used to apply pressure inside the tunnel by balloon inflation mimicked some pathomechanical phenomena associated with pressure elevation inside the carpal tunnel. The relationships between carpal tunnel pressure and carpal tunnel morphological parameters at the cross section of the hook of the hamate were derived.
Fig. 1.
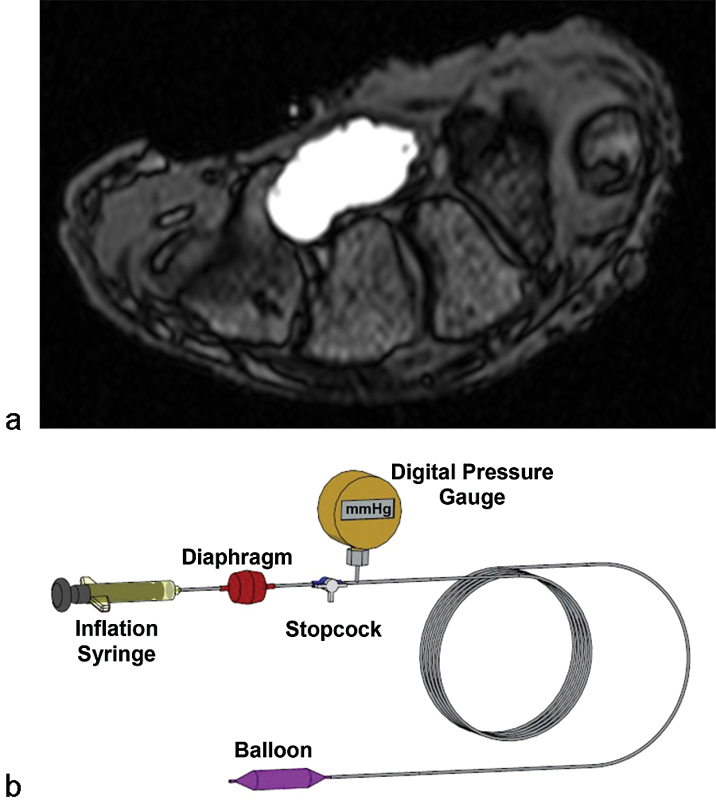
(a) MRI of the pressurized carpal tunnel (b) with a custom balloon device.
The results showed that the carpal tunnel had considerable compliance to accommodate pressure variation inside the tunnel. Specifically, the cross-sectional area of the distal tunnel increased by 9% and 15% at 100 mm Hg and 200 mm Hg of tunnel pressure, respectively (Fig. 2). This increase in carpal tunnel area was mainly attributed to the carpal arch formed by the TCL. For example, at carpal tunnel pressure of 200 mm Hg, the carpal arch area increased by 39% as opposed to the relatively small increase of 9% in the bony arch area. Furthermore, the circularity and aspect ratio of the tunnel's cross section were positively and negatively correlated with tunnel pressure, respectively. This means that as carpal tunnel pressure increased, the tunnel's cross section became more rounded. The aspect ratio changed from 2.05 at 0 mm Hg to 1.78 at 200 mm Hg. The change in cross-sectional shape explains the change in cross-sectional area even though the perimeter remained unchanged. Further analyses revealed that the compliance at the proximal tunnel (pisiform) level was greater than that at the distal tunnel level,28 which is consistent with a previous study demonstrating that the carpal tunnel is less deformable at the distal than proximal level.19
Fig. 2.
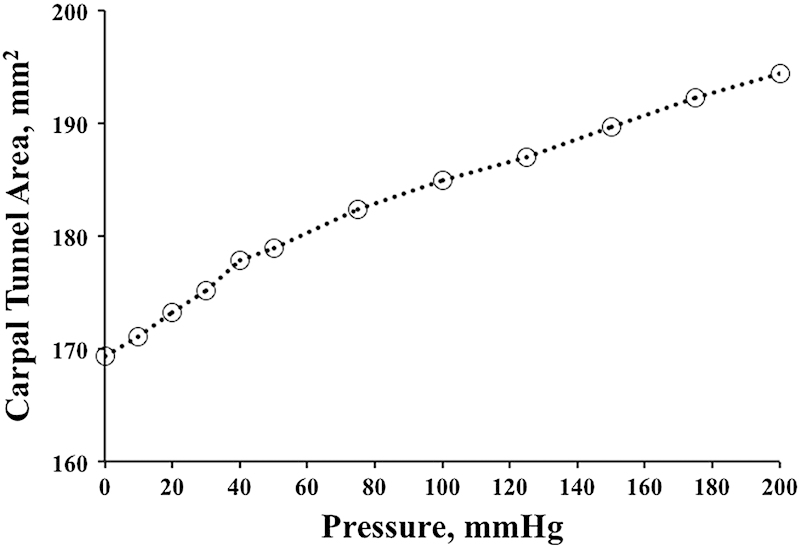
Change in total carpal tunnel area with respect to carpal tunnel pressure.
Change of Structural Compliance after Carpal Tunnel Release
Although the carpal tunnel demonstrates some ability to accommodate pressure increases, the structural compliance of the intact carpal tunnel is limited. In the case of carpal tunnel syndrome, the TCL is viewed as an unfavorable mechanical constraint, and it is routinely transected during carpal tunnel release. Morphological changes to the carpal tunnel structure are known to occur as a result of transecting the TCL. MRI studies have shown that after carpal tunnel release there is an increase in carpal tunnel cross-sectional area and circularity, as well as tunnel volume.29 30 31 Notably, Kato et al reported that endoscopic carpal tunnel release caused a 33% increase in the cross-sectional area of the carpal tunnel and a 3.6 times increase in the carpal arch area.32 The change in the carpal arch width after carpal tunnel release remains controversial,33 as some studies reported a widening of the carpal arch,34 35 while other studies found no width changes at postoperative follow-up.29 30 It is known that carpal tunnel release effectively reduces carpal tunnel pressure15; however, postoperative pressure may remain higher than normal and even surpass 100 mm Hg during hand activities.15 36 Therefore, it is expected that the compromised tunnel structure after carpal tunnel release exhibits different biomechanical properties, particularly in regard to its implications on carpal tunnel pressure.
To understand the compliance of the released carpal tunnel, Kim et al investigated the pressure-morphology relationship of the carpal tunnel after endoscopically releasing the TCL in cadaveric hands.37 After the release, carpal tunnel pressure was increased by infusing water into the lumen of the tunnel until a pressure of 120 mm Hg was achieved (Fig. 3). While the pressure was increased, cross-sectional ultrasound images at the hamate hook level were simultaneously recorded. The imaging and pressure data were analyzed to determine the pressure-morphology relationship of the carpal arch.
Fig. 3.
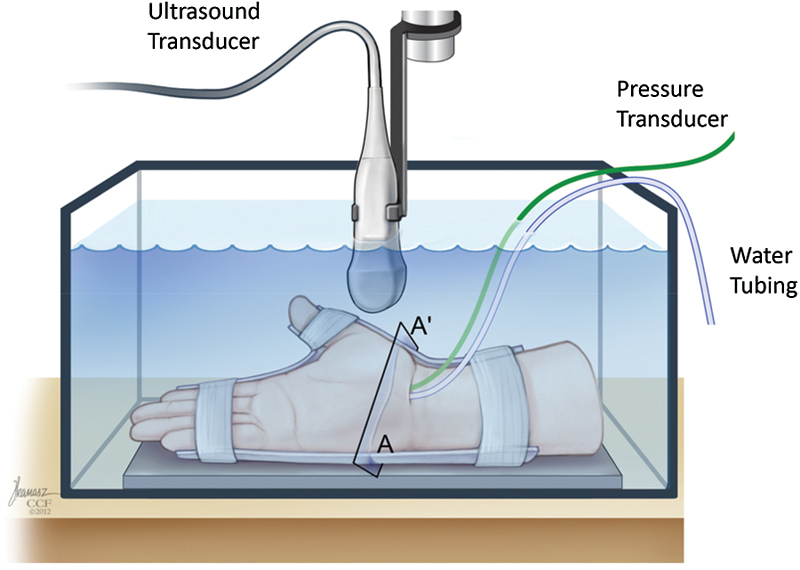
Ultrasound imaging of the carpal tunnel cross section (denoted by A-A′) while pressure was applied inside the tunnel.
As carpal tunnel pressure increased, the TCL increasingly separated at its transected edges (Fig. 4), and the carpal arch shape became more convex (Fig. 5). The carpal arch area increased by 62.4 ± 20.2 mm2 when the pressure reached 120 mm Hg, relative to the carpal arch area at the baseline pressure of 10 mm Hg. The increased carpal arch area of the released carpal tunnel was due to increases in the carpal arch length and height. The carpal arch area increased linearly within a low-pressure range and plateaued around 70 mm Hg. This demonstrates that the released carpal arch is relatively compliant at low pressure and becomes less compliant as pressure increases and the tunnel expands. In the linear region (10–70 mm Hg) of the pressure-area plot, the compliance of the carpal arch quantified by the slope of the regression line was 0.89 mm2/mm Hg (Fig. 6). When compared with the intact carpal arch within a similar pressure range,28 the released carpal arch had a 9 times greater compliance (Fig. 6). The increased compliance of the released carpal arch is expected because the leaflets of the TCL are more flexible to deform at the cutting edges. The dramatic increase in carpal arch compliance after carpal tunnel release helps explain the mechanisms of median nerve decompression in alleviating symptoms of carpal tunnel syndrome.
Fig. 4.
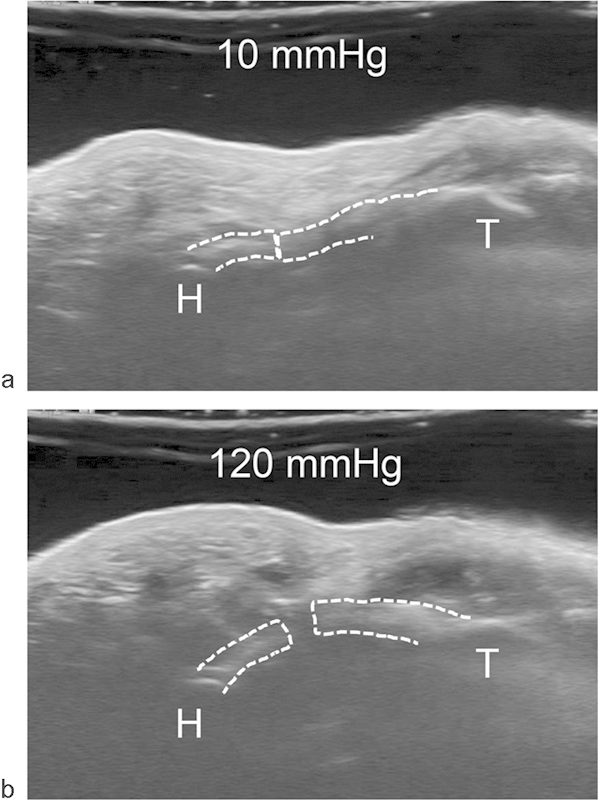
Carpal tunnel cross-sectional images showing the transected TCL (dotted line) at (a) 10 mm Hg and (b) 120 mm Hg (H = hamate, T = trapezium).
Fig. 5.
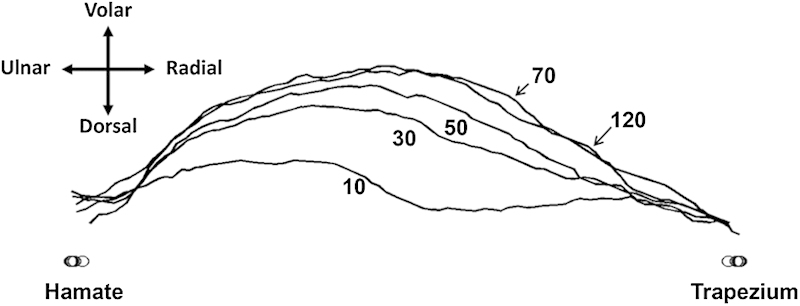
Representative outlines of the TCL volar boundary in response to pressure changes.
Fig. 6.
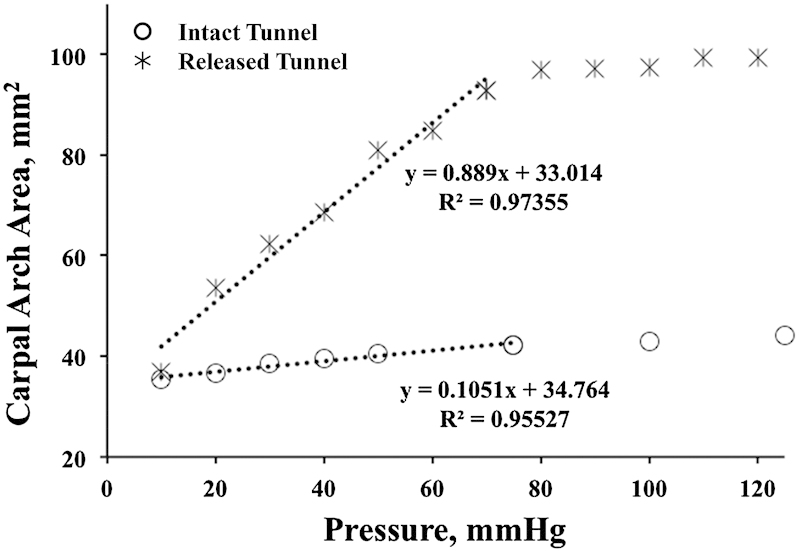
Change in carpal arch area as a function of tunnel pressure for the intact (O) and released (*) carpal tunnels with dotted lines indicating respective linear regressions in the pressure range of 10–70 mm Hg.
The TCL: Present and Future
The TCL has attracted extensive research attention for the past two decades, and considerable knowledge has been gained from basic science research and its clinical implications in hand and wrist surgery. The TCL is known to have functional significance in stabilizing the carpal tunnel structure,19 22 38 serving as a pulley for the flexor tendons,39 40 anchoring the thenar and hypothenar muscles for hand strength,2 3 as well as providing wrist proprioception.41 Although this article focuses on the TCL's biomechanical role in carpal tunnel compliance, the science of this ligament has progressively evolved with regard to its cellular mechanisms,42 histological composition,43 44 fiber architecture,44 45 46 neural anatomy,41 morphological characteristics,47 48 49 material properties,50 51 52 and structural mechanics.3 19 38 53 54 55 56 57
Clinically, transecting the TCL has been shown to benefit patients by relieving symptoms of carpal tunnel syndrome. However, disrupting the tunnel structure may compromise other aspects of hand function; these implications remain to be further clarified. Alternative surgical techniques58 59 60 61 62 and nonsurgical strategies related to the TCL63 64 65 continue to be explored, each warranting rigorous scientific investigation to establish evidence-based interventions. Regarding the many etiological factors of carpal tunnel syndrome, TCL thickening and stiffening are suggested as possible mechanisms, causing pathomechanics for median nerve compression and carpal tunnel syndrome.16 Future studies are needed to elucidate the potential mechanobiological effects on the TCL resulting from repetitive hand use that involves biomechanical interactions among tissues (e.g., TCL, flexor tendons, and thenar muscles). For carpal tunnel biomechanics and its relationship to the TCL, a validated computational model of the wrist with high-fidelity constitutive components is valuable to assist our understanding of pathomechanisms and to provide patient-specific simulation and surgical procedures. More research is needed to investigate wrist function in the in vivo, physiological environment with the aid of dynamic imaging modalities, such as ultrasound, fluoroscopy, computed tomography (CT), and MRI. Such advancement of knowledge will further improve clinical management of hand and wrist conditions.
Acknowledgments
This publication is made possible by Grant Number R21AR062753 from the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
Conflict of Interest None
References
- 1.Fuss F K, Wagner T F. Biomechanical alterations in the carpal arch and hand muscles after carpal tunnel release: a further approach toward understanding the function of the flexor retinaculum and the cause of postoperative grip weakness. Clin Anat. 1996;9(2):100–108. doi: 10.1002/(SICI)1098-2353(1996)9:2<100::AID-CA2>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 2.Kung J, Budoff J E, Wei M L, Gharbaoui I, Luo Z P. The origins of the thenar and hypothenar muscles. J Hand Surg [Br] 2005;30(5):475–476. doi: 10.1016/j.jhsb.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 3.Shen Z L, Li Z M. Biomechanical interaction between the transverse carpal ligament and the thenar muscles. J Appl Physiol (1985) 2013;114(2):225–229. doi: 10.1152/japplphysiol.01273.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silverstein B A, Fine L J, Armstrong T J. Occupational factors and carpal tunnel syndrome. Am J Ind Med. 1987;11(3):343–358. doi: 10.1002/ajim.4700110310. [DOI] [PubMed] [Google Scholar]
- 5.Rempel D, Keir P J, Smutz W P, Hargens A. Effects of static fingertip loading on carpal tunnel pressure. J Orthop Res. 1997;15(3):422–426. doi: 10.1002/jor.1100150315. [DOI] [PubMed] [Google Scholar]
- 6.Gelberman R H, Hergenroeder P T, Hargens A R, Lundborg G N, Akeson W H. The carpal tunnel syndrome. A study of carpal canal pressures. J Bone Joint Surg Am. 1981;63(3):380–383. [PubMed] [Google Scholar]
- 7.Luchetti R, Schoenhuber R, Nathan P. Correlation of segmental carpal tunnel pressures with changes in hand and wrist positions in patients with carpal tunnel syndrome and controls. J Hand Surg [Br] 1998;23(5):598–602. doi: 10.1016/s0266-7681(98)80009-0. [DOI] [PubMed] [Google Scholar]
- 8.Nakamichi K, Tachibana S. The use of ultrasonography in detection of synovitis in carpal tunnel syndrome. J Hand Surg [Br] 1993;18(2):176–179. doi: 10.1016/0266-7681(93)90100-t. [DOI] [PubMed] [Google Scholar]
- 9.Lluch A L. Thickening of the synovium of the digital flexor tendons: cause or consequence of the carpal tunnel syndrome? J Hand Surg [Br] 1992;17(2):209–212. doi: 10.1016/0266-7681(92)90091-f. [DOI] [PubMed] [Google Scholar]
- 10.Campiglio G L, Di Giuseppe P, Migliorini L, Cazzaniga M, Lamperti E, Romorini A. Histopathology of the flexor tendon sheaths and its relevance in idiopathic carpal tunnel syndrome. Eur J Plast Surg. 1999;22:230–233. [Google Scholar]
- 11.Cobb T K, An K N, Cooney W P. Effect of lumbrical muscle incursion within the carpal tunnel on carpal tunnel pressure: a cadaveric study. J Hand Surg Am. 1995;20(2):186–192. doi: 10.1016/S0363-5023(05)80005-X. [DOI] [PubMed] [Google Scholar]
- 12.Fuchs P C, Nathan P A, Myers L D. Synovial histology in carpal tunnel syndrome. J Hand Surg Am. 1991;16(4):753–758. doi: 10.1016/0363-5023(91)90208-s. [DOI] [PubMed] [Google Scholar]
- 13.Keir P J, Bach J M. Flexor muscle incursion into the carpal tunnel: a mechanism for increased carpal tunnel pressure? Clin Biomech (Bristol, Avon) 2000;15(5):301–305. doi: 10.1016/s0268-0033(99)00092-3. [DOI] [PubMed] [Google Scholar]
- 14.Weiss N D, Gordon L, Bloom T, So Y, Rempel D M. Position of the wrist associated with the lowest carpal-tunnel pressure: implications for splint design. J Bone Joint Surg Am. 1995;77(11):1695–1699. doi: 10.2106/00004623-199511000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Okutsu I, Ninomiya S, Hamanaka I, Kuroshima N, Inanami H. Measurement of pressure in the carpal canal before and after endoscopic management of carpal tunnel syndrome. J Bone Joint Surg Am. 1989;71(5):679–683. [PubMed] [Google Scholar]
- 16.Moore J S. Biomechanical models for the pathogenesis of specific distal upper extremity disorders. Am J Ind Med. 2002;41(5):353–369. doi: 10.1002/ajim.10037. [DOI] [PubMed] [Google Scholar]
- 17.John V, Nau H E, Nahser H C, Reinhardt V, Venjakob K. CT of carpal tunnel syndrome. AJNR Am J Neuroradiol. 1983;4(3):770–772. [PMC free article] [PubMed] [Google Scholar]
- 18.Li Z M, Tang J, Chakan M, Kaz R. Carpal tunnel expansion by palmarly directed forces to the transverse carpal ligament. J Biomech Eng. 2009;131(8):81011. doi: 10.1115/1.3148469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xiu K H, Kim J H, Li Z M. Biomechanics of the transverse carpal arch under carpal bone loading. Clin Biomech (Bristol, Avon) 2010;25(8):776–780. doi: 10.1016/j.clinbiomech.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gabra J N, Domalain M, Li Z M. Movement of the distal carpal row during narrowing and widening of the carpal arch width. J Biomech Eng. 2012;134(10):101004. doi: 10.1115/1.4007634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Taleisnik J. The ligaments of the wrist. J Hand Surg Am. 1976;1(2):110–118. doi: 10.1016/s0363-5023(76)80004-4. [DOI] [PubMed] [Google Scholar]
- 22.Tengrootenhuysen M, van Riet R, Pimontel P, Bortier H, Van Glabbeek F. The role of the transverse carpal ligament in carpal stability: an in vitro study. Acta Orthop Belg. 2009;75(4):467–471. [PubMed] [Google Scholar]
- 23.Garcia-Elias M, An K N, Cooney W P III, Linscheid R L, Chao E Y. Stability of the transverse carpal arch: an experimental study. J Hand Surg Am. 1989;14(2 Pt 1):277–282. doi: 10.1016/0363-5023(89)90021-x. [DOI] [PubMed] [Google Scholar]
- 24.Uchiyama S, Itsubo T, Yasutomi T, Nakagawa H, Kamimura M, Kato H. Quantitative MRI of the wrist and nerve conduction studies in patients with idiopathic carpal tunnel syndrome. J Neurol Neurosurg Psychiatry. 2005;76(8):1103–1108. doi: 10.1136/jnnp.2004.051060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monagle K, Dai G, Chu A, Burnham R S, Snyder R E. Quantitative MR imaging of carpal tunnel syndrome. AJR Am J Roentgenol. 1999;172(6):1581–1586. doi: 10.2214/ajr.172.6.10350293. [DOI] [PubMed] [Google Scholar]
- 26.Horch R E Allmann K H Laubenberger J Langer M Stark G B Median nerve compression can be detected by magnetic resonance imaging of the carpal tunnel Neurosurgery 199741176–82., discussion 82–83 [DOI] [PubMed] [Google Scholar]
- 27.Mesgarzadeh M, Schneck C D, Bonakdarpour A, Mitra A, Conaway D. Carpal tunnel: MR imaging. Part II. Carpal tunnel syndrome. Radiology. 1989;171(3):749–754. doi: 10.1148/radiology.171.3.2541464. [DOI] [PubMed] [Google Scholar]
- 28.Li Z M, Masters T L, Mondello T A. Area and shape changes of the carpal tunnel in response to tunnel pressure. J Orthop Res. 2011;29(12):1951–1956. doi: 10.1002/jor.21468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Richman J A, Gelberman R H, Rydevik B L. et al. Carpal tunnel syndrome: morphologic changes after release of the transverse carpal ligament. J Hand Surg Am. 1989;14(5):852–857. doi: 10.1016/s0363-5023(89)80089-9. [DOI] [PubMed] [Google Scholar]
- 30.Ablove R H, Peimer C A, Diao E, Oliverio R, Kuhn J P. Morphologic changes following endoscopic and two-portal subcutaneous carpal tunnel release. J Hand Surg Am. 1994;19(5):821–826. doi: 10.1016/0363-5023(94)90194-5. [DOI] [PubMed] [Google Scholar]
- 31.Lee C H, Kim T K, Yoon E S, Dhong E S. Postoperative morphologic analysis of carpal tunnel syndrome using high-resolution ultrasonography. Ann Plast Surg. 2005;54(2):143–146. doi: 10.1097/01.sap.0000143799.88513.47. [DOI] [PubMed] [Google Scholar]
- 32.Kato T, Kuroshima N, Okutsu I, Ninomiya S. Effects of endoscopic release of the transverse carpal ligament on carpal canal volume. J Hand Surg Am. 1994;19(3):416–419. doi: 10.1016/0363-5023(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 33.Brooks J J, Schiller J R, Allen S D, Akelman E. Biomechanical and anatomical consequences of carpal tunnel release. Clin Biomech (Bristol, Avon) 2003;18(8):685–693. doi: 10.1016/s0268-0033(03)00052-4. [DOI] [PubMed] [Google Scholar]
- 34.Gartsman G M, Kovach J C, Crouch C C, Noble P C, Bennett J B. Carpal arch alteration after carpal tunnel release. J Hand Surg Am. 1986;11(3):372–374. doi: 10.1016/s0363-5023(86)80144-7. [DOI] [PubMed] [Google Scholar]
- 35.Flores L P, Cavalcante T F, Neto O R, Alcântara F S. Quantitative analysis of the variation in angles of the carpal arch after open and endoscopic carpal tunnel release. Clinical article. J Neurosurg. 2009;111(2):311–316. doi: 10.3171/2008.9.JNS08457. [DOI] [PubMed] [Google Scholar]
- 36.Goss B C, Agee J M. Dynamics of intracarpal tunnel pressure in patients with carpal tunnel syndrome. J Hand Surg Am. 2010;35(2):197–206. doi: 10.1016/j.jhsa.2009.09.019. [DOI] [PubMed] [Google Scholar]
- 37.Kim D H, Marquardt T L, Gabra J N. et al. Pressure-morphology relationship of a released carpal tunnel. J Orthop Res. 2013;31(4):616–620. doi: 10.1002/jor.22271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guo X, Fan Y, Li Z M. Effects of dividing the transverse carpal ligament on the mechanical behavior of the carpal bones under axial compressive load: a finite element study. Med Eng Phys. 2009;31(2):188–194. doi: 10.1016/j.medengphy.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 39.Kline S C, Moore J R. The transverse carpal ligament. An important component of the digital flexor pulley system. J Bone Joint Surg Am. 1992;74(10):1478–1485. [PubMed] [Google Scholar]
- 40.Wehbé M A. Transverse carpal ligament. An important component of the digital flexor pulley system. J Bone Joint Surg Am. 1993;75(10):1575–1576. doi: 10.2106/00004623-199310000-00019. [DOI] [PubMed] [Google Scholar]
- 41.Mashoof A A, Levy H J, Soifer T B, Miller-Soifer F, Bryk E, Vigorita V. Neural anatomy of the transverse carpal ligament. Clin Orthop Relat Res. 2001;(386):218–221. doi: 10.1097/00003086-200105000-00028. [DOI] [PubMed] [Google Scholar]
- 42.Allampallam K, Chakraborty J, Robinson J. Effect of ascorbic acid and growth factors on collagen metabolism of flexor retinaculum cells from individuals with and without carpal tunnel syndrome. J Occup Environ Med. 2000;42(3):251–259. doi: 10.1097/00043764-200003000-00005. [DOI] [PubMed] [Google Scholar]
- 43.Nakamichi K, Tachibana S. Histology of the transverse carpal ligament and flexor tenosynovium in idiopathic carpal tunnel syndrome. J Hand Surg Am. 1998;23(6):1015–1024. doi: 10.1016/s0363-5023(98)80009-9. [DOI] [PubMed] [Google Scholar]
- 44.Stecco C, Macchi V, Lancerotto L, Tiengo C, Porzionato A, De Caro R. Comparison of transverse carpal ligament and flexor retinaculum terminology for the wrist. J Hand Surg Am. 2010;35(5):746–753. doi: 10.1016/j.jhsa.2010.01.031. [DOI] [PubMed] [Google Scholar]
- 45.Prantil R K, Xiu K, Kim K E. et al. Fiber orientation of the transverse carpal ligament. Clin Anat. 2012;25(4):478–482. doi: 10.1002/ca.21257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Isogai S, Murakami G, Wada T, Akita K, Yamashita T, Ishii S. Laminar configuration of the transverse carpal ligament. J Orthop Sci. 2002;7(1):79–83. doi: 10.1007/s776-002-8424-2. [DOI] [PubMed] [Google Scholar]
- 47.Pacek C A, Chakan M, Goitz R J, Kaufmann R A, Li Z M. Morphological analysis of the transverse carpal ligament. Hand (NY) 2010;5(2):135–140. doi: 10.1007/s11552-009-9219-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Manley M, Boardman M, Goitz R J. The carpal insertions of the transverse carpal ligament. J Hand Surg Am. 2013;38(4):729–732. doi: 10.1016/j.jhsa.2013.01.015. [DOI] [PubMed] [Google Scholar]
- 49.Shen Z L, Li Z M. Ultrasound assessment of transverse carpal ligament thickness: a validity and reliability study. Ultrasound Med Biol. 2012;38(6):982–988. doi: 10.1016/j.ultrasmedbio.2012.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen Z L, Vince D G, Li Z M. In vivo study of transverse carpal ligament stiffness using acoustic radiation force impulse (ARFI) imaging. PLoS ONE. 2013;8(7):e68569. doi: 10.1371/journal.pone.0068569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Main E K, Goetz J E, Baer T E, Klocke N F, Brown T D. Volar/dorsal compressive mechanical behavior of the transverse carpal ligament. J Biomech. 2012;45(7):1180–1185. doi: 10.1016/j.jbiomech.2012.01.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmes M W, Howarth S J, Callaghan J P, Keir P J. Carpal tunnel and transverse carpal ligament stiffness with changes in wrist posture and indenter size. J Orthop Res. 2011;29(11):1682–1687. doi: 10.1002/jor.21442. [DOI] [PubMed] [Google Scholar]
- 53.Zheng Y P, Li Z M, Choi A P, Lu M H, Chen X, Huang Q H. Ultrasound palpation sensor for tissue thickness and elasticity measurement—assessment of transverse carpal ligament. Ultrasonics. 2006;44 01:e313–e317. doi: 10.1016/j.ultras.2006.06.018. [DOI] [PubMed] [Google Scholar]
- 54.Li Z M. Gender difference in carpal tunnel compliance. J Musculoskeletal Res. 2005;9(3):153–159. [Google Scholar]
- 55.Miyamoto H, Miura T, Morizaki Y, Uehara K, Ohe T, Tanaka S. Comparative study on the stiffness of transverse carpal ligament between normal subjects and carpal tunnel syndrome patients. Hand Surg. 2013;18(2):209–214. doi: 10.1142/S0218810413500251. [DOI] [PubMed] [Google Scholar]
- 56.Carrigan S D, Whiteside R A, Pichora D R, Small C F. Development of a three-dimensional finite element model for carpal load transmission in a static neutral posture. Ann Biomed Eng. 2003;31(6):718–725. doi: 10.1114/1.1574027. [DOI] [PubMed] [Google Scholar]
- 57.Ishiko T, Puttlitz C M, Lotz J C, Diao E. Scaphoid kinematic behavior after division of the transverse carpal ligament. J Hand Surg Am. 2003;28(2):267–271. doi: 10.1053/jhsu.2003.50055. [DOI] [PubMed] [Google Scholar]
- 58.Jakab E, Ganos D, Cook F W. Transverse carpal ligament reconstruction in surgery for carpal tunnel syndrome: a new technique. J Hand Surg Am. 1991;16(2):202–206. doi: 10.1016/s0363-5023(10)80097-8. [DOI] [PubMed] [Google Scholar]
- 59.Seitz W H, Lall A. Open carpal tunnel release with median neurolysis and Z-plasty reconstruction of the transverse carpal ligament. Curr Orthop Pract. 2013;24(1):53–57. [Google Scholar]
- 60.Netscher D, Dinh T, Cohen V, Thornby J. Division of the transverse carpal ligament and flexor tendon excursion: open and endoscopic carpal tunnel release. Plast Reconstr Surg. 1998;102(3):773–778. doi: 10.1097/00006534-199809030-00023. [DOI] [PubMed] [Google Scholar]
- 61.Netscher D, Mosharrafa A, Lee M. et al. Transverse carpal ligament: its effect on flexor tendon excursion, morphologic changes of the carpal canal, and on pinch and grip strengths after open carpal tunnel release. Plast Reconstr Surg. 1997;100(3):636–642. doi: 10.1097/00006534-199709000-00015. [DOI] [PubMed] [Google Scholar]
- 62.Netscher D T. The benefit of transverse carpal ligament reconstruction following open carpal tunnel release. Plast Reconstr Surg. 2003;111(6):2020–2022. doi: 10.1097/01.PRS.0000056836.14559.09. [DOI] [PubMed] [Google Scholar]
- 63.Sucher B M, Hinrichs R N, Welcher R L, Quiroz L D, St Laurent B F, Morrison B J. Manipulative treatment of carpal tunnel syndrome: biomechanical and osteopathic intervention to increase the length of the transverse carpal ligament: part 2. Effect of sex differences and manipulative “priming.”. J Am Osteopath Assoc. 2005;105(3):135–143. [PubMed] [Google Scholar]
- 64.Li Z M, Gabra J N, Marquardt T L, Kim D H. Narrowing carpal arch width to increase cross-sectional area of carpal tunnel—a cadaveric study. Clin Biomech (Bristol, Avon) 2013;28(4):402–407. doi: 10.1016/j.clinbiomech.2013.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Porrata H Porrata A Sosner J New carpal ligament traction device for the treatment of carpal tunnel syndrome unresponsive to conservative therapy J Hand Ther 200720120–27., quiz 28 [DOI] [PubMed] [Google Scholar]


