Abstract
Colorectal cancer (CRC) remains a significant cause of mortality. Inhibitors of cyclooxygenase (COX) and thus prostaglandin E2, are promising CRC preventives, but have significant toxicities. Ginger has been shown to inhibit COX, to decrease the incidence and multiplicity of adenomas, and decrease PGE2 concentrations in subjects at normal risk for CRC. This study was conducted to determine the effects of 2.0 g/d of ginger given orally on the levels of PGE2, leukotriene B4 (LTB4), 13-hydroxy-octadecadienoic acids, and 5-, 12-, & 15-hydroxy-eicosatetraenoic acid, in the colonic mucosa of subjects at increased risk for CRC. We randomized 20 subjects to 2.0 g/d ginger or placebo for 28 d. At baseline and Day 28, a flexible sigmoidoscopy was used to obtain colon biopsies. A liquid chromatography mass spectrometry method was used to determine eicosanoid levels in the biopsies, and levels were expressed per amount of protein or free arachidonic acid (AA). There was a significant decrease in AA between baseline and Day 28 (P = 0.05) and significant increase in LTB4 (P = 0.04) when normalized to protein, in subjects treated with ginger versus placebo. No other changes in eicosanoids were observed. There was no difference between the groups in total adverse events (AE; P = 0.06). Ginger lacks the ability to decrease eicosanoid levels in people at increased risk for CRC. Ginger did appear to be both tolerable and safe; and could have chemopreventive effects through other mechanisms. Further investigation should focus on other markers of CRC risk in those at increased CRC risk.
Keywords: cancer risk reductive, prostaglandins, lipoxygenase, inflammation, zingiber
INTRODUCTION
Despite recent decreases in mortality, colorectal cancer (CRC) remains the third most prevalent and second most deadly cancer in the United States [1]. Non-steroidal anti-inflammatory drugs (NSAIDs), which inhibit cyclooxygenase 1 & 2 (COX-1 & 2) enzymes and thus lower the levels of the inflammatory prostaglandin E2 (PGE2) are promising colorectal cancer (CRC) chemopreventive agents [2]. However, the gastrointestinal and cardiovascular side-effects of aspirin and other NSAIDs have raised concerns for their daily prescription to an otherwise healthy population [3,4]. Also, there is some thought that the inhibition of COX enzymes by these drugs could cause the shunting of arachidonic acid (AA), the substrate for COX, towards the production of other inflammatory eicosanoids [5]. In particular, various eicasanoids produced by the lipoxygenase (LOX) enzymes (5-, 12-, & 15-LOX) from AA, which are leukotriene B4 (LTB4) and the hydroxy-eicosatetraenoic acids (HETEs), 5-, 12-, & 15-HETE and 13-hydroxy-octadecadienoic acid (13-HODE) produced from linoleic acid. Leukotriene B4 and 5-& 12-HETE have all been implicated in the development of CRC [6-12], while both 15-HETE and 13-HODE appear to have anti-inflammatory and anti-tumorigenic activities [13-15]. Therefore, using natural nutritional components with low toxicity, which have the potential to affect COX and LOX, and their products is a potential area of investigation for the prevention of CRC.
One such natural nutritional compound is ginger root (Zingiber officinale),which has been shown to inhibit 5-LOX [16-19] and COX-1 & -2 [18,20-22]. Ginger root decreases inflammation in various murine models [16,23-26], and reduces serum concentrations of PGE2 in rats [27]. Ginger root has also demonstrated preventative effects by decreasing tumor size, incidence and multiplicity in chemically induced animal models of colon carcinogenesis [28-30]. When ginger was administered in the post-initiation stage, it did not suppress aberrant crypt foci formation nor did ginger significantly change the proliferative or apoptotic indexes of the colonic crypt [31]. In our recent studies in participants at normal and increased risk for CRC we found that ginger significantly lowered COX-1 protein expression in increased risk participants, but not in normal risk participants [32]. In another study, ginger significantly reduced gut tissue concentrations of PGE2 and 5-HETE and with a trend toward significant decreases in 12-HETE and 15-HETE in participants at normal risk for CRC [33].
The purpose of this study was to expand on our previous work in subjects at normal risk for CRC by examining the effect of 2.0 g of ginger taken daily for 28 days compared to placebo on eicosanoids in the colonic mucosa of subjects at increased risk for developing colorectal cancer. Secondary objectives were to evaluate the safety, tolerability, adherence and blinding of ginger supplementation.
MATERIALS AND METHODS
Study Participants
Fliers and word-of mouth were used to recruit 21 participants from the Ann Arbor, MI area between June 2009 and January 2010. Eligible participants had to be generally healthy individuals 18 years or older, who were at increased risk for CRC defined as having at least one of the following: (1) a first degree relative with CRC before the age of 60; (2) a previous adenomatous polyp; (3) or early stage resected (Dukes A, B, or C) colon cancer. Subjects were excluded if they were: lactose intolerant; had a diagnosis of peptic ulcer disease, gastrointestinal bleeding, or gastrin secreting tumors; had a known allergy to ginger; were taking supplements or medications which could obscure the ability to detect anti-inflammatory effects; and pregnant or lactating women. Also persons with hereditary non-polyposis colon cancer or familial adenomatous polyposis (HNPCC/FAP), inflammatory bowel disease, or coagulopathy disorders were excluded. Participants were told to stop eating any foods containing ginger within 14 days before drug administration and given a list of gingerrich foods to avoid. All participants were reimbursed for their time.
The University of Michigan Institutional Review Board approved this study and all participants gave written, informed consent before beginning any study procedures. This study was conducted at the University of Michigan Clinical Research Unit (MCRU).
Ginger Intervention
Details on quality control of the ginger extract have been previously published [33]. Briefly, a 2.0 g dose of powered ginger root extract (Z. officinale) standardized to 15 mg (5%) of total gingerols and manufactured by Pure Encapsulations® (Sudbury, MA) was used in the study. This was the same ginger product used in our previous trials with identical amount (5%) of total gingerols. The 2.0 g dose of ginger was based on the maximum tolerated dose of ginger in a phase 1 study in healthy volunteers [34]. Lactose was used for the placebo capsules. Both the lactose and ginger powder were placed into identical opaque red capsules by the Investigational Drug Service (IDS) of the University of Michigan.
Toxicity of the intervention was evaluated at weekly intervals using The National Cancer Institute (NCI) Common Toxicity Scale V 4.02 [35].
Randomization, Allocation, Adherence, and Blinding
Participants were randomized equally into the placebo or ginger group. The study biostatistician generated the randomization code, which was kept by the University of Michigan IDS. The next available randomization number was assigned by the IDS as eligible participants were identified. Participants and study personnel were unaware of the randomization list or treatment assignment. To determine if participants were blind to treatment allocation, participants were asked at their final visit which treatment they received (“ginger,” “placebo” or “don’t know”).
Adherence was assessed by weekly telephone calls, self-report, and pill counts at the end of the study. Adherence was defined as taking at least 70% of capsules as prescribed.
Flexible Sigmoidoscopy and Tissue Collection
Two flexible sigmoidoscopies, one at baseline and the second within 24 h of the last ginger/placebo dose on Day 28 were performed. Participants were not asked to fast or to undergo any bowel cleansing preparation. Participants were placed in a left lateral decubitus position and a flexible sigmoidoscope was passed at least 15 cm above the anal sphincter and eight tissue samples were obtained. Each biopsy specimen was taken 2 cm or more from other biopsy sites in the distal sigmoid colonic mucosa by opening and pressing the biopsy forceps perpendicular to the mucosal surface with mild pressure.
Tissue Handling and Disposition
Biopsies were frozen in liquid nitrogen and then stored at −70°C after being placed into a sterile 1.5-mL Eppendorf tube. Biopsy samples were taken at precisely 50 s after the time the biopsy forceps were closed. Biopsies weighed approximately 5 mg and contained between 400 and 600 μg protein. Eicosanoids assays were run in triplicate and required around 10–20 μg of colon tissue, which is the equivalent of two biopsies.
Analytical Methods
Eicosanoids (PGE2, 5-HETE, 12-HETE, 15-HETE and 13-HODE)
Eicosanoids were assayed according to previously reported methods [33,36,37] Briefly, reverse-phase LC electrospray ionization mass spectrometry (LC/MS/MS) analyses were used for quantitation of PGE2, LTB4, 5-HETE, 12-HETE, 15-HETE, and 13-HODE. LC/MS/MS analyses were performed using a Quattro Ultima tandem mass spectrometer (Micromass, Beverly, MA) equipped with an Agilent HP 1100 binary pump HPLC inlet. Eicosanoids were separated using a Luna 3 μ Phenyl-Hexyl 2 mm × 150 mm LC column (Phenomenex, Torrance, CA). The mobile phase consisted of 10 mM ammonium acetate (pH 8.5) and methanol. For the analysis of PGE2, HETEs, and 13-HODE, the separation was achieved using a linear methanol gradient from 40% to 60% over 18 min followed by a methanol flush. The flow rate was 250 μL/min with a column temperature of 50°C. The sample injection volume was 25 μL. Samples were kept at 4°C during the analysis. All eicosanoids were detected using electrospray negative ionization and multiple-reaction monitoring of the transition ions for the metabolites and their internal standards [38].
The mass spectrometer (Thermo Finnigan TSQ Quantum, San Jose, CA) was operated in the electrospray negative ion mode with a cone voltage of 2300 V, a cone gas flow rate of 117l/h, and a devolution gas flow rate of 998l/h. The temperature of the desolvation region was 350°C, and the temperature of the source region was 120°C. Fragmentation for all compounds was performed using argon as the collision gas at a collision cell pressure of 2.10 × 10−3 Torr. The collision energy ranged from 16 to 31 V depending on the analyte. The results were either expressed as nanogram (ng) of eicosanoid per milligram (mg) of protein or as ng of eicosanoid per microgram (μg) of free AA. All of the biopsy samples from a given individual were assayed in the same batch to eliminate any batch effects over time.
Statistical Methods and Sample Size
Statistical analyses were conducted using SAS 9.2 statistical software (SAS Institute Inc., Cary, NC). A P-value ≤0.05 (two-sided) was considered statistically significant. Baseline characteristics stratified by treatment group were analyzed and reported as mean standard deviations (SD) for continuous variables, and as counts and percentages for categorical variables. Balance between treatment groups on baseline characteristics was tested using independent sample t-tests for continuous variables and Pearson’s Chi-square and Fisher exact tests, as appropriate, for categorical variables.
We calculated the mean percent change within treatment group, for PGE2, LTB4, 5-HETE, 12-HETE, 15-HETE, and 13-HODE from baseline to Day 28. Results are reported as mean ± SD. Pearson’s Chi-square or Fisher’s exact test were used to calculate between group differences for adverse events.
Assessment of blinding and adherence were determined using an independent sample t-test to determine the difference in the proportion of participants in each group who correctly guessed their correct group assignment or who took 70% or greater of their study medication. Mean and SD for percent adherent per group was tested using independent sample t-test.
We determined using a two sample t-test that a sample size of 10 per treatment group would have better than 80% power and a 5% level of significance to detect a reduction of at least 25% mean difference in PGE2 mucosal concentration in the ginger group compared to the placebo group at the end of a 28d intervention. This is based on previously reported data on PGE2 levels in participants at increased risk of CRC human colon tissue who had baseline PGE2 mucosal concentrations of 14.4, ± 1.7 pg/μg protein, which decreased after 28 d of 81 mg of asprin to a mean concentration of 4.7 ± 0.70 pg/μg protein which was roughly a 70% reduction compared to the placebo group [39].
RESULTS
Subjects and Toxicity
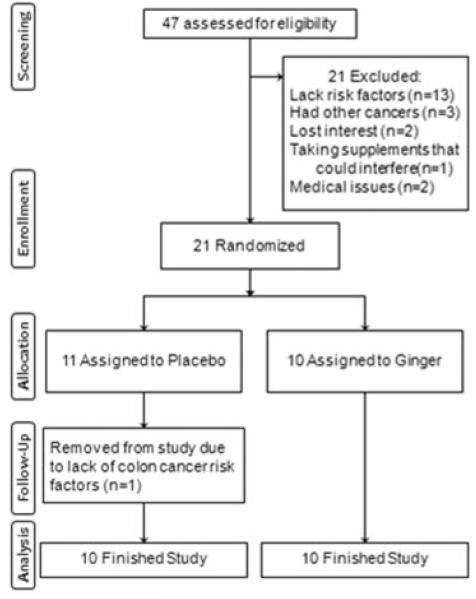
We screened 47 persons of whom 21 met all eligibility criteria and were randomized: 11 to placebo and 10–2.0 g ginger, for 28 d. However, one participant randomized to the placebo group was removed from study after it was determined that he was not at increased risk for CRC. Figure 1 documents the numbers of participants, reasons for exclusions and reasons for discontinuing the intervention.
Figure 1.
Flow diagram of a trial of ginger supplementation over 4 wk on eicosanoids in normal-appearing colorectal mucosa of individuals at increased risk of for colorectal cancer
Table 1 reports demographic and clinical characteristics by group. No significant differences for any demographic or clinical characteristic between treatment groups were found. The mean age of the study subjects was 51.0 ± 12.9 (range 29–73 yr) with less than half of the participants being male (N = 7, 35.0%). The majority of the participants self-reported as being Caucasian (N = 15, 75.0%), with two (25%) indicating that they were African American (N = 2), American Indian (N = 2), or mixed race (N = 1), while none of the participants reported being of Hispanic ethnicity. The majority of participants (N = 10, 50.0%) were at increased risk for CRC due to having had a prior adenoma (N = 12, 60.0%), and seven subjects had a first degree relative with a diagnosis of CRC before the age of 60. Only one participant had a history of early stage colon cancer (5%), and three participants had multiple reasons (both a prior adenoma and a first degree relative) for being at increased colon cancer risk.
Table 1.
Baseline Characteristics of the Randomization Groups
| Characteristics | Ginger (N = 10) |
Placebo (N = 10) |
P-value |
|---|---|---|---|
| Sex, no. (%) | |||
| Men | 4 (40) | 3 (30) | 0.64a |
| Women | 6 (60) | 7 (70) | |
| Race, no. (%) | |||
| White | 8 (80) | 7 (70) | 0.38a |
| Age, mean (SD), yr | 51.1 (11.7) | 50.8 (14.6) | 0.95b |
| Reason for being high risk for CRCc, no. (%) | |||
| First degree relatived | 4 (40.0) | 6 (60.0) | 0.47b |
| Previous adenoma | 5 (50.0) | 4 (40.0) | |
| Previous CRC | 1 (10.0) | 0 (0.0) | |
P-value is based on an independent samples t-test.
P-value is based on a Chi-Squared.
CRC, colorectal cancer.
First degree relative diagnosed with colorectal cancer before the age of 60 yr.
Possible, probably or likely treatment-related toxicities are reported by participants in Table 2. All adverse events were non-serious and reported as grade 1 per the NCI Common Toxicity Criteria (v. 4.02) [35]. No significant difference was observed between the groups for total adverse events (N = 13, P = 0.06) or specific categories of adverse events such as gastrointestinal (GI) toxicities (N = 10, P = 0.18).
Table 2.
Adverse Events Reported by Person
| Adverse Events | Ginger (n = 10) |
Placebo (n = 10) |
P-valuea |
|---|---|---|---|
| All participants with any AE, No. (%) |
9 (90.0) | 4 (40.0) | 0.06 |
| CGIb | 7 (70.0) | 3 (30.0) | 0.18 |
| Otherc | 3 (30.0) | 1 (10.0) | 0.58 |
P-value: Chi-Square or Fisher’s exact test as appropriate.
GI symptoms includes: bloating, urgency, gas, nausea, heartburn, sores in mouth & anorexia.
Other includes: allergic reaction, nose bleed, skin rash, struck with water tube after biopsy procedure.
Eicosanoids (PGE2, LTB4, 5-HETE, 12-HETE, 15-HETE, and 13-HODE)
In Table 3, all continuous outcomes and mean percent change from baseline to Day 28 of PGE2, LTB4, 5-, 12-, 15-HETE, 13-HODE, and AA are shown normalized to both protein and AA, with the exception of AA. The baseline values of PGE2, LTB4, 5-HETE, 12-HETE, 15-HETE, and 13-HODE in colon biopsies across both groups were 11.8 ± 12.8, 2.60 ± 1.7, 6.3 ± 6.3, 2.7 ± 1.8, 10.1 ± 13.6, and 47.2 ± 77.5 pg/μg protein, respectively (mean ± SD, n = 20). Baseline values normalized to AA of PGE2, LTB4, 5-HETE, 12-HETE, 15-HETE, and 13-HODE were 1.4 ± 1.2, 0.5 ± 0.7, 1.9 ± 5.0, 0.5 ± 0.6, 1.9 ± 3.2, and 4.9 ± 8.1 ng/μg, respectively.
Table 3.
Eicasanoids Levels in Normal Mucosa in Participants at Increased Risk for Colorectal Cancer [Mean (SD)*]
| Placebo (n = 10) |
Ginger (n = 10) |
||||||
|---|---|---|---|---|---|---|---|
| Eicosanoid | Baseline | After 28 d | Mean % changeb | Baseline | After 28 d | Mean % changeb | P-valuea |
| Standardized to protein (pg/μg) | |||||||
| PGE2 | 12.9 (15.2) | 12.8 (16.8) | 37.0 (113.4) | 10.6 (10.5) | 23.6 (21.2) | 333.5 (773.6) | 0.26 |
| LTB4 | 2.9 (1.7) | 2.6 (2.1) | −4.7 (54.9) | 2.4 (1.9) | 3.5 (2.3) | 54.0 (63.2) | 0.04 |
| HETE5 | 8.9 (8.0) | 9.0 (8.0) | 39.1 (108.9) | 3.7 (2.0) | 15.2 (14.0) | 412.1 (755.6) | 0.16 |
| HETE12 | 3.2 (2.2) | 5.1 (5.5) | 59.4 (149.1) | 2.2 (1.3) | 4.5 (4.4) | 101.7 (196.1) | 0.60 |
| HETE15 | 14.7 (17.7) | 11.1 (9.8) | 82.1 (189.5) | 5.5 (5.6) | 20.6 (18.8) | 602.5 (1047.8) | 0.16 |
| HODE13 | 41.4 (50.8) | 30.9 (22.0) | 27.7 (105.2) | 53.0 (100.1) | 35.0 (32.6) | 55.7 (122.4) | 0.59 |
| AA (ng/pg)c | 1.0 (1.3) | 1.4 (1.4) | 229.4 (413.7) | 1.6 (1.1) | 0.7 (0.4) | −44.2 (41.5) | 0.05 |
| Standardized to arachidonic acid (ng/μg) | |||||||
| PGE2 | 1.7 (1.4) | 3.70 (8.4) | 147.6 (368.7) | 1.0 (1.0) | 3.0 (2.9) | 1149.2 (3194.5) | 0.17 |
| LTB4 | 0.7 (1.0) | 0.34 (0.3) | 84.3 (205.9) | 0.2 (0.2) | 0.5 (0.4) | 173.8 (248.9) | 0.27 |
| HETE5 | 3.4 (6.9) | 1.72 (2.5) | 362.7 (718.9) | 0.4 (0.3) | 2.4 (3.7) | 1079.6 (2297.0) | 0.15 |
| HETE12 | 0.7 (0.7) | 0.57 (0.5) | 247.1 (598.9) | 0.2 (0.1) | 0.6 (0.7) | 279.2 (487.4) | 0.38 |
| HETE15 | 3.3 (4.1) | 2.00 (3.2) | 290.6 (545.8) | 0.5 (0.5) | 3.2 (5.2) | 1406.3 (2993.3) | 0.13 |
| HODE13 | 6.8 (10.9) | 4.98 (6.3) | 140.5 (453.7) | 3.1 (3.4) | 4.4 (4.5) | 114.1 (164.02) | 0.23 |
SD,±standard deviation.
independent t-test of the difference between the mean percent change from baseline to Day 28.
Mean percent change between baseline and Week 4 is calculated as [(eicosanoid at time 2/eicosanoid at time 1)/eicosanoid at time 1)] per participant and then an average is obtained. Mean percent change may not appear reflective of change in baseline and 28-d follow-up mean values. This is due to the large amount of variability in the baseline measures.
AA, arachidonic acid; PGE2, prostaglandin E2; 5-HETE, 12-HETE, 15-HETE, 5-, 12- & 15-hydroxyeicosatetraenoicacid; 13-HODE, 13-hydroxy-octadecadienoic acids.
There was no significant difference in mean percent change between the placebo and ginger group for any of the eicosanoids when normalized to AA after 28 d. In contrast, when normalized to protein there was a significant increase (P = 0.04) in LTB4 in the ginger group (−4.7 ± 54.9% placebo versus 54.0 ± 63.2% ginger) and a significant (P = 0.05) decrease in AA in the ginger group (229.4 ± 413.7% placebo versus −44.2 ± 41.5% ginger). There were no other significant differences in the other eicosanoids.
Blinding and Adherence
Participants were unable to determine whether they had received ginger or placebo (P = 0.53). The majority of participants (N = 9, 45.0%) indicated they were taking placebo, with seven participants reporting ginger (35.0%) and four (20.0%) being unable to decide to which treatment they were randomized.
Participants on average took 79.1 ± 7.4% of their capsules and all participants were adherent per our definition of taking at least 70% of their capsules with a mean ± SD of 78.4 ± 8.6% in the placebo group and 79.8 ± 6.6% in the ginger group. There was no significant difference in adherence between treatment groups (P = 0.70).
DISCUSSION
We found that ingesting 2.0 g per d of ginger root extract for 28 d significantly decreased AA and significantly increased concentrations of LTB4, when normalized to protein, in normal appearing gut mucosa in participants who were at increased risk of developing colon cancer. Ginger had no significant effect on any other eicosanoid including PGE2 in gut tissue whether normalized to protein or AA. Although not statistically significant, the concentrations of all eicosanoids were increased, and in some cases to a large extent, in the ginger treatment group compared to the placebo group at the end of a 28d intervention using either method of normalization. Of import, however, mean percent increases in eicosanoid concentrations were accompanied by a high level of variability implying that the response to ginger was highly heterogeneous with some participants experiencing no change, others decreases in and others large increases in eicosanoid concentrations.
These findings are in contrast to previous studies in humans as well as those conducted in a rat model and in vitro. Whole ginger root and various ginger constituents have been shown to inhibit leukotriene synthesis by blocking 5-LOX activity [21,40], reduce COX-1 and COX-2 activity and subsequently reduce concentrations of PGE2 in a variety of cell lines [20-22,41-47], and significantly reduce serum levels of PGE2 in female Sprague–Dawley rats given 50 mg/kg ginger extract daily [27]. Our previous work in participants at normal risk for CRC showed that a 2.0 g dose of ginger root extract given for 28 d significantly reduced PGE2 concentrations in colonic mucosa [33]. We also demonstrated that COX-1 protein expression was significantly reduced in participants at increased risk for CRC (those reported on, in this study), although it remained unchanged in participants at normal risk [32]. In contrast, a study by Black and colleagues saw no significant difference in plasma PGE2 levels in healthy volunteers after 28 d of taking 2.0 g of either raw or heat treated ginger root [48]. Different to previous studies, but in agreement with our results, a study in 21 people with knee and hip osteoarthritis found that 28 d of ingesting a 340 mg of a standardized ginger extract (EV.EXT 35) significantly increased levels of several prostaglandins including PGE2 in the stomach mucosa [49].
Differences between these various studies could be due to different doses and formulations of the ginger products, the absorption and metabolism of ginger in in vivo environments; or differential effects of ginger on different tissue types or in situations of underlying inflammation. A clear challenge with natural health products, such as ginger, is the heterogeneity of ginger preparations. A few in vitro studies have shown significant differences between various ginger constituents in impacting COX enzyme activity and PGE2 production [22,42,46]. However, systematic differences in structure and function of various ginger constituents have not been examined in animal models or humans, thus making it unclear if different ginger formulations would behave differently in in vivo situations.
Underlying the importance of examining the effect of ginger in humans and on tissues of interest is the possible differential effects of ginger on different tissue cell types or in situations of underlying inflammation. While ginger and its components appear to decrease the production of inflammatory products such as PGE2 and LTB4, many of these studies were conducted in isolated cell lines and quite a few in murine macrophages (RAW 264.7) using lipopolysaccharide (LPS)-elicited production of PGE2. In vitro studies such as these are useful for exploring possible mechanisms, but they do not model the complex biological interactions that occur in animals or humans. For instance the study by Drozdov and colleagues [49] found that PGE2 and related eicosanoids were elevated in gastric tissue after ginger consumption and they also found an increased concentration of PGE2 in the gastric mucosa.
Another interpretation of our findings, if eciosanoids are good biomarkers of CRC risk, is that ginger may not be an effective CRC prevention agent. Alternately, although a controversial idea, is that inhibition of inflammatory eciosanoids may not equate with prevention of CRC. There is a robust and growing body of evidence that NSAIDs may not act via their conventional anti-COX effects for colon cancer prevention [50]. Consequently, it may be more fruitful for future investigations to focus on ginger’s effect on underlying biological pathways rather than basing ginger’s evaluation on biochemistry such as eicosanoid levels. Numerous in vitro studies have shown significant impacts on various cell cycle markers [51,52]. Moreover, research in people at increased risk of CRC have demonstrated significant effects of ginger on proliferation, differentiation, and apoptosis markers [53].
This study had several limitations, which include a small sample size, a short intervention period, and a fairly large amount of variability in eicosanoid levels. This was intended to be a pilot study for a larger human trial. Thus, it is possible that extended ginger consumption and more study participants might provide additional power to detect the effects of dietary ginger root intake on various prostaglandin pathways. Further, the small sample size did not allow us to conduct additional subgroup analyses by risk type, for example, family history, previous adenoma, previous CRC nor by sociodemographic variables such as age, race, and gender. This study would need to be further replicated in larger trials, which would have adequate power to examine several independent groups defined by baseline colonic mucosal eicosanoid concentrations to account for the presence of low levels of inflammation. Also, future studies may need to focus on outcomes with less variability than tissue eicosanoids such as proliferation, differentiation, and apoptosis markers, which have already been demonstrated to be significantly affected in humans by ginger [53].
In summary, 28 d of supplementation with ginger root extract in participants who were at increased risk for CRC caused a significant decrease in normal colonic mucosa of AA and significantly increased concentrations of LTB4, when normalized to protein. Ginger had no significant effect on any other eicosanoid including PGE2 in colon tissue whether normalized to protein or AA. Ginger did increase eicasanoids other than PGE2, as compared to placebo, when normalized to either AA or protein, but these increases were not statistically significant. Future, larger studies with ginger supplementation should perhaps focus on other biomarker outcomes.
ACKNOWLEDGMENTS
The ginger extract was generously donated by Pure Encapsulations® (Sudbury, MA). We would also like to thank Kate Brummett for assistance with figures. Research reported in this publication was supported by National Cancer Institute of the National Institutes of Health under award number P30 CA047904, P30 CA 48592, K24 CA80846, and K07CA102592 from the National Cancer Institute (NCI) and University of Michigan Clinical Research Center UL1RR024986, and the Kutsche Family Memorial Endowment. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Grant sponsor: National Cancer Institute (NCI); Grant numbers: P30 CA047904; P30 CA 48592; K24 CA80846; K07CA102592; Grant sponsor: University of Michigan Clinical Research Center; Grant number: UL1RR024986; Grant sponsor: Kutsche Family Memorial Endowment
Abbreviations
- CRC
colorectal cancer
- COX
cyclooxygenase
- PGE2
prostaglandin E2
- LTB4
leukotriene B4
- 13-HODE
13-hydroxy-octadecadienoic acids
- 5-, 12-, & 15-HETE
5-, 12-, & 15-hydroxyeicosatetraenoic acid
- AA
arachidonic acid
- AE
adverse events
- NSAIDs
non-steroidal anti-inflammatory drugs
- COX-1 & 2
cyclooxygenase 1 & 2
- LOX
lipoxygenase
- 5-, 12-, & 15-LOX
enzymes
- HETEs
hydroxyeicosatetraenoic acids
- MCRU
University of Michigan Clinical Research Unit
- IDS
Investigational Drug Service
- NCI
National Cancer Institute
- LC/MS/MS
reverse-phase LC electrospray ionization mass spectrometry
- ng
nanogram
- mg
milligram
- μg
microgram
- SD
standard deviations
Footnotes
Conflict of interest: none.
ClinicalTrials.gov Identifier: NCT01344538.
REFERENCES
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Elwood PC, Gallagher AM, Duthie GG, Mur LA, Morgan G. Aspirin, salicylates, and cancer. Lancet. 2009;373:1301–1309. doi: 10.1016/S0140-6736(09)60243-9. [DOI] [PubMed] [Google Scholar]
- 3.Solomon SD, McMurray JJ, Pfeffer MA, et al. Cardiovascular risk associated with celecoxib in a clinical trial for colorectal adenoma prevention. N Engl J Med. 2005;352:1071–1080. doi: 10.1056/NEJMoa050405. [DOI] [PubMed] [Google Scholar]
- 4.James MW, Hawkey CJ. Assessment of non-steroidal anti-inflammatory drug (NSAID) damage in the human gastroin-testinal tract. Br J Clin Pharmacol. 2003;56:146–155. doi: 10.1046/j.1365-2125.2003.01934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cianchi F, Cortesini C, Fantappie O, et al. Cyclooxygenase-2 activation mediates the proangiogenic effect of nitric oxide in colorectal cancer. Clin Cancer Res. 2004;10:2694–2704. doi: 10.1158/1078-0432.ccr-03-0192. [DOI] [PubMed] [Google Scholar]
- 6.Bortuzzo C, Hanif R, Kashfi K, Staiano-Coico L, Shiff SJ, Rigas B. The effect of leukotrienes B and selected HETEs on the proliferation of colon cancer cells. Biochim Biophys Acta. 1996;1300:240–246. doi: 10.1016/0005-2760(96)00003-3. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen CK, Campbell JI, Ohd JF, et al. A novel localization of the G-protein-coupled CysLT1 receptor in the nucleus of colorectal adenocarcinoma cells. Cancer Res. 2005;65:732–742. [PubMed] [Google Scholar]
- 8.Tong WG, Ding XZ, Talamonti MS, Bell RH, Adrian TE. LTB4 stimulates growth of human pancreatic cancer cells via MAPK and PI-3 kinase pathways. Biochem Biophys Res Commun. 2005;335:949–956. doi: 10.1016/j.bbrc.2005.07.166. [DOI] [PubMed] [Google Scholar]
- 9.Soumaoro LT, Iida S, Uetake H, et al. Expression of 5-lipoxygenase in human colorectal cancer. World J Gastro-enterol. 2006;12:6355–6360. doi: 10.3748/wjg.v12.i39.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Melstrom LG, Bentrem DJ, Salabat MR, et al. Overexpression of 5-lipoxygenase in colon polyps and cancer and the effect of 5-LOX inhibitors in vitro and in a murine model. Clin Cancer Res. 2008;14:6525–6530. doi: 10.1158/1078-0432.CCR-07-4631. [DOI] [PubMed] [Google Scholar]
- 11.Ihara A, Wada K, Yoneda M, Fujisawa N, Takahashi H, Nakajima A. Blockade of leukotriene B4 signaling pathway induces apoptosis and suppresses cell proliferation in colon cancer. J Pharmacol Sci. 2007;103:24–32. doi: 10.1254/jphs.fp0060651. [DOI] [PubMed] [Google Scholar]
- 12.Gao P, Guan L, Zheng J. Role of leukotriene B4 in celecoxib-mediated anticancer effect. Biochem Biophys Res Commun. 2010;402:308–311. doi: 10.1016/j.bbrc.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharya S, Mathew G, Jayne D, Pelengaris S, Khan M. 15-Lipoxygenase-1 in colorectal cancer: A review. Tumor Biol. 2009;30:185–199. doi: 10.1159/000236864. [DOI] [PubMed] [Google Scholar]
- 14.Il Lee S, Zuo X, Shureiqi I. 15-Lipoxygenase-1 as a tumor suppressor gene in colon cancer: Is the verdict in? Cancer Metastasis Rev. 2011;30:481–491. doi: 10.1007/s10555-011-9321-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shureiqi I, Chen D, Day RS, et al. Profiling lipoxygenase metabolism in specific steps of colorectal tumorigenesis. Cancer Prev Res (Phila) 2010;3:829–838. doi: 10.1158/1940-6207.CAPR-09-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mascolo N, Jain R, Jain SC, Capasso F. Ethnopharmacologic investigation of ginger (Zingiber officinale) J Ethnopharmacol. 1989;27:129–140. doi: 10.1016/0378-8741(89)90085-8. [DOI] [PubMed] [Google Scholar]
- 17.Kiuchi F, Shibuya M, Sankawa U. Inhibitors of prostaglandin biosynthesis from ginger. Chem Pharm Bull (Tokyo) 1982;30:754–757. doi: 10.1248/cpb.30.754. [DOI] [PubMed] [Google Scholar]
- 18.Koo KL, Ammit AJ, Tran VH, Duke CC, Roufogalis BD. Gingerols and related analogues inhibit arachidonic acid-induced human platelet serotonin release and aggregation. Thromb Res. 2001;103:387–397. doi: 10.1016/s0049-3848(01)00338-3. [DOI] [PubMed] [Google Scholar]
- 19.Sharma JN, Srivastava KC, Gan EK. Suppressive effects of eugenol and ginger oil on arthritic rats. Pharmacology. 1994;49:314–318. doi: 10.1159/000139248. [DOI] [PubMed] [Google Scholar]
- 20.Nurtjahja-Tjendraputra E, Ammit AJ, Roufogalis BD, Tran VH, Duke CC. Effective anti-platelet and COX-1 enzyme inhibitors from pungent constituents of ginger. Thromb Res. 2003;111:259–265. doi: 10.1016/j.thromres.2003.09.009. [DOI] [PubMed] [Google Scholar]
- 21.Kiuchi F, Iwakami S, Shibuya M, Hanaoka F, Sankawa U. Inhibition of prostaglandin and leukotriene biosynthesis by gingerols and diarylheptanoids. Chem Pharm Bull (Tokyo) 1992;40:387–391. doi: 10.1248/cpb.40.387. [DOI] [PubMed] [Google Scholar]
- 22.Tjendraputra E, Tran VH, Liu-Brennan D, Roufogalis BD, Duke CC. Effect of ginger constituents and synthetic analogues on cyclooxygenase-2 enzyme in intact cells. Bioorg Chem. 2001;29:156–163. doi: 10.1006/bioo.2001.1208. [DOI] [PubMed] [Google Scholar]
- 23.Surh YJ, Lee E, Lee JM. Chemoprotective properties of some pungent ingredients present in red pepper and ginger. Mutat Res. 1998;402:259–267. doi: 10.1016/s0027-5107(97)00305-9. [DOI] [PubMed] [Google Scholar]
- 24.Ojewole JA. Analgesic, antiinflammatory and hypoglycaemic effects of ethanol extract of Zingiber officinale (Roscoe) rhizomes (Zingiberaceae) in mice and rats. Phytother Res. 2006;20:764–772. doi: 10.1002/ptr.1952. [DOI] [PubMed] [Google Scholar]
- 25.Park KK, Chun KS, Lee JM, Lee SS, Surh YJ. Inhibitory effects of [6]-gingerol, a major pungent principle of ginger, on phorbol ester-induced inflammation, epidermal ornithine decarboxylase activity and skin tumor promotion in ICR mice. Cancer Lett. 1998;129:139–144. doi: 10.1016/s0304-3835(98)00081-0. [DOI] [PubMed] [Google Scholar]
- 26.Suekawa M, Aburada M, Hosoya E. Pharmacological studies on ginger. II. Pressor action of (6)-shogaol in anesthetized rats, or hindquarters, tail and mesenteric vascular beds of rats. J Pharmacobiodyn. 1986;9:842–852. doi: 10.1248/bpb1978.9.842. [DOI] [PubMed] [Google Scholar]
- 27.Thomson M, Al-Qattan KK, Al-Sawan SM, Alnaqeeb MA, Khan I, Ali M. The use of ginger (Zingiber officinale Rosc.) as a potential anti-inflammatory and antithrombotic agent. Prostaglandins Leukot Essent Fatty Acids. 2002;67:475–478. doi: 10.1054/plef.2002.0441. [DOI] [PubMed] [Google Scholar]
- 28.Kim M, Miyamoto S, Yasui Y, Oyama T, Murakami A, Tanaka T. Zerumbone, a tropical ginger sesquiterpene, inhibits colon and lung carcinogenesis in mice. Int J Cancer. 2009;124:264–271. doi: 10.1002/ijc.23923. [DOI] [PubMed] [Google Scholar]
- 29.Manju V, Nalini N. Chemopreventive efficacy of ginger, a naturally occurring anticarcinogen during the initiation, post-initiation stages of 1,2 dimethylhydrazine-induced colon cancer. Clin Chim Acta. 2005;358:60–67. doi: 10.1016/j.cccn.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 30.Yoshimi N, Wang A, Morishita Y, et al. Modifying effects of fungal and herb metabolites on azoxymethane-induced intestinal carcinogenesis in rats. Jpn J Cancer Res. 1992;83:1273–1278. doi: 10.1111/j.1349-7006.1992.tb02758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dias MC, Spinardi-Barbisan AL, Rodrigues MA, de Camargo JL, Teran E, Barbisan LF. Lack of chemopreventive effects of ginger on colon carcinogenesis induced by 1,2-dimethylhydrazine in rats. Food Chem Toxicol. 2006;44:877–884. doi: 10.1016/j.fct.2005.11.015. [DOI] [PubMed] [Google Scholar]
- 32.Jiang Y, Turgeon DK, Wright BD, et al. Effect of ginger root on cyclooxygenase-1 and 15-hydroxy-prostaglandin dehydrogenase expression in colonic mucosa of humans at normal and increased risk for colorectal cancer. Eur J Cancer Prev. 2012;5:455–460. doi: 10.1097/CEJ.0b013e32835c829b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zick SM, Turgeon DK, Vareed SK, et al. Phase II study of the effects of ginger root extract on eicosanoids in colon mucosa in people at normal risk for colorectal cancer. Cancer Prev Res (Phila) 2011;4:1929–1937. doi: 10.1158/1940-6207.CAPR-11-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zick SM, Djuric Z, Ruffin MT, et al. Pharmacokinetics of 6-gingerol, 8-gingerol, 10-gingerol, and 6-shogaol and conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1930–1936. doi: 10.1158/1055-9965.EPI-07-2934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.U.S., Department of Health and Human Services NCI Commmon Terminology Criteria for Adverse Events (CTCAE) 2009 Sep 15; 2009 v4.02 [cited 2013 January 22]; Version 4.0:[Available from: http://www.acrin.org/Portals/0/Administration/Regulatory/CTCAE_4.02_2009-09-15_QuickReference_5×7.pdf.
- 36.Yang P, Chan D, Felix E, et al. Determination of endogenous tissue inflammation profiles by LC/MS/MS: COX- and LOX-derived bioactive lipids. Prostaglandins Leukot Essent Fatty Acids. 2006;75:385–395. doi: 10.1016/j.plefa.2006.07.015. [DOI] [PubMed] [Google Scholar]
- 37.Vareed SK, Kakarala M, Ruffin MT, et al. Pharmacokinetics of curcumin conjugate metabolites in healthy human subjects. Cancer Epidemiol Biomarkers Prev. 2008;17:1411–1417. doi: 10.1158/1055-9965.EPI-07-2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Buczynski MW, Stephens DL, Bowers-Gentry RC, Grkovich A, Deems RA, Dennis EA. TLR-4 and sustained calcium agonists synergistically produce eicosanoids independent of protein synthesis in RAW264.7 cells. J Biol Chem. 2007;282:22834–22847. doi: 10.1074/jbc.M701831200. [DOI] [PubMed] [Google Scholar]
- 39.Krishnan K, Ruffin MT, Normolle D, et al. Colonic mucosal prostaglandin E2 and cyclooxygenase expression before and after low aspirin doses in subjects at high risk or at normal risk for colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2001;10:447–453. [PubMed] [Google Scholar]
- 40.Flynn DL, Rafferty MF, Boctor AM. Inhibition of human neutrophil 5-lipoxygenase activity by gingerdione, shogaol, capsaicin and related pungent compounds. Prostaglandins Leukot Med. 1986;24:195–198. doi: 10.1016/0262-1746(86)90126-5. [DOI] [PubMed] [Google Scholar]
- 41.Lantz RC, Chen GJ, Sarihan M, Solyom AM, Jolad SD, Timmermann BN. The effect of extracts from ginger rhizome on inflammatory mediator production. Phytomedicine. 2007;14:123–128. doi: 10.1016/j.phymed.2006.03.003. [DOI] [PubMed] [Google Scholar]
- 42.van Breemen RB, Tao Y, Li W. Cyclooxygenase-2 inhibitors in ginger (Zingiber officinale) Fitoterapia. 2011;82:38–43. doi: 10.1016/j.fitote.2010.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kim SO, Chun KS, Kundu JK, Surh YJ. Inhibitory effects of [6]-gingerol on PMA-induced COX-2 expression and activation of NF-kappaB and p38 MAPK in mouse skin. Biofactors. 2004;21:27–31. doi: 10.1002/biof.552210107. [DOI] [PubMed] [Google Scholar]
- 44.Jolad SD, Lantz RC, Chen GJ, Bates RB, Timmermann BN. Commercially processed dry ginger (Zingiber officinale): Composition and effects on LPS-stimulated PGE2 production. Phytochemistry. 2005;66:1614–1635. doi: 10.1016/j.phytochem.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 45.Han YA, Song CW, Koh WS, et al. Anti-inflammatory effects of the Zingiber officinale roscoe constituent 12-dehydrogingerdione in lipopolysaccharide-stimulated Raw 264.7 cells. Phytother Res. 2013;27:1200–1205. doi: 10.1002/ptr.4847. [DOI] [PubMed] [Google Scholar]
- 46.Li F, Nitteranon V, Tang X, et al. In vitro antioxidant and anti-inflammatory activities of 1-dehydro-[6]-gingerdione, 6-shogaol, 6-dehydroshogaol and hexahydrocurcumin. Food Chem. 2012;135:332–337. doi: 10.1016/j.foodchem.2012.04.145. [DOI] [PubMed] [Google Scholar]
- 47.Ha SK, Moon E, Ju MS, et al. 6-Shogaol, a ginger product, modulates neuroinflammation: A new approach to neuro-protection. Neuropharmacology. 2012;63:211–223. doi: 10.1016/j.neuropharm.2012.03.016. [DOI] [PubMed] [Google Scholar]
- 48.Black CD, Herring MP, Hurley DJ, O’Connor PJ. Ginger (Zingiber officinale) reduces muscle pain caused by eccentric exercise. J Pain. 2010;11:894–903. doi: 10.1016/j.jpain.2009.12.013. [DOI] [PubMed] [Google Scholar]
- 49.Drozdov VN, Kim VA, Tkachenko EV, Varvanina GG. Influence of a specific ginger combination on gastropathy conditions in patients with osteoarthritis of the knee or hip. J Altern Complement Med (NY) 2012;18:583–588. doi: 10.1089/acm.2011.0202. [DOI] [PubMed] [Google Scholar]
- 50.Liggett JL, Zhang X, Eling TE, Baek SJ. Anti-tumor activity of non-steroidal anti-inflammatory drugs: Cyclooxygenase-independent targets. Cancer Lett. 2014;346:217–224. doi: 10.1016/j.canlet.2014.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Baliga MS, Haniadka R, Pereira MM, et al. Update on the chemopreventive effects of ginger and its phytochemicals. Crit Rev Food Sci Nutr. 2011;51:499–523. doi: 10.1080/10408391003698669. [DOI] [PubMed] [Google Scholar]
- 52.Butt MS, Sultan MT. Ginger and its health claims: Molecular aspects. Crit Rev Food Sci Nutr. 2011;51:383–393. doi: 10.1080/10408391003624848. [DOI] [PubMed] [Google Scholar]
- 53.Citronberg J, Bostick RM, Ahearn TU, et al. Effects of ginger supplementation on cell cycle biomarkers in the normal-appearing colonic mucosa: Results from a pilot, randomized, controlled trial. Cancer Prev Res (Phila) 2013;4:271–281. doi: 10.1158/1940-6207.CAPR-12-0327. [DOI] [PMC free article] [PubMed] [Google Scholar]